Abstract
Reaction of 4,5,6-trichloropyrimidine-2-carbonitrile (1) with concentrated sulfuric acid at ca. 20 °C gave 4,5,6-trichloropyrimidine-2-carboxamide (5) in 91% yield. The new compound was fully characterized by IR, MALDI-TOF, NMR and elemental analysis.
1. Introduction
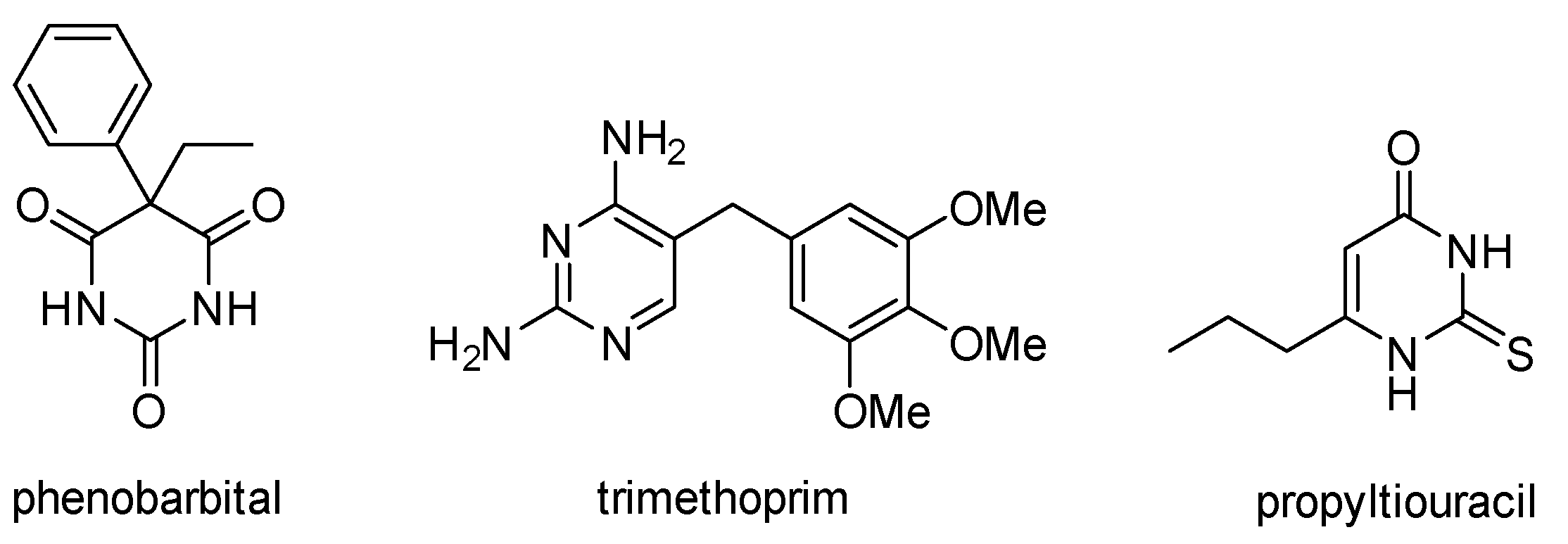
Pyrimidines are important aromatic N-heterocycles that exist in nature; for example, as components of pyrimidine nucleotides (cytosine, thymine and uracil) [1]. Pyrimidines are also frequently used in pharmaceuticals as they rank 10th in the most frequently used nitrogen heterocycles in U.S. FDA approved drugs [2]. Examples of pyrimidine drugs are the CNS depressant phenobarbital, the antibacterial trimethoprim, and the hyperthyroidism drug propylthiouracil (Figure 1). Additional pharmaceutical applications include uses as diuretics [3], anti-inflammatory [4], anti-malarial [5], and anti-tumor [6] agents. The chemistry of pyrimidines has been reviewed [7].
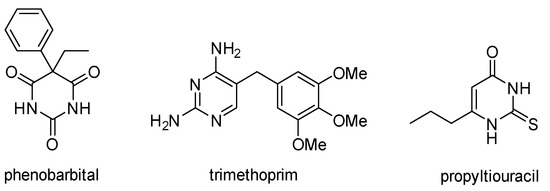
Figure 1.
Pyrimidine containing drugs.
2. Results and Discussion
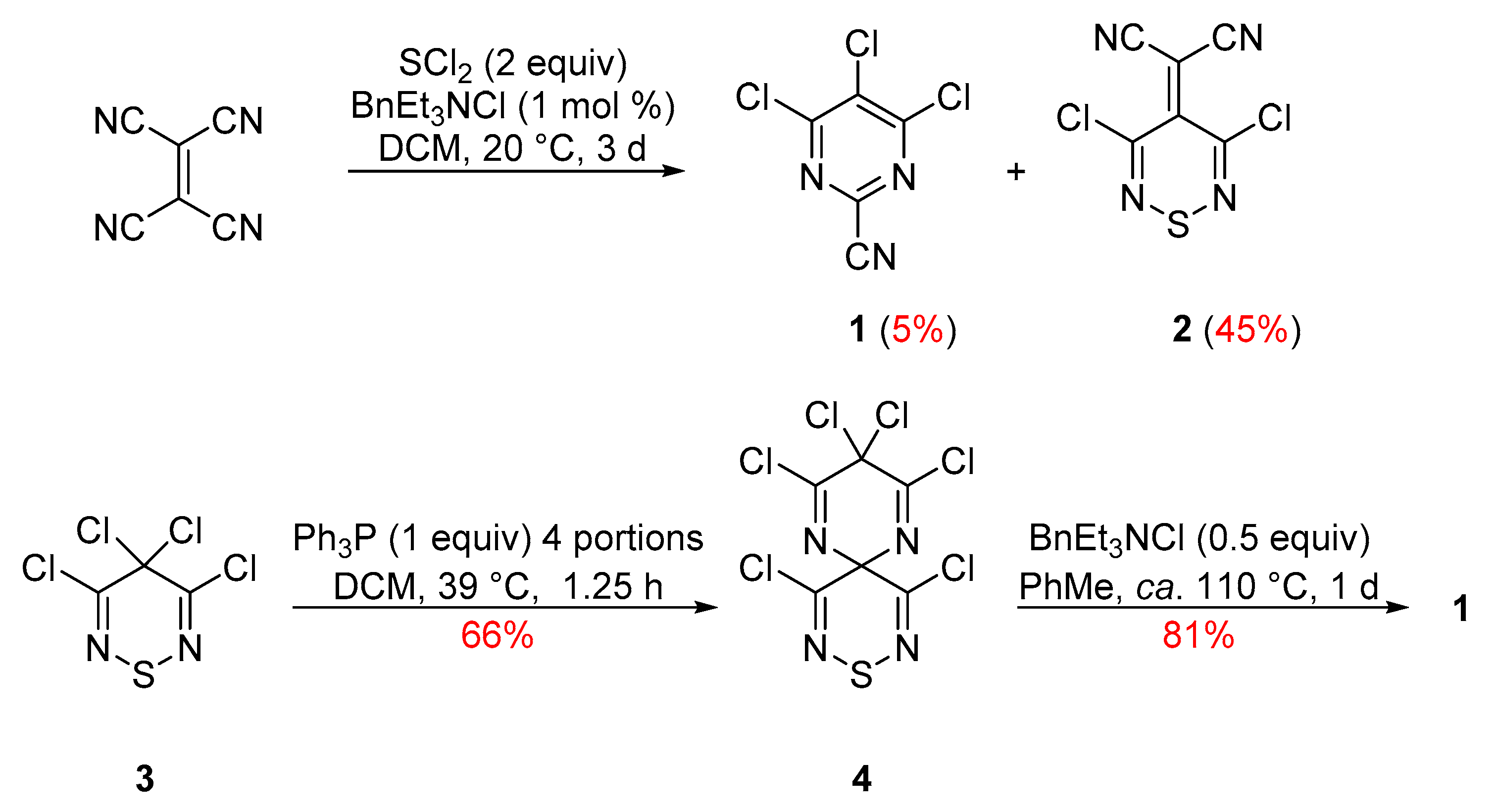
Our interest in pyrimidines began with 4,5,6-trichloropyrimidine-2-carbonitrile (1), a compound that was first isolated as an unexpected minor product from the reaction of tetracyanoethene (TCNE) with SCl2 during the preparation of 2-(3,5-dichloro-4H-1,2,6-thiadiazin-4-ylidene)malononitrile (2) [8] (Scheme 1). A more efficient synthesis of pyrimidine 1 was subsequently developed, starting from the less readily available but highly reactive tetrachlorothiadiazine 3 via perchloro-9-thia-1,5,8,10-tetraazaspiro[5.5]undeca-1,4,7,10-tetraene (4) with a 53% overall yield [9] (Scheme 1). Other efforts to develop an independent synthesis of pyrimidine 1 [10,11] or investigate its chemistry [12] were also reported.
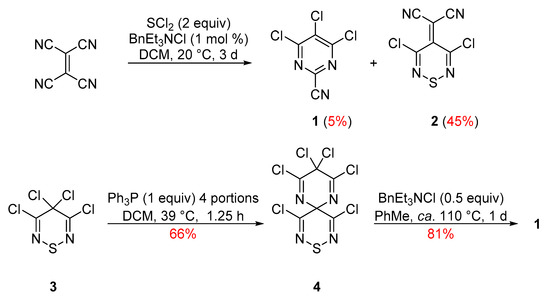
Scheme 1.
Preparation of trichloropyrimidine 1 from TCNE and from tetrachlorothiadiazine 3.
We are interested in studying the use of trichloropyrimidine 1 as a synthetic scaffold as it offers multiple sites of reactivity towards heteroatom nucleophiles or organometallic reagents. To identify any potential side products from the chemistry of pyrimidine 1, we investigated its hydration to 4,5,6-trichloropyrimidine-2-carboxamide (5). Having frequently worked with cyano-substituted heterocycles, we often encountered the hydration products of the nitrile group [13]; therefore, we considered preparing, isolating, and characterizing this carboxamide worthwhile.
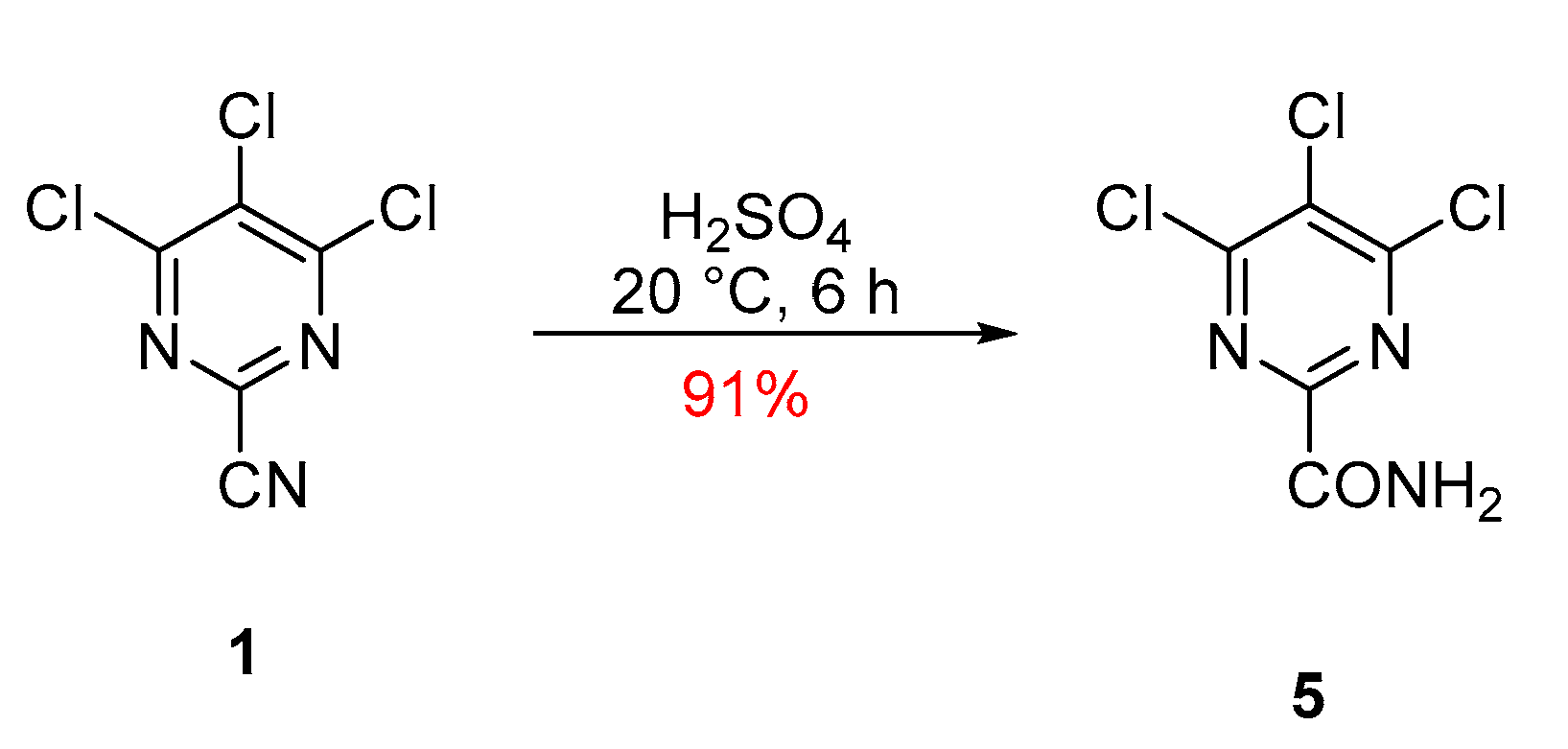
The reaction involved stirring a solution of trichloropyrimidine 1 in concentrated sulfuric acid for 6 h that, after workup, gave the desired compound with a 91% yield (Scheme 2). Product 5 was then isolated as colorless plates, melting point (mp) 162–164 °C (from c-hexane/DCE), while FTIR spectroscopy showed ν(N-H) stretches at 3402, 3291, 3219 and 3167 cm−1, along with a C=O stretch at 1686 cm−1, indicative of an amide (see Supplementary Material). Two diastereotopic protons in 1H NMR (acetone-d6) were present as broad singlets, 8.04 and 7.32 ppm, assigned to the amide functionality, while 13C NMR (acetone-d6) showed the presence of four quaternary carbon resonances at 161.9, 160.6, 155.8, and 131.1 ppm. Compared to the starting material 1, which had a C≡N resonance at 113.4 ppm, the new product 5 lacked this signal and displayed a new down-field signal at 160.6 ppm, which is typical for an amide C=O resonance supporting the hydration of the nitrile functionality. Finally, a correct elemental analysis (CHN) was obtained for the molecular formula C5H2Cl3N3O.
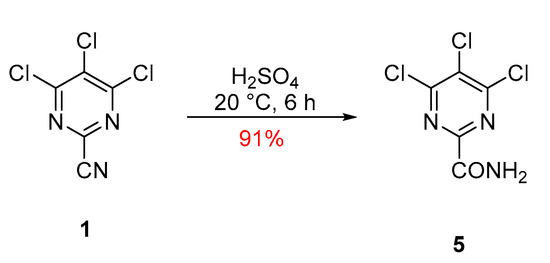
Scheme 2.
Synthesis of 4,5,6-trichloropyrimidine-2-carboxamide (5).
Pyrimidine 5 is potentially a useful synthetic scaffold and could be used instead of trichloropyrimidine 1 as it has one less leaving group that could lead to more regioselective substitution chemistry, while its higher melting point (162–164 °C vs. 62–63 °C for cyano 1 [10]) would make it more stable in storage.
3. Materials and Methods
The reaction mixture was monitored by thin layer chromatography (TLC) using commercial glass-backed TLC plates (Merck Kieselgel 60 F254). The plates were observed under UV light at 254 and 365 nm. The melting point was determined using a PolyTherm-A, Wagner & Munz, Kofler hot-stage Microscope apparatus (Wagner & Munz, Munich, Germany). The solvent used for recrystallization is indicated after the melting point. The UV-vis spectrum was obtained using a Perkin–Elmer Lambda-25 UV/Vis spectrophotometer (Perkin–Elmer, Waltham, MA, USA); inflections are identified by the abbreviation “inf”. The IR spectrum was recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer (Shimadzu, Kyoto, Japan) with a Pike Miracle Ge ATR accessory (Pike Miracle, Madison, WI, USA); strong, medium, and weak peaks are represented by s, m, and w, respectively. A Bruker Avance 500 machine (Bruker, Billerica, MA, USA) was used at 500 and 125 MHz to record the 1H and 13C NMR spectra, respectively. Deuterated solvents were used for the homonuclear lock; the signals are referenced to the deuterated solvent peaks. Attached proton test (APT) NMR studies were used for the assignment of the 13C peaks as CH3, CH2, CH, and Cq (quaternary). The MALDI-TOF mass spectrum (+ve mode) was recorded on a Bruker Autoflex III Smartbeam instrument (Bruker). The elemental analysis was run by the London Metropolitan University Elemental Analysis Service. 4,5,6-Trichloropyrimidine-2-carbonitrile (1) was prepared according to the literature procedure [9].
4,5,6-Trichloropyrimidine-2-carboxamide (5)
To stirred concentrated sulfuric acid (2 mL) at ca. 20 °C was added 4,5,6-trichloropyrimidine-2-carbonitrile (1) (104 mg, 0.500 mmol), and the mixture was stirred at this temperature until complete consumption of the starting material (TLC, 6 h). The mixture was then poured into crushed ice and the mixture was then extracted with DCM (5 × 10 mL) and dried (Na2SO4). The solvent was then evaporated in vacuo to give the title compound 5 (103 mg, 91%) as colorless plates, mp 162–164 °C (from c-hexane/DCE); Rf 0.21 (DCM); (found: C, 26.47; H, 0.72; N, 18.43. C5H2Cl3N3O requires C, 26.52; H, 0.89; N, 18.56%); λmax(DCM)/nm 245 (log ε 3.01), 265 inf (2.84); vmax/cm−1 3402w, 3291w, 3219w and 3167w (N-H), 1686s (C=O), 1601m, 1508m, 1497s, 1439w, 1304s, 1234w, 1111w, 1055m, 881w, 822m, 808s, 760m; δH(500 MHz; CDCl3) 7.53 (1H, br s, NH2), 6.33 (1H, br s, NH2); δC(125 MHz; CDCl3) 161.3 (Cq), 160.8 (Cq), 153.5 (Cq), 131.4 (Cq); δH[500 MHz; (CD3)2CO] 8.04 (1H, br s, NH2), 7.32 (1H, br s, NH2); δC[125 MHz; (CD3)2CO] 161.9 (Cq), 160.6 (Cq), 155.8 (Cq), 131.1 (Cq); m/z (MALDI-TOF) 229 (M+ + 4, 34%), 226 (M+ − H + 2, 100%), 224 (M+ − H, 91), 207 (M+ − H2O, 37).
Supplementary Materials
The following are available online: mol file, IR, mass spectrometry, 1H and 13C NMR spectra in CDCl3 and (CD3)2CO, UV/Vis spectrum.
Author Contributions
P.A.K. and A.S.K. conceived the experiments; A.S.K. designed and performed the experiments, analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Cyprus Research Promotion Foundation, grant numbers ΣΤΡΑΤΗΙΙ/0308/06, NEKYP/0308/02 ΥΓΕΙΑ/0506/19 and ΕΝΙΣΧ/0308/83.
Acknowledgments
The authors thank the following organizations and companies in Cyprus for generous donations of chemicals and glassware: The State General Laboratory, the Agricultural Research Institute, the Ministry of Agriculture, MedoChemie Ltd., Medisell Ltd., and Biotronics Ltd. Furthermore, we thank the A. G. Leventis Foundation for helping to establish the NMR facility at the University of Cyprus.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Soukup, G.A. Nucleic Acids: General Properties. In Encyclopedia of Life Sciences; Maccarrone, M., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2003. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Tugaibei, I.A.; Bereznykova, N.L.; Karvechenko, V.N.; Turov, A.V. 4-Hydroxy-2-quinolones 144. Alkyl-, arylalkyl-, and arylamides of 2-hydroxy-4-oxo-4H-pyrido[1,2-a]pyrimidine-3-carboxylic acid and their diuretic properties. Chem. Heterocycl. Com. 2008, 44, 565–575. [Google Scholar] [CrossRef]
- Amr, A.E.; Nermien, M.S.; Abdulla, M.M. Synthesis, reactions, and anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon. Monatsh. Chem. 2007, 138, 699–707. [Google Scholar] [CrossRef]
- Gorlitzer, K.; Herbig, S.; Walter, R.D. Indeno[1,2-d]pyrimidin-4-yl-amines. Pharmazie 1997, 52, 670–672. [Google Scholar]
- Wagner, E.; Al-Kadasi, K.; Zimecki, M.; Sawka-Dobrowolska, W. Synthesis and pharmacological screening of derivatives of isoxazolo[4,5-d]pyrimidine. Eur. J. Med. Chem. 2008, 43, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.J. Pyrimidines and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Oxford, UK, 1984; Volume 3, pp. 57–155. [Google Scholar]
- Koutentis, P.A.; Rees, C.W. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. J. Chem. Soc. Perkin Trans. 1 2000, 1089–1094. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Manoli, M.; Koutentis, P.A. Two-step conversion of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine into 4,5,6-trichloropyrimidine-2-carbonitrile. Tetrahedron Lett. 2017, 58, 2618–2621. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Kourtellaris, A.; Koutentis, P.A. Synthesis of 4,5,6-trichloropyrimidine-2-carbonitrile from 4,6-dichloro-2-(methylthio)pyrimidine. ARKIVOC 2020, vii, 27–35. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. Synthesis of 2-Cyanopyrimidines. Molbank 2019, 2019, M1086. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. Reactions of Polychlorinated Pyrimidines with DABCO. Molbank 2019, 2019, M1084. [Google Scholar] [CrossRef]
- Christoforou, I.C.; Kalogirou, A.S.; Koutentis, P.A. The preparation of dicyano-1,3,4-thiadiazole and tricyanothiazole via 1,2,3-dithiazole chemistry. Tetrahedron 2009, 65, 9967–9972. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).