Abstract
Three new Schiff bases of isoniazid were synthesized using microwave-assisted synthesis and conventional condensation with aromatic aldehydes. Synthesized compounds were characterized using elemental analysis, IR, NMR, and Mass spectroscopy. Synthesized compounds were evaluated for antiproliferative activity against MCF-7 cell line. The IC50 values were from 125 to 276 µM. The compounds were also evaluated for antibacterial activity against Staphylococcus aureus and Escherichia coli. Results showed that the synthesized compounds produce significant antibacterial activity in vitro. Inhibition of compounds ranged from 13 to 18 mm.
1. Introduction
The Schiff bases of isoniazid and their derivatives exhibited antitubercular [1,2,3], antibacterial, antifungal and cytotoxic activities [4], antimicrobial and urease inhibitory activity [5] and antidepressant and analgesic properties [6]. The carvone Schiff base of isoniazid was evaluated for its pharmacokinetic profile in rat [7]. Schiff bases are the compounds that possess azomethine or imine (-C=N-) as their functional group serving as key pharmacophore for the development of biologically active compounds, which can be synthesized by the condensation reaction of primary amine with carbonyl compounds. It was observed that in recent years, isoniazid and its derivatives have received attention and are being widely studied. The derivatives of isoniazid are evaluated for their various biological activities, such as anti-inflammatory [8], anticancer [9,10], antimicrobial [11,12,13], anti-tubercular [2,11,14,15,16,17], and in the treatment of Alzheimer’s disease [18].
In men, the most common place of cancer occurrence is in the lungs, prostrate, colorectum, stomach and liver. In women, it occurs in breast, colorectum, stomach, lungs and cervix [19]. Breast cancer is the most common cancer and is the second highest cause of death in women. At present, the treatment includes surgical removal of the tumor followed by radiation therapy with systemic chemotherapy. Chemotherapy is the most common and widely used therapy for cancer at all stages. The role of a medicinal chemist is to develop new molecules that exhibit various biological activities, such as anticancer and antibacterial; keeping this in view, there is a need to develop new synthetic anticancer drugs that minimize or overcome the side effects or target the cell cycle. There is also need to develop new chemical entities that can counteract the infection caused by microorganisms that are developing resistance to the existing therapeutics. These properties encouraged us to undertake the synthesis of Schiff Bases of isoniazid that might exhibit bioactive properties against breast cancer MCF-7 cell lines and combat the drug-resistant bacterial infection. In the present communication, we are reporting the conventional and economical microwave assisted synthesis of Schiff bases of isoniazid. The percent yield and time required to complete the synthesis are being compared.
2. Results and Discussion
2.1. Chemical Section
Conventional and microwave-assisted synthesis produced the anticipated product with an average yield of 95.0% and 98.5% respectively (Scheme 1). The microwave-assisted synthesis is 20 to 45 times faster than the conventional method, which takes 6 to 8 h. With respect to the yield and purity of the product, the microwave-assisted synthesis requires less time and energy than the conventional method [20]. With the help of a Robotic Biotage® Initiator+Alstra™ microwave plus peptide synthesizer, several compounds can be synthesized in a few hours. The percent yield of the product using both methods is presented in the experimental section. The NMR, LCMS/MS, IR spectroscopic, and Elemental analysis techniques were used to characterize the synthesized compounds. The spectral data are presented as Supplementary data. The 1H-NMR data of Schiff bases indicate that the N=CH proton resonates between 8.35 to 8.54 ppm. In case of IR spectroscopy, the carbonyl functional group of (CO-NHN=) was observed between 1657 to 1674 cm−1 and the N-H bending between 1533 to 1578 cm−1. The LCMS/MS spectroscopic data indicate the facile synthesis of the Schiff bases of isoniazid.
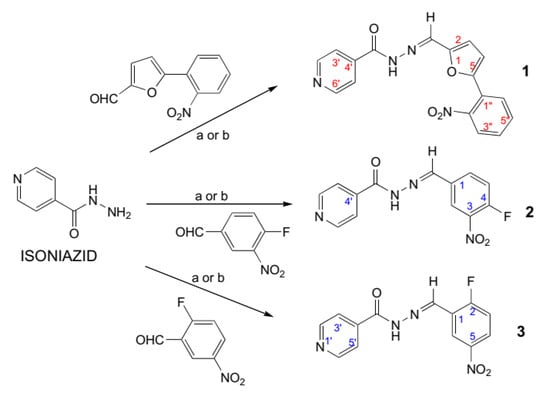
Scheme 1.
Synthesis of the Schiff bases of isoniazid. Conditions (a): absolute ethanol, acetic acid, microwave irradiation (5–10 W, 0–1 atm. pressure), 10 to 20 min, at 85 °C. (b) Absolute ethanol, acetic acid, and reflux 6–8 h.
2.2. Biological Studies
2.2.1. Antiproliferative Activity
The human breast adenocarcinoma MCF-7 cells were used to study the preliminary anti-proliferative activity of the compounds. The antiproliferative activities of the compounds were determined as described elsewhere by our group without modification [21,22]. Results are reported in Table 1. The IC50 values of the synthesized compound ranged from 125 to 276 µM. Compound (E)-N’-(4-fluoro-3-nitrobenzylidene)isonicotinohydrazide (2) showed superior activity (IC50 125 ± 3µM) when compared to the other compounds. Vincristine sulfate was used as standard antiproliferative agent against the MCF-7 cell line. It is a well-known anticancer drug; in the present study, the synthesized compounds showed low to moderate activity, compared to standard. The vincristine sulfate inhibits the microtubule formation in mitotic spindle, resulting in an arrest of dividing cells at the metaphase stage.

Table 1.
Cytotoxic activity (IC50) against MCF-7 cell lines and antibacterial activity of Schiff Bases.
2.2.2. Antibacterial Screening
The compound (E)-N’-((5-(2-nitrophenyl)furan-2-yl)methylene)isonicotinohydrazide (1) showed better antibacterial activity than compounds (2) and (3) against Gram-positive and Gram-negative strains. The compounds showed weak activity against Gram-positive and Gram-negative microorganism with respect to the standard drugs, such as amoxicillin, gentamycin, and tobramycin.
The preliminary results indicate that more structural modification is required to enhance the anti-proliferative and antibacterial activities. With this limited data, it can be noted that compound (1), with a furan ring system, produces better antibacterial activity than the aromatic ring.
3. Materials and Methods
3.1. General
Starting reagents were purchased from Sigma Aldrich (St. Louis, Missouri, USA). USA) and were used without any purification. 1H-NMR were recorded on Bruker DPX-300 (Billerica, MA, USA) instrument (300 MHz) and Bruker 500 MHz-Avance III (500 MHz) spectrometer (Billerica, MA, USA), while 13C-NMR spectra were recorded on Bruker 500 MHz-Avance III (at 125 MHz) at room temperature using TMS as reference. The solvent used for NMR was DMSO-d6. Chemical shifts are reported in parts per million (δ/ppm). Coupling constants are reported in Hertz (J/Hz). The peak patterns are indicated as follows: s, singlet; d, doublet; t, triplet; q, quadruplet; m, multiplet; dd, doublet of doublets, br/m, broad multiplet and br/s, broad singlet. Infrared spectra were measured on an Shimadzu Prestige-21 FT-IR instrument (Shimadzu Corporation, Kyoto, Japan). The signals are reported in reciprocal centimeters (cm−1). Mass spectra were recorded with LC-MS/MS-4500Qtrap Triple quadrupole liquid chromatograph mass-spectrometer from ABSciex USA (ESI) (AB Sciex LLC, Framingham, MA, USA) using methanol and 1% formic acid as mobile phase for infusion. Elemental analyses (C, H, and N) were performed on a Perkin-Elmer model 2400 analyzer (PerkinElmer, Shelton, CT, USA). Microwave-assisted synthesis was performed on Biotage® Initiator+ Alstra™, which is a fully automated microwave plus peptide synthesizer (Biotage®, Uppsala, Sweden). The melting points were determined in open capillary and are uncorrected.
General Procedure for the Synthesis of Schiff Bases of Isoniazid
(a) Microwave-assisted synthesis. Various aldehydes (1mM) each in ethanol were added to isoniazid (1mM) in ethanol with intermittent shaking separately. To this mixture, glacial acetic acid (GAA, 2 drops) was added dropwise with shaking. The microwave glass tubes were closed with the help of Teflon septum and aluminum caps. The microwave synthesis was performed by irradiating the reaction mixture under microwave (5–10 W, 0–1 atm. pressure), at 85 °C until the completion of the reaction (10 to 20 min.). TLC was used to monitor the progress of the reaction.
(b) Conventional method. Various aldehydes (2mM) each in ethanol were added to isoniazid (2mM) in ethanol with intermittent shaking separately. To this mixture, glacial acetic acid (GAA) was added dropwise with shaking and then refluxed for 6 to 8 h. The completion of reaction was monitored by thin layer chromatography using chloroform:methanol:acetic acid (90:10:0.5 v/v/v) as solvent system. The reaction mixture was concentrated, and the residue obtained washed with water and dried. The crude product obtained was recrystallized using ethyl alcohol.
(E)-N’-((5-(2-Nitrophenyl)furan-2-yl)methylene)isonicotinohydrazide (1): (yield, time (a) 99%, 15 min. (b) 95%, 6h, yellow crystalline), m.p. 180–182 °C (decomposed). 1H-NMR (300 MHz, DMSO-d6): δ: 12.04 (s, 1H, NH-N=); 8.76–8.74 (br/m, 2H, H2′ and H6′ of pyr); 8.35 (s, 1H, N=CH-); 7.94–7.91(br/m, 2H, H3′’ and H6′’ of ArH); 7.72–7.85 (m, 3H, H3′, H5′ of pyr and H5′’ of ArH); 7.58–7.63 (br, 1H, H4′’ of ArH); 7.03 (d, 1H, J = 12 Hz, H4 of furan), 6.99 (d, 1H, J = 12 Hz, H3 of furan). LCMS/MS for C17H12N4O4 (ESI+ion): m/z = 337.6 [M + 1], 336.6[M+], 319.2, 216, 148, 121, 104.8, 80, 79. IR (KBr, cm−1): 3442.9 (N-H), 3209.6 (C-H, arom.), 1660.7 (C=O), 1616.4 (C=C), 1533.4 (N-H), 1410.0 (C-N), and 690.5 (C-H, arom.). Elemental Analysis C17H12N4O4, Calculated C, 60.71; H, 3.60; N, 16.66; Found C, 60.49; H, 3.59; N 16.62. Rf value = 0.69 (chloroform:methanol:acetic acid: 90:10:0.5 v/v/v).
(E)-N’-(4-Fluoro-3-nitrobenzylidene)isonicotinohydrazide (2): (yield, time (a) 98%, 10 min. (b) 95%, 6 h, brown crystals), m.p. 200–205 °C (decomposed). 1H-NMR (500 MHz, DMSO-d6): δ: 12.32 (1H; s; NH-N=); 8.81(d, 2H, J = 5.0 Hz, H-2′ and H-6′, pyr); 8.54 (1H, s, N=C-H), 8.51 (d, 1H, J = 6.8 Hz, H-2), 8.19 (d, 1H, J = 7.0 Hz, H-6), 7.83 (d, 2H, J = 5.0 Hz, H-3′ and H-5′, pyr), 7.69 (dd, 1H, 3JH-F = 10.5 Hz, H-5); 13C-NMR (125 MHz, DMSO-d6): δ 162.38 (C=O), 155.77 (d, 1JC-F = 263 Hz, C-4), 150.84 (C-2′ and C-6′, pyr), 146.15 (C′′), 140.64 (C-4′), 137.78 (d, 2JC-F = 7.50 Hz, C-2), 134.95 (d, 2JC-F = 9.5 Hz, C-3), 131.98 (C-1), 124.80 (C-6), 122.02 (C-3′ and C-5′, pyr), 119.74 (d, 2JC-F = 21.50 Hz, C-5). LC-MS/MS for C13H9FN4O3 (ESI+ion): m/z = 289.6 [M+1], 288.6, 242.8, 123, 121, 104, 80, 79. IR (KBr, cm−1): 3446.8 (N-H), 3201.8, 3118.9, 3086.1 (C-H, arom.), 1674.2 (C=O), 1618.3 (C=C), 1577.8 (N-H), 1537.3 (C=C), 1411.9 (C-N), 1159.2 (C-F), and 752.2 and 694.4 (C-H, arom.). Elemental Analysis C13H9FN4O3, Calculated C, 54.17; H, 3.15; N, 19.44; Found C, 54.29; H, 3.16; N 19.51. Rf value = 0.52 (chloroform: methanol: acetic acid:90:10:0.5 v/v/v).
(E)-N’-(2-Fluoro-5-nitrobenzylidene)isonicotinohydrazide (3): (yield, time (a) 98.5%, 20 min. (b) 95.0%, 8 h, off white, fine crystals), m.p. 208–210 °C; 1H-NMR (300 MHz, DMSO-d6): δ: 12.38 (s, 1H, NH-N=C); 8.82(d, J = 5.1 Hz, 2H, H-2′ and H-6′, pyr), 8.70 (s/br, N=C-H, overlapped with 1H, H-6), 8.35-8.37 (m/br, 1H, H-4), 7.85 (d, J = 5.1 Hz, 2H, H-3′ and H-5′, pyr), 7.64 (dd, 1H, 3JH-F =10.5 Hz, H-3). LCMS/MS for C13H9FN4O3 (ESI+ion): m/z = 289.8 [M + 1], 288.8, 242.8, 126, 123, 121, 105, 80, 79. IR (KBr, cm−1): 3427.5 (N-H), 3228.8, 3203.8, 3074.5 (C-H, arom.), 1656.9(C=O), 1626.0 (C=C), 1602.9 (C=C), 1556.6 (N-H), 1525.7 (C=C), 1423.5 (C-N), 1143.8 (C-F), 746.5 (C-H, arom.), and 682.8 (C-H, arom.); Elemental Analysis C13H9FN4O3, Calculated: C, 54.17; H, 3.15; N, 19.44; Found C, 54.32; H, 3.14; N 19.56. Rf value = 0.63 (chloroform: methanol: acetic acid:90:10:0.5 v/v/v).
3.2. Chemicals and Cell Lines
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] was purchased from Sigma Chemicals (St. Louis, Missouri, USA). The human MCF-7 cells were procured from The University of Jordan, Amman. Cells developed in an ideal media magnified with 10% warmth inactivated fetal bovine serum (FBS), 100 μg of streptomycin/mL, 100 U/mL of penicillin, and were incubated in humidified environment with 5% CO2 at 37 °C.
3.2.1. Antiproliferative Activity
The human breast adenocarcinoma MCF-7cells were used to study the preliminary anti-proliferative activity of the compounds [21,22]. Each experiment was carried out in triplicate.
3.2.2. Antibacterial Screening
Synthetic compounds were tested for antibacterial activity [23] against Gram negative (E. coli ATCC 8739) and Gram-positive bacteria (Staphylococcus aureus ATCC 6538).
Determination of inhibition zones—briefly, standardized fresh inoculums (direct suspension of colonies) were swabbed onto the surface of sterile nutrient agar plates (9 cm). Sigma-Aldrich microbiological paper disc (6 mm) were impregnated in the solution of compounds (50 µg/mL) overnight. These impregnated disks were placed on to the agar media surface along with standard antibiotics disc (amoxicillin 25 μg, Gentamycin 10 μg, Tobramycin 30 μg/disk). Plates were then incubated at 37 °C for 24 h. The zones of inhibition were determined as the diameter of the zone of inhibition. Disks impregnated with DMSO were used as control. Each experiment was carried out in duplicate.
3.3. Statistical Analysis
Statistical analysis was accomplished by a one-way ANOVA conveyed by Bonferroni’s multiple comparison test. This was used to examine the correlation p < 0.05 and was used to signify statistical significance when computing the IC50 of all the compounds. Data were analyzed utilizing GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA).
4. Conclusions
We have successfully developed an efficient protocol for the synthesis of a Schiff basis in excellent yields, using microwave irradiation and conventional conditions. The microwave-assisted method presented here is rapid and economical. All the synthesized compounds were thoroughly characterized using elemental analysis, NMR, IR, and LC-MS/MS spectrometric analyses. The synthesized compounds were then utilized for anti-proliferative and biological studies. The synthesized compound exhibited low to moderate anti-proliferative and weak antibacterial activities; more structural modifications are required to enhance the efficacy of the synthesized compounds.
Supplementary Materials
The following data are available online, Figure S1: Representative 1H-NMR of (E)-N’-((5-(2-nitrophenyl)furan-2-yl)methylene)isonicotinohydrazide; Figure S2: Representative 1H-NMR of (E)-N’-(4-fluoro-3-nitrobenzylidene)isonicotinohydrazide; Figure S3: Representative 13C-NMR of (E)-N’-(4-fluoro-3-nitrobenzylidene)isonicotinohydrazide; Figure S4: Representative DEPT-13C-NMR of (E)-N’-(4-fluoro-3-nitrobenzylidene)isonicotinohydrazide, Figure S5: Representative DEPT-13C-NMR of (E)-N’-(4-fluoro-3-nitrobenzylidene)isonicotinohydrazide (enlarged View); Figure S6: Representative 1H-NMR of (E)-N’-(2-fluoro-5-nitrobenzylidene)isonicotinohydrazide; Figure S7: ESI-LC-MS-MS spectrum and fragmentation pattern of (E)-N’-((5-(2-nitrophenyl)furan-2-yl)methylene)isonicotinohydrazide in KBr, Figure S8: ESI-LC-MS-MS spectrum and fragmentation pattern of (E)-N’-(4-fluoro-3-nitro benzylidene)isonicotinohydrazide in KBr; Figure S9: ESI-LC-MS-MS spectrum and fragmentation pattern (E)-N’-(2-fluoro-5-nitro-benzylidene)isonicotinohydrazide; Figure S10: IR of (E)-N’-((5-(2-nitro-phenyl)furan-2-yl)methylene)isonicotinohydrazide in KBr; Figure S11: IR spectrum of (E)-N’-(4-fluoro-3-nitrobenzylidene)isonicotinohydrazide in KBr; Figure S12: IR spectrum of (E)-N’-(2-fluoro-5-nitrobenzylidene)isonicotinohydrazide.
Author Contributions
Synthesis and characterization were carried out by A.K.S., B.A.A.-H. and R.R.N.; writing the research paper, editing, and biological activity experiments and their interpretation were carried out by B.A.A.-H., A.K.S., R.R.N., and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
No external fund was received during this research.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors wish to thank the Deanship, Faculty of Pharmacy, Medical Sciences and the Dean of Research and Higher Education, Al-Ahliyya Amman University, Amman, Jordan, for providing necessary facilities. The research is supported by Al-Ahliyya Amman University, Amman, Jordan. The present work is Dedicated to my late colleague Adnan Al Labadi for his suggestion and encouragement.
Conflicts of Interest
The authors declare no potential conflict of interest.
References
- Aboul-Fadl, T.; Mohammed, F.A.; Hassan, E.A. Synthesis, antitubercular activity and pharmacokinetic studies of some Schiff bases derived from 1-alkylisatin and isonicotinic acid hydrazide (INH). Arch. Pharm. Res. 2003, 26, 778–784. [Google Scholar] [CrossRef]
- Hearn, M.J.; Cynamon, M.H.; Chen, M.F.; Coppins, R.; Davis, J.; Joo-On Kang, H.; Noble, A.; Tu-Sekine, B.; Terrot, M.S.; Trombino, D.; et al. Preparation and antitubercular activities in vitro and in vivo of novel Schiff bases of isoniazid. Eur. J. Med. Chem. 2009, 44, 4169–4178. [Google Scholar] [CrossRef]
- Nazim, U.; Ali, S.I.; Ishrat, G.; Hassan, A.; Ahmed, M.; Ali, M.; Ali, Z.; Noori, M.Y. Synthesis, characterization and SEM studies of novel 1-indanyl isoniazid and hydrazide Schiff base derivatives as new anti-tubercular agents. Pak. J. Pharm. Sci. 2020, 33, 1095–1103. [Google Scholar]
- Chohan, Z.H.; Arif, M.; Shafiq, Z.; Yaqub, M.; Supuran, C.T. In vitro antibacterial, antifungal & cytotoxic activity of some isonicotinoylhydrazide Schiff’s bases and their cobalt (II), copper (II), nickel (II) and zinc (II) complexes. J Enzym. Inhib. Med. Chem. 2006, 21, 95–103. [Google Scholar] [CrossRef]
- Habala, L.; Varényi, S.; Bilková, A.; Herich, P.; Valentová, J.; Kožíšek, J.; Devínsky, F. Antimicrobial Activity and Urease Inhibition of Schiff Bases Derived from Isoniazid and Fluorinated Benzaldehydes and of Their Copper(II) Complexes. Molecules 2016, 21, 1742. [Google Scholar] [CrossRef]
- Uddin, N.; Rashid, F.; Ali, S.; Tirmizi, S.A.; Ahmad, I.; Zaib, S.; Zubair, M.; Diaconescu, P.L.; Tahir, M.N.; Iqbal, J.; et al. Synthesis, characterization, and anticancer activity of Schiff bases. J. Biomol. Struct. Dyn. 2020, 38, 3246–3259. [Google Scholar] [CrossRef]
- Iqbal, M.; Bhat, M.A.; Shakeel, F. Development and validation of UHPLC-MS/MS assay for rapid determination of a carvone Schiff base of isoniazid (CSB-INH) in rat plasma: Application to pharmacokinetic study. Biomed. Chromatogr. 2015, 29, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Jia, Z.L.; Ma, R.J.; Wang, X.F.; Chen, W.Y.; Liu, K.C. Isoniazid promotes the anti-inflammatory response in zebrafish associated with regulation of the PPARgamma/NF-kappaB/AP-1 pathway. Chem. Biol. Interact. 2020, 316, 108928. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Oliveira, A.C.; Cavalcanti, B.C.; Pessoa, C.; Pinheiro, A.C.; de Souza, M.V. Biological evaluation of isoniazid derivatives as an anticancer class. Sci. Pharm. 2014, 82, 21–28. [Google Scholar] [CrossRef]
- Firmino, G.S.; de Souza, M.V.; Pessoa, C.; Lourenco, M.C.; Resende, J.A.; Lessa, J.A. Synthesis and evaluation of copper(II) complexes with isoniazid-derived hydrazones as anticancer and antitubercular agents. Biometals 2016, 29, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.S.; Kasare, S.L.; Haval, N.B.; Khedkar, V.M.; Dixit, P.P.; Rekha, E.M.; Sriram, D.; Haval, K.P. Novel isoniazid embedded triazole derivatives: Synthesis, antitubercular and antimicrobial activity evaluation. Bioorg. Med. Chem. Lett. 2020, 30, 127434. [Google Scholar] [CrossRef]
- Jardosh, H.H.; Patel, M.P. Design and synthesis of biquinolone-isoniazid hybrids as a new class of antitubercular and antimicrobial agents. Eur. J. Med. Chem. 2013, 65, 348–359. [Google Scholar] [CrossRef]
- Vigorita, M.G.; Ottana, R.; Maccari, R.; Monforte, F.; Bisignano, G.; Pizzimenti, F.C. Synthesis and in vitro antimicrobial and antitumoral screening of novel lipophilic isoniazid analogues. VI. Boll. Chim. Farm. 1998, 137, 267–276. [Google Scholar]
- Volynets, G.P.; Tukalo, M.A.; Bdzhola, V.G.; Derkach, N.M.; Gumeniuk, M.I.; Tarnavskiy, S.S.; Yarmoluk, S.M. Novel isoniazid derivative as promising antituberculosis agent. Future Microbiol. 2020, 15, 869–879. [Google Scholar] [CrossRef]
- Rozycka, D.; Korycka-Machala, M.; Zaczek, A.; Dziadek, J.; Gurda, D.; Orlicka-Plocka, M.; Wyszko, E.; Biniek-Antosiak, K.; Rypniewski, W.; Olejniczak, A.B. Novel Isoniazid-Carborane Hybrids Active in Vitro Against Mycobacterium tuberculosis. Pharmaceuticals 2020, 13, 465. [Google Scholar] [CrossRef]
- Rani, A.; Johansen, M.D.; Roquet-Baneres, F.; Kremer, L.; Awolade, P.; Ebenezer, O.; Singh, P.; Sumanjit; Kumar, V. Design and synthesis of 4-Aminoquinoline-isoindoline-dione-isoniazid triads as potential anti-mycobacterials. Bioorg. Med. Chem. Lett. 2020, 30, 127576. [Google Scholar] [CrossRef]
- Jeyaraman, P.; Alagarraj, A.; Natarajan, R. In silico and in vitro studies of transition metal complexes derived from curcumin-isoniazid Schiff base. J. Biomol. Struct. Dyn. 2020, 38, 488–499. [Google Scholar] [CrossRef]
- Santos, D.C.; Henriques, R.R.; Junior, M.; Farias, A.B.; Nogueira, T.; Quimas, J.V.F.; Romeiro, N.C.; Silva, L.L.D.; Souza, A.L.F. Acylhydrazones as isoniazid derivatives with multi-target profiles for the treatment of Alzheimer’s disease: Radical scavenging, myeloperoxidase/acetylcholinesterase inhibition and biometal chelation. Bioorg. Med. Chem. 2020, 28, 115470. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. Ca. Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Thomas, A.B.; Tupe, P.N.; Badhe, R.V.; Nanda, R.K.; Kothapalli, L.P.; Paradkar, O.D.; Sharma, P.A.; Deshpande, A.D. Green route synthesis of Schiff’s bases of isonicotinic acid hydrazide. Green Chem. Lett. Rev. 2009, 2, 23–27. [Google Scholar] [CrossRef]
- Elagbar, Z.A.; Naik, R.R.; Shakya, A.K.; Bardaweel, S.K. Fatty Acids Analysis, Antioxidant and Biological Activity of Fixed Oil of Annona muricata L. Seeds. J. Chem. 2016, 2016, 6948098. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Tawaha, K.A.; Hudaib, M.M. Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. BMC Complementary Altern. Med. 2014, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C. Antimicrobial Susceptibility Testing☆. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2019; pp. 166–175. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).