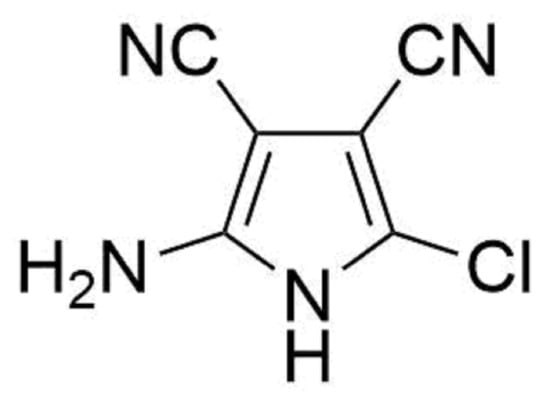
2-Amino-5-chloro-1H-pyrrole-3,4-dicarbonitrile
Abstract
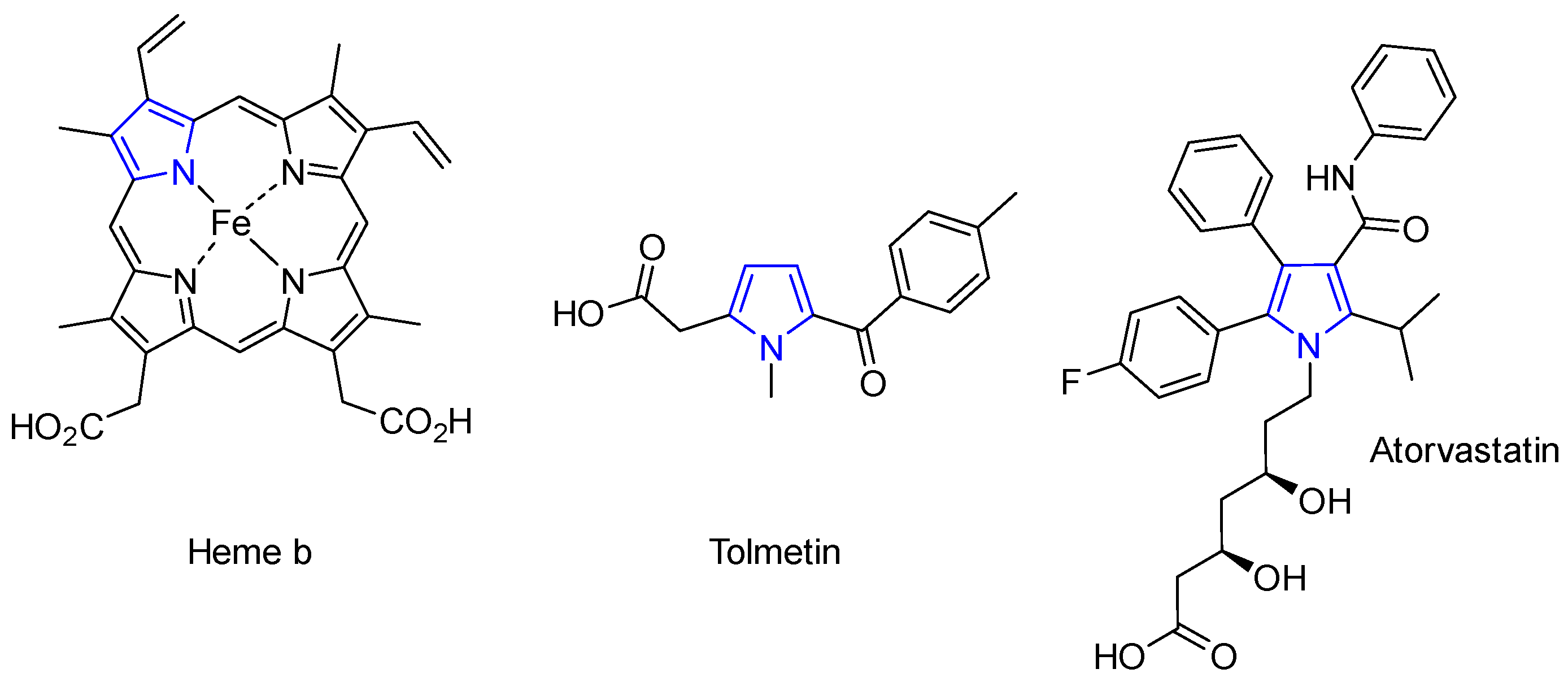
1. Introduction
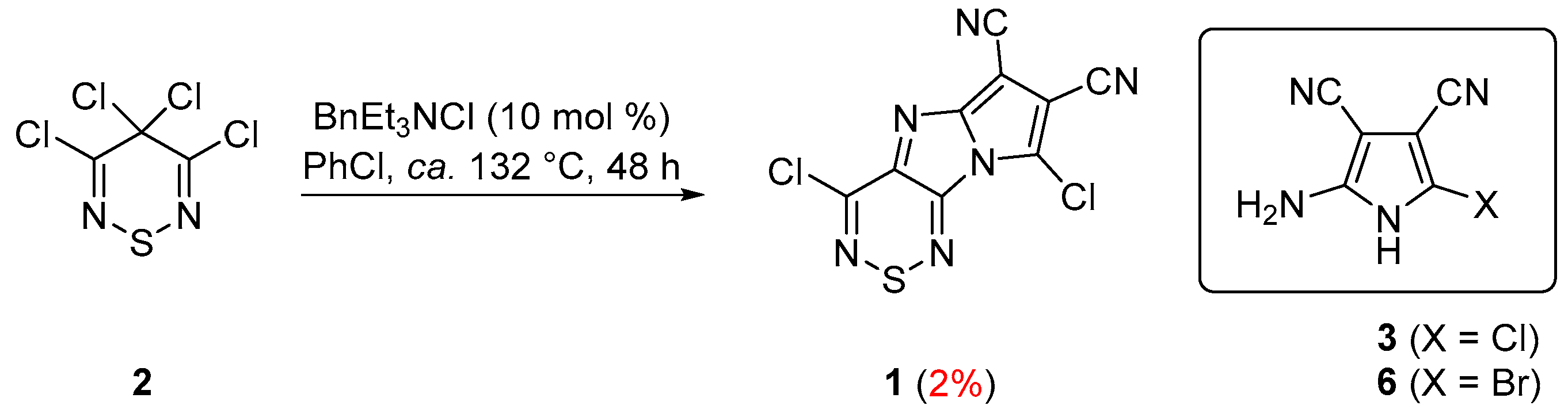
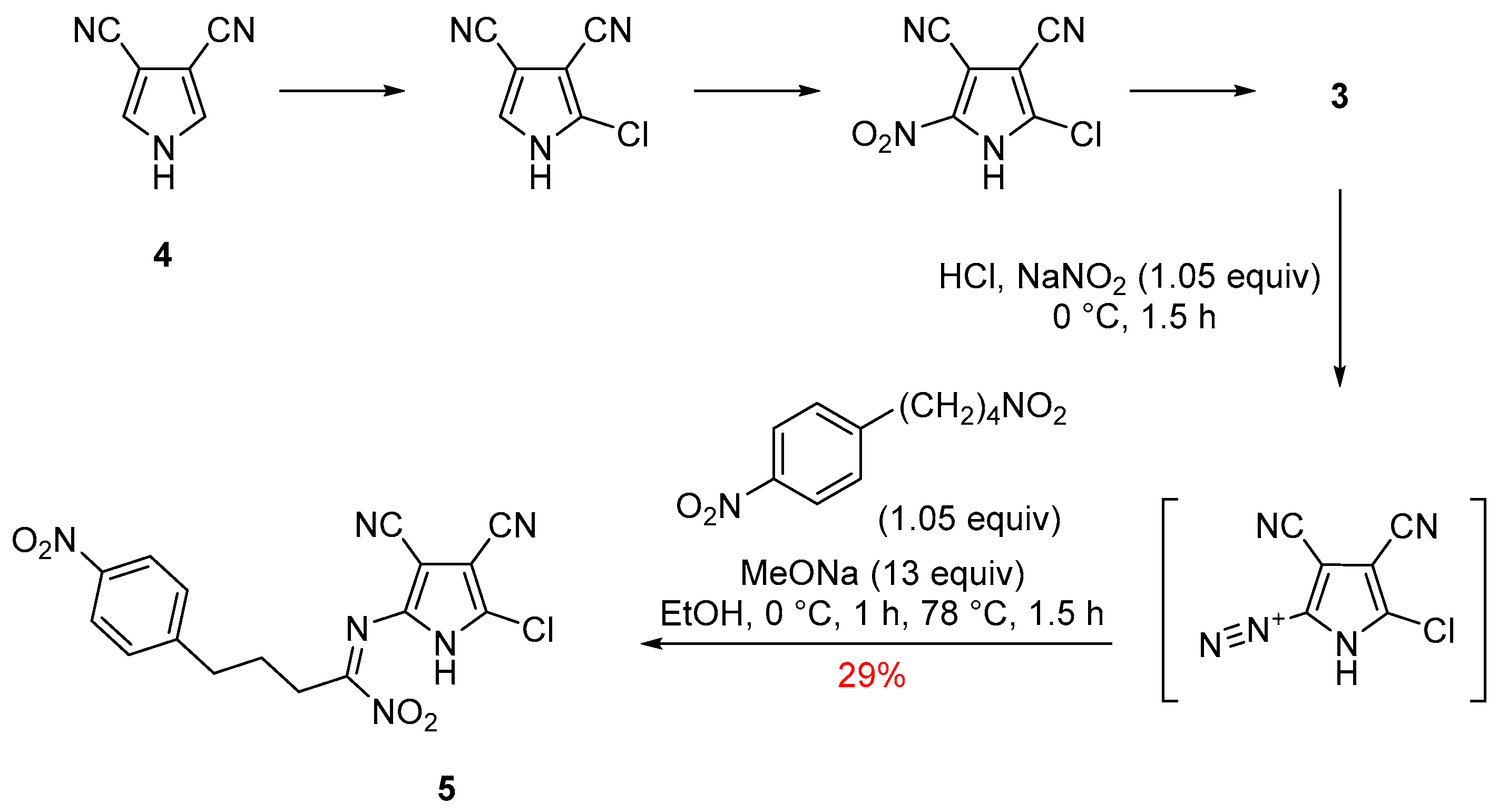
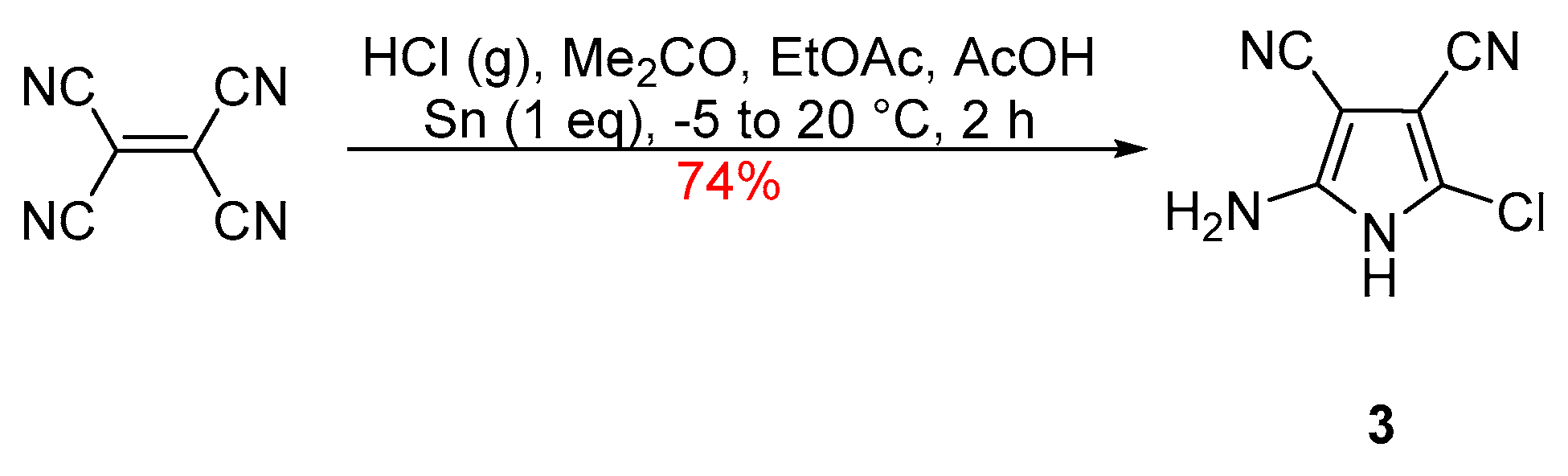
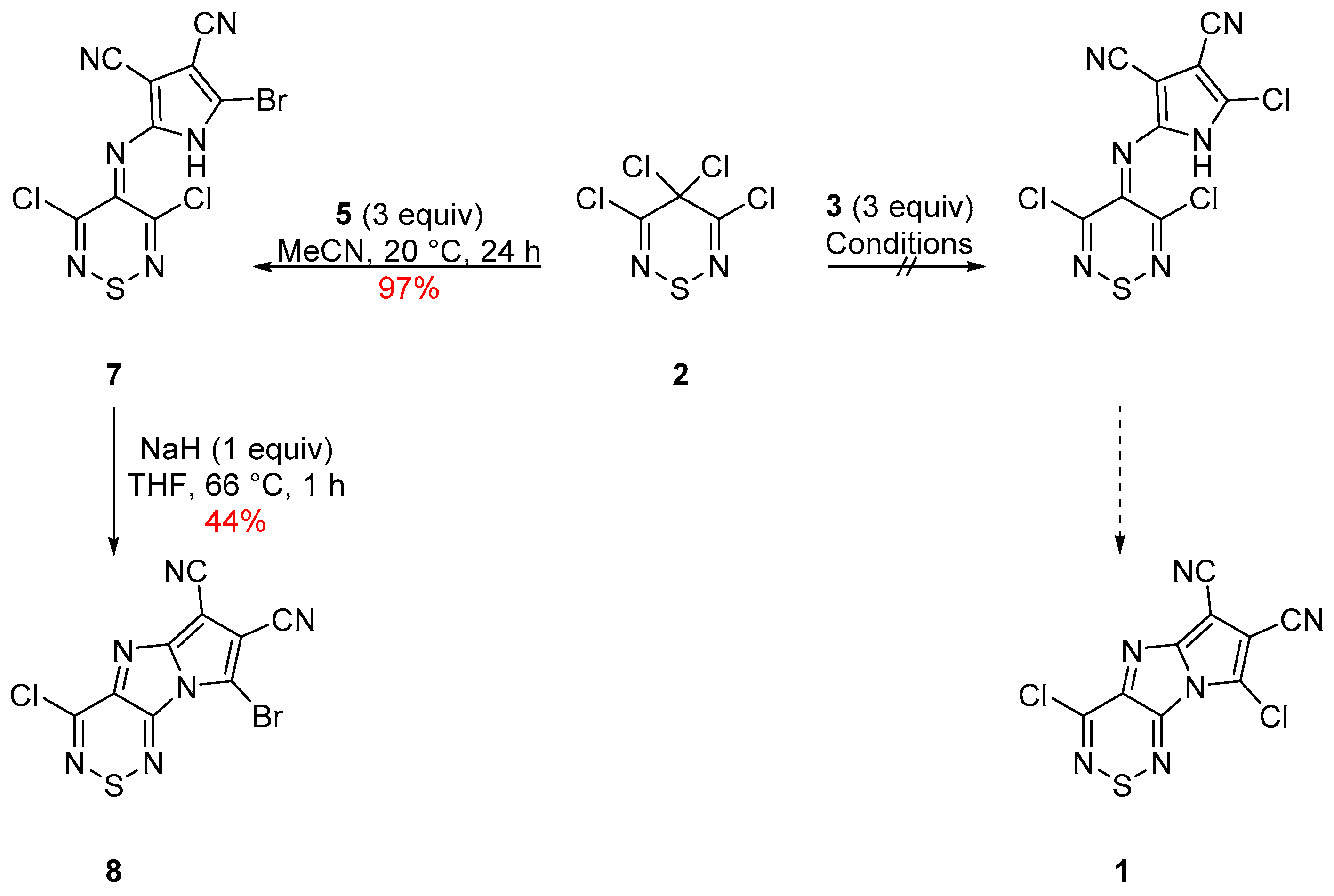
2. Results and Discussion
3. Materials and Methods
2-Amino-5-chloro-1H-pyrrole-3,4-dicarbonitrile (3)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loughlin, W.A.; Murphy, M.E.; Elson, K.E.; Henderson, L.C. Synthesis of a Novel Pyrrole Oxazole Analogue of the Insecticide Pirate. Aust. J. Chem. 2004, 57, 227–232. [Google Scholar] [CrossRef]
- Chou, S.-S.P.; Hsu, G.-T.; Lin, H.-C. Synthesis and second-order nonlinearities of sulfonyl-substituted pyrrole imino dyes. Tetrahedron Lett. 1999, 40, 2157–2160. [Google Scholar] [CrossRef]
- Wuang, S.C.; Neoh, K.G.; Kang, E.-T.; Pack, D.W.; Leckband, D.E. Polypyrrole Nanospheres with Magnetic and Cell-Targeting Capabilities. Macromol. Rapid Commun. 2007, 28, 816–821. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Nedolya, N.A. Pyrroles and their Benzo Derivatives: Reactivity. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Pergamon Press: Oxford, UK, 2008; Volume 3, pp. 45–268. [Google Scholar]
- Kalogirou, A.S.; Manoli, M.; Koutentis, P.A. Reaction of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine with benzyltriethylammonium chloride. Tetrahedron Lett. 2018, 59, 3589–3593. [Google Scholar] [CrossRef]
- Hayashi, H. Processing Method for Color Photographic Material. Japan Patent 05,341,470, 24 December 1993. [Google Scholar]
- Asami, M. Color Photographic Photosensitive Material and Color Image-Forming Method Using Same. Japan Patent 05,297,537, 12 November 1993. [Google Scholar]
- Kawai, K. Method for Forming a Color Image. European Patent 556,858, 25 August 1993. [Google Scholar]
- Morigaki, M.; Yoshioka, Y.; Seto, N. Silver Halide Color Photographic Material. European Patent 544,317, 2 June 1993. [Google Scholar]
- Hayashi, H. Method for Processing Color Photographic Material. European Patent 557,851, 1 September 1993. [Google Scholar]
- Naruse, H.; Suzuki, M.; Sato, T. Silver Halide Color Photographic Material. European Patent 544,319, 2 June 1993. [Google Scholar]
- Suzuki, M.; Naruse, H.; Sato, T. Silver Halide Color Photographic Material. European Patent 544,323, 2 June 1993. [Google Scholar]
- Naruse, H.; Suzuki, M. Silver Halide Color Photographic Material. European Patent 544,322, 2 June 1993. [Google Scholar]
- Seto, N.; Yoshioka, Y.; Suzuki, M.; Morigaki, M. Silver Halide Color Photographic Material. European Patent 544,316, 2 June 1993. [Google Scholar]
- Middleton, W.J.; Engelhardt, V.A.; Fisher, B.S. Cyanocarbon Chemistry. VIII. Heterocyclic Compounds from Tetracyanoethylene. J. Am. Chem. Soc. 1958, 80, 2822–2829. [Google Scholar] [CrossRef]
- Wilding, B.; Winkler, M.; Petschacher, B.; Kratzer, R.; Glieder, A.; Klempier, N. Nitrile Reductase from Geobacillus kaustophilus: A Potential Catalyst for a New Nitrile Biotransformation Reaction. Adv. Synth. Catal. 2012, 354, 2191–2198. [Google Scholar] [CrossRef]
- Carboni, R.A. Tetracyanoethylene. Org. Synth. 1959, 39, 64. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Koutentis, P.A. 2-Amino-5-chloro-1H-pyrrole-3,4-dicarbonitrile. Molbank 2021, 2021, M1191. https://doi.org/10.3390/M1191
Kalogirou AS, Koutentis PA. 2-Amino-5-chloro-1H-pyrrole-3,4-dicarbonitrile. Molbank. 2021; 2021(1):M1191. https://doi.org/10.3390/M1191
Chicago/Turabian StyleKalogirou, Andreas S., and Panayiotis A. Koutentis. 2021. "2-Amino-5-chloro-1H-pyrrole-3,4-dicarbonitrile" Molbank 2021, no. 1: M1191. https://doi.org/10.3390/M1191
APA StyleKalogirou, A. S., & Koutentis, P. A. (2021). 2-Amino-5-chloro-1H-pyrrole-3,4-dicarbonitrile. Molbank, 2021(1), M1191. https://doi.org/10.3390/M1191