Abstract
2,4,6-Tris(4-iodophenyl)-1,3,5-trimethylbenzene was synthesized from 2,4,6-triphenyl-1,3,5-trimethylbenzene, using [bis(trifluoroacetoxy)iodo]benzene as the iodinating agent. The title compound was characterized by means of NMR, IR, and mass spectrometry, as well as TG analysis.
1. Introduction
Iodinated organics are important derivatives in synthetic chemistry. Although more expensive than their brominated counterparts, they are often preferred over the latter in coupling reactions, as they lead to better yields and shorter reaction times. Polyiodinated aromatics are often used as intermediaries in the design of new materials, whether they are polymers [1], organic cages [2], covalent organic frameworks (COFs) [3], metal organic frameworks (MOFs) [4,5,6,7], light- harvesting complexes [8,9], dendrimers [10], etc. Most often, the iodination of aromatic rings is performed using electrophilic iodine species [11,12,13], although in some cases radical [14] or nucleophilic species [15] are used. Our continued interest in the design of new organic molecules which can act as linkers in MOF design has led us to synthesize tris(4-iodophenyl)-1,3,5-trimethylbenzene 2 from the corresponding hydrocarbon 1, using the hypervalent iodine derivative [bis(trifluoroacetoxy)iodo]benzene (PIFA), according to Scheme 1:
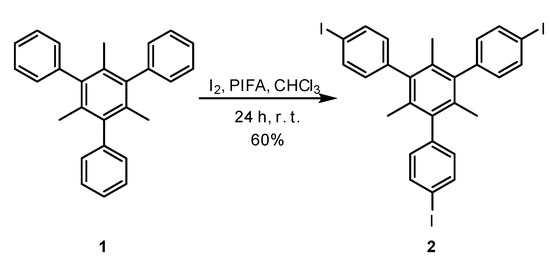
Scheme 1.
The synthetic pathway to the title compound.
2. Results and Discussion
The title compound was obtained by iodinating 2,4,6-triphenyl-1,3,5-trimethylbenzene 1 with PIFA and iodine in chloroform, at room temperature, according to a procedure already established [16]. Unlike other iodination reactions, this particular system does not use strong acids, such as the sulfuric acid/nitric acid/iodine system [17], potentially explosive reagents—as found in the case of the sulfuric acid/iodine/periodic acid system [18]—or toxic metals, such as mercury acetate [19]. Although the hypervalent iodine reagent PIFA is moisture sensitive, the reaction did not require any special precautions and was run under an open atmosphere leading to the desired compound in a 60% yield after purification. During the course of the reaction, the formation of a precipitate could be observed. After stirring for 24 h, the precipitate was filtered, washed with hexane and dried. A preliminary 1H NMR analysis indicated that the isolated solid was the title compound 2. Moreover, no traces of other byproducts could be identified. An analytically pure sample was obtained by recrystallization from toluene.
1H NMR analysis of the pure sample, performed in CDCl3, confirmed the proposed structure. The six protons situated ortho to the iodine substituents can be found as a doublet at 7.82 ppm, with a coupling constant 3J 7.8 Hz. The remaining six aromatic protons, situated meta to the iodine substituents, are present as a doublet at 6.97 ppm, with a coupling constant 3J 7.9 Hz. Finally, the nine aliphatic protons belonging to the three methyl groups give rise to a singlet found at 1.70 ppm. The 13C NMR spectrum displays the signal belonging to the three carbon atoms directly bound to the iodine substituents at 92.3 ppm, while the three aliphatic carbon atoms can be found at 19.5 ppm. The Mass spectrum of 2, obtained using electron ionization, displayed the molecular ion [M]+ at m/z 726.1. Moreover, several other fragments could also be identified. As such, the signal found at m/z 599.0 belongs to the [M-I]+ ion. This in turn loses two methyl groups, as proven by the m/z 584.1 and m/z 569.1 signals, belonging to the [M-CH3I]+ and [M-C2H6I]+ ions respectively. The loss of two iodine substituents ([M-I2]+ fragment) gives a signal at m/z 472.2. Further loss of a methyl group leads to the [M-CH3I2]+ fragment with an m/z 457.1. The recorded thermogravimetric plots of 2 show one degradation step that started at about 320 °C and finished at 600 °C attributed to molecular chain scission. Decomposition onset temperature (Tonset) is about 364 °C. The DTA curve reveals two endothermic events at approximately 360–420 °C accompanied by a mass loss. The endotherm even at 365 °C is in good correlation with the visual melting point determination. At 700 °C, the charcoal residual weight is 16.7%. NMR, IR, and mass spectra, as well as the TG curve can be found as separate figures in the Supplementary Materials section.
The synthesis of iodinated derivative 2 opens up the way to a number of interesting potential ligands for MOF/COF design. Thus, by coupling it with functionalized aromatic boronic acids (Suzuki Coupling), alkynes (Sonogashira Coupling) or alkenes (Heck Coupling), extended linkers capable of forming frameworks with large pores will become accessible. Moreover, 2 can serve as a building block in polymer synthesis.
3. Materials and Methods
All solvents and commercially available reagents were purchased and used without further purification. NMR spectra were recorded in CDCL3 using a Bruker Avance III 400 (Bruker BioSpin, Rheinstetten, Germany) instrument operated at 25 °C. Chemical shifts (δ, ppm) are described in relation to tetramethylsilane. The IR spectrum was recorded on a Bruker Tensor 27 (Bruker Optik GmbH, Ettlingen, Germany) equipped with an ATR probe head. The mass spectrum was recorded using a Thermo Scientific ISQ LT (Thermo Fisher Scientific Inc., Waltham, MA, USA). TG analysis was performed using a STA 330 Jupiter (Netzsch, GmbH, Selb, Germany) thermal analyzer in N2 flow with a linear heating rate of 10 °C/min and a temperature range of 30–700 °C. The Netzsch Proteus software packages (Netzsch, GmbH, Selb, Germany) were used for data analysis. Elemental analysis (C, H) was performed on a CE440 Elemental Analyser (Exeter Analytical, Coventry, United Kingdom). The melting point was determined using a KSP1 Melting-Point Meter (A.KRÜSS Optronic, Hamburg, Germany). The UV spectrum was recorded using a Carry 100 Bio UV-Visible spectrophotometer (Agilent Technologies, Santa Clara, CA, USA), with chloroform as a solvent, at a concentration of 10−5 M.
Synthesis of 2,4,6-Tris(4-Iodophenyl)-1,3,5-trimethylbenzene (2)
To a stirred solution of 2,4,6-triphenyl-1,3,5-trimethylbenzene 1 (0.875 g, 2.5 mmol) in chloroform (25 mL), at room temperature, iodine (1.9 g, 7.5 mmol) and then PIFA (3.23 g, 7.5 mmol) were added and the resulting mixture was left to stir for 24 h. The formed precipitate was then filtered, washed with hexane (3 × 5 mL) and air dried to yield a pink solid. Recrystallisation gave the title compound 2 (1.09 g, 60%) as colorless prisms; m.p. 351–353 °C (decomp.) (PhMe). 1H NMR (400 MHz, CDCl3): δ 7.82 (d, 6H, 3J 7.8 Hz), 6.97 (d, 6H, 3J 7.9 Hz), 1.70 (s, CH3, 9H) ppm. 13C NMR (100 MHz, CDCl3): δ 141.2 (Cq), 138.8 (Cq), 137.8 (CH), 133.2 (Cq), 131.4 (CH), 92.3 (CI), 19.5 (CH3) ppm. IR-ATR (cm−1): ν = 1483 (m), 1384 (m), 1098 (w), 1058 (m), 1006 (s), 957 (m), 823 (s), 764 (s), 513 (s), 497 (w), 480 (w), 421 (w). EI-MS (m/z, M+): 726.1. Elemental analyses for C27H21I3 calc. C 44.66%, H 2.91%, found C 44.43%, H 2.97%. UV/Vis (CHCl3): λmax (log ε) 242 (4.83).
Supplementary Materials
The following are available online. Figure S1: 1H NMR spectrum of 2, Figure S2: 13C NMR spectrum of 2, Figure S3: IR spectrum of 2, Figure S4: Mass spectrum of 2, Figure S5: TG curve of 2. Figure S6: UV-Vis spectrum of 2.
Author Contributions
All authors contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Social Fund for Regional Development, Competitiveness Operational Programme Axis 1—Project “Novel Porous Coordination Polymers with Organic Ligands of Variable Length for Gas Storage”, POCPOLIG (ID P_37_707, Contract 67/08.09.2016, MySMIS: 104810).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morisaki, Y.; Gon, M.; Tsuji, Y.; Kajiwara, Y.; Chujo, Y. Synthesis and Characterization of [2.2]Paracyclophane-Containing Conjugated Microporous Polymers. Macromol. Chem. Phys. 2012, 213, 572–579. [Google Scholar] [CrossRef]
- Jiao, T.; Chen, L.; Yang, D.; Li, X.; Wu, G.; Zeng, P.; Zhou, A.; Yin, Q.; Pan, Y.; Wu, B.; et al. Trapping White Phosphorus within a Purely Organic Molecular Container Produced by Imine Condensation. Angew. Chem. Int. Ed. 2017, 56, 14545–14550. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, X.; Li, T.; Zhang, W.D.; Fu, Q.T.; Lu, H.S.; Wang, X.; Gu, Z.G. Three-dimensional porphyrin-based covalent organic frameworks with tetrahedral building blocks for single-site catalysis. New J. Chem. 2019, 43, 16907–16914. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Bejan, D.; Shova, S.; Gdaniec, M.; Fronc, M.; Lozan, V.; Janiak, C. Alkali- and alkaline-earth metal–organic networks based on a tetra(4-carboxyphenyl)bimesitylene-linker. Polyhedron 2019, 173, 114128. [Google Scholar] [CrossRef]
- Bumstead, A.M.; Cordes, D.B.; Dawson, D.M.; Chakarova, K.K.; Mihaylov, M.Y.; Hobday, C.L.; Duren, T.; Hadjiivanov, K.I.; Slawin, A.M.Z.; Ashbrook, S.E.; et al. Modulator-Controlled Synthesis of Microporous STA-26, an interpenetrated 8,3-Connected Zirconium MOF with the the-iTopology, and its Reversible Lattice Shift. Chem. Eur. J. 2018, 24, 6115–6126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Feng, D.; Liu, T.F.; Li, J.R.; Zhou, H.C. Pore Surface Engineering with Controlled Loadings of Functional Groups via Click Chemistry in Highly Stable Metal–Organic Frameworks. J. Am. Chem. Soc. 2012, 134, 14690–14693. [Google Scholar] [CrossRef] [PubMed]
- Bejan, D.; Bahrin, L.G.; Shova, S.; Sardaru, M.; Clima, L.; Nicolescu, A.; Marangoci, N.; Lozan, V.; Janiak, C. Spontaneous resolution of non-centrosymmetric coordination polymers of zinc(II) with achiral imidazole-biphenyl-carboxylate ligands. Inorg. Chim. Acta 2018, 482, 275–283. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Satake, A.; Kobuke, Y. Light-Harvesting Macroring Accommodating a Tetrapodal Ligand Based on Complementary and Cooperative Coordinations. J. Am. Chem. Soc. 2004, 126, 8668–8669. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, Y.; Satake, A.; Sandanayaka, A.S.D.; Araki, Y.; Ito, O.; Kobuke, Y. Fullerene- and Pyromellitdiimide-Appended Tripodal Ligands Embedded in Light-Harvesting Porphyrin Macrorings. Inorg. Chem. 2011, 50, 10249–10258. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Eich, O.; Housecroft, C.E. High-nuclearity cobaltadendrimers. J. Chem. Soc. Dalton Trans. 1999, 1363–1364. [Google Scholar] [CrossRef]
- Felix, L.; Sezer, U.; Arndt, M.; Mayor, M. Synthesis of Highly Fluoroalkyl-Functionalized Oligoporphyrin Systems. Eur. J. Org. Chem. 2014, 6884–6895. [Google Scholar] [CrossRef]
- Ohshiro, N.; Takei, F.; Onitsuka, K.; Takahashi, S. Synthesis of organometallic dendrimers with a backbone composed of platinum-acetylide units. J. Organomet. Chem. 1998, 569, 195–202. [Google Scholar] [CrossRef]
- Boudjada, A.; Hernandez, O.; Meinnel, J.; Mani, M.; Paulus, W. 1,3,5-Triiodo-2,4,6-trimethylbenzene at 293 K. Acta Cryst. 2001, C57, 1106–1108. [Google Scholar] [CrossRef] [PubMed]
- da Silva Correa, C.M.M.; Oliviera, M.A.B.C.S. Reaction of arenesulphonyl halides with free radicals. Part 2. J. Chem. Soc. Perkin Trans. 2 1983, 711–715. [Google Scholar] [CrossRef]
- Krasnokutskaya, E.A.; Semenischeva, N.I.; Filimonov, V.D.; Knochel, P. A New, One-Step, Effective Protocol for the Iodination of Aromatic and Heterocyclic Compounds via Aprotic Diazotization of Amines. Synthesis 2007, 81–84. [Google Scholar] [CrossRef]
- Heering, C.; Francis, B.; Nateghi, B.; Makhloufi, G.; Lüdekeb, S.; Janiak, C. Syntheses, structures and properties of group 12 element (Zn, Cd, Hg) coordination polymers with a mixed-functional phosphonate-biphenylcarboxylate linker. CrystEngComm. 2016, 18, 5209–5223. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Natarajan, R.; Venugopalan, P. Three-Dimensional Four-Connecting Organic Scaffolds with a Twist: Synthesis and Self-Assembly. J. Org. Chem. 2005, 70, 8568–8571. [Google Scholar] [CrossRef] [PubMed]
- Łapok, Ł.; Gut, A.; Nowakowska, M. Synthesis and spectroscopic propertiesof 5-tert-butyl-3-(trifluoromethyl)phthalonitrile: A novel precursorfor the synthesis of phthalocyanines. Tetrahedron Lett. 2013, 54, 4388–4391. [Google Scholar] [CrossRef]
- He, H.M.; Fanwick, P.E.; Wood, K.; Cushman, M. A Novel 1,3 O .fwdarw. C Silyl Shift and Deacylation Reaction Mediated by Tetra-n-butylammonium Fluoride in an Aromatic System. J. Org. Chem. 1995, 60, 5905–5909. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).