(E)-1,1,1-Trifluoro-6,6-bis(4-methoxyphenyl)hexa-3,5-dien-2-one
Abstract
1. Introduction
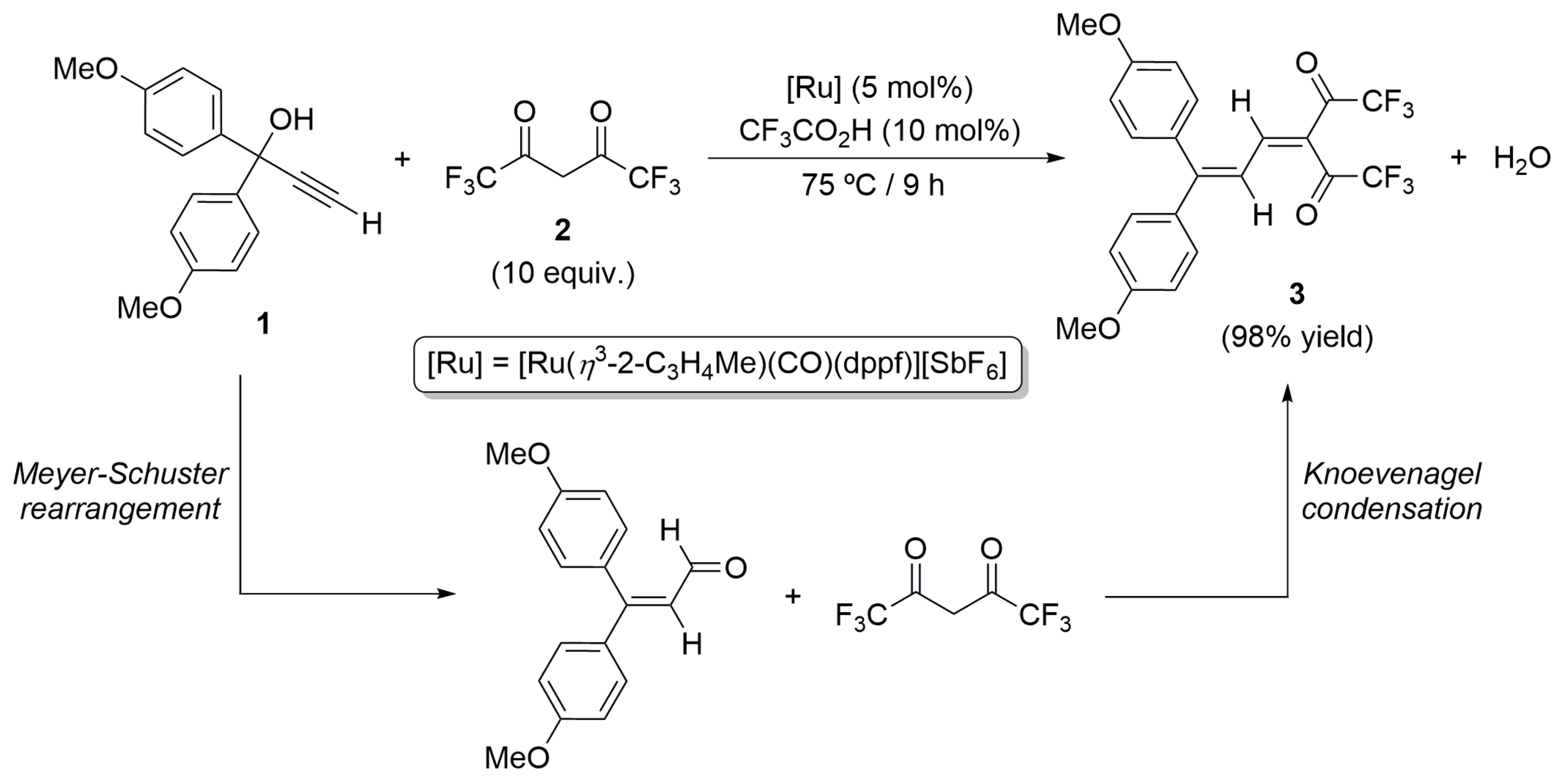
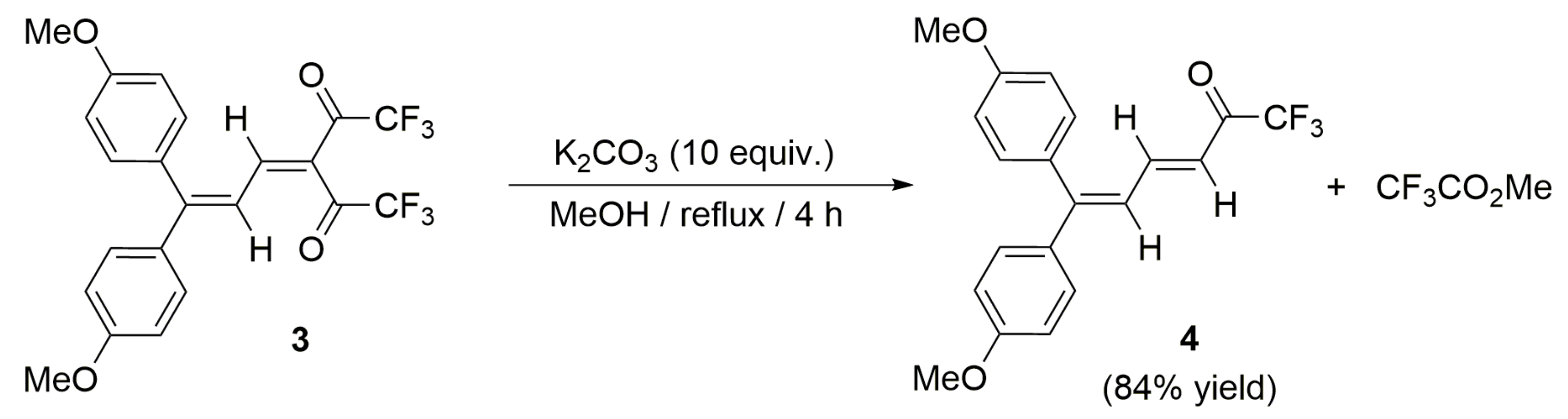
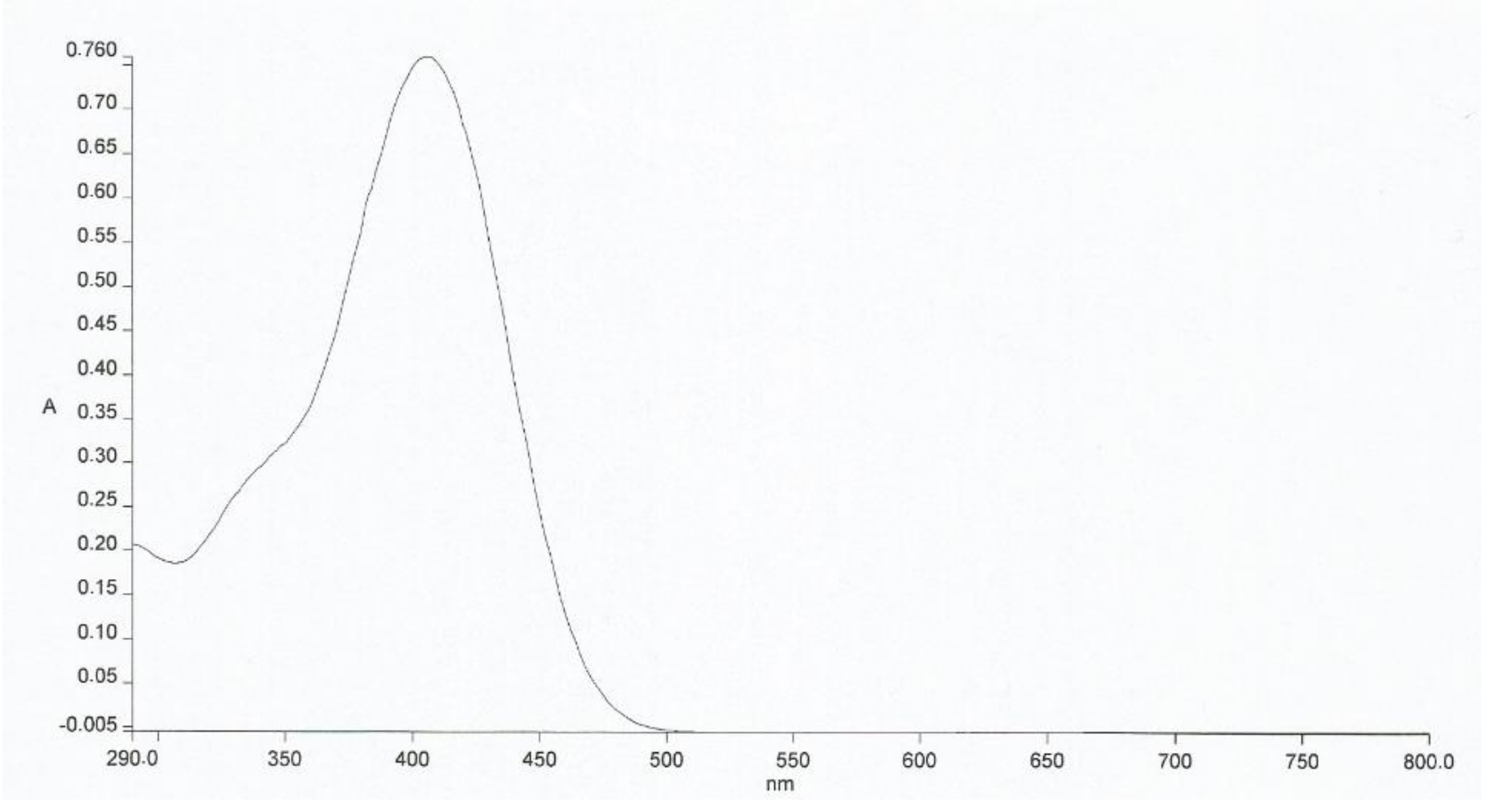
2. Results and Discussion
3. Materials and Methods
(E)-1,1,1-Trifluoro-6,6-bis(4-methoxyphenyl)hexa-3,5-dien-2-one (4)
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Kelly, C.B.; Mercadante, M.A.; Leadbeater, N.E. Trifluoromethyl ketones: Properties, preparation, and application. Chem. Commun. 2013, 49, 11133–11148. [Google Scholar] [CrossRef] [PubMed]
- Nenajdenko, V.G.; Sanin, A.V.; Balenkova, E.S. Methods for the synthesis of α,β-unsaturated trifluoromethyl ketones and their use in organic synthesis. Russ. Chem. Bull. 1999, 68, 437–458. [Google Scholar] [CrossRef]
- Druzhinin, S.V.; Balenkova, E.S.; Nenajdenko, V.G. Recent advances in the chemistry of α,β-unsaturated trifluoromethylketones. Tetrahedron 2007, 63, 7753–7808. [Google Scholar] [CrossRef]
- Nenajdenko, V.G.; Balenkova, E.S. α,β-Unsaturated trifluoromethyl ketones in the synthesis of fluorinated heterocycles. Targets Heterocycl. Syst. 2010, 14, 213–265. [Google Scholar]
- Nenajdenko, V.G.; Balenkova, E.S. Preparation of α,β-unsaturated trifluoromethyl ketones and their application in the synthesis of heterocycles. Arkivoc 2011, (i), 246–328. [Google Scholar]
- Jiang, B. A stereocontrolled synthesis of conjugated dienyl trifluoromethyl ketones via the Claisen rearrangement of allyl 2-phenylsulfanyl-1-(trifluoromethyl) vinyl ethers. Chem. Commun. 1996, 861–862. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, Y.; Yuan, C. A new and facile stereocontrolled synthesis of conjugated dienyl trifluoromethyl ketones. Perkin 1 2000, 4240–4241. [Google Scholar] [CrossRef]
- Peng, W.; Zhu, S. The synthesis of 2,4-dienyl fluoroalkyl ketones by a tandem three-stage sequence of propargyl 2-fluoroalkylvinyl ether formation, Claisen rearrangement, and allene-to-conjugated diene isomerization. Tetrahedron 2003, 59, 4641–4649. [Google Scholar] [CrossRef]
- Kohn, B.L.; Rovis, T. Cobaltate anion couples terminal dienes with trifluoroacetic anhydride: A direct fluoroacylation of 1,3-dienes. Chem. Sci. 2014, 5, 2889–2892. [Google Scholar] [CrossRef]
- Cadierno, V.; Díez, J.; García-Garrido, S.E.; Gimeno, J.; Nebra, N. Efficient Access to conjugated dienones and diene-diones from propargylic alcohols and enolizable ketones: A tandem isomerization/condensation process catalyzed by the sixteen-electron allyl-ruthenium(II) complex [Ru(η3-2-C3H4Me)(CO)(dppf)][SbF6]. Adv. Synth. Catal. 2006, 348, 2125–2132. [Google Scholar] [CrossRef]
- Borge, J.; Cadierno, V.; Díez, J.; García-Garrido, S.E.; Gimeno, J. Novel push-pull butadienes derived from 1,1-diaryl-2-propyn-1-ols and 1,1,1,5,5,5-hexafluoro-2,4-pentanedione: Synthesis, absorption spectra and solvatochromic behaviour. Dyes Pigm. 2010, 87, 209–217. [Google Scholar] [CrossRef]
- Francos, J.; Borge, J.; Díez, J.; García-Garrido, S.E.; Cadierno, V. Easy entry to donor/acceptor butadiene dyes through a MW-assisted InCl3-catalyzed coupling of propargylic alcohols with indan-1,3-dione in water. Catal. Commun. 2015, 63, 10–14. [Google Scholar] [CrossRef]
- Francos, J.; García-Garrido, S.E.; Borge, J.; Suárez, F.J.; Cadierno, V. Butadiene dyes based on 3-(dicyanomethylidene)indan-1-one and 1,3-bis(dicyanomethylidene)indane: Synthesis, characterization and solvatochromic behaviour. RSC Adv. 2016, 6, 6858–6867. [Google Scholar] [CrossRef]
- Rezgui, F.; El Gaïed, M.M. Réaction régiospécifique des carbanions stabilisés avec la 2-(acétoxyméthyl)cyclohex-2-én-1-one: Synthèse de diénones bicycliques. Tetrahedron 1997, 53, 15711–15716. [Google Scholar] [CrossRef]
- Bray, D.J.; Jolliffe, K.A.; Lindoy, L.F.; McMurtrie, J.C. Tris-β-diketones and related keto derivatives for use as building blocks in supramolecular chemistry. Tetrahedron 2007, 63, 1953–1958. [Google Scholar] [CrossRef]
- Amijs, C.H.M.; López-Carrillo, V.; Raducan, M.; Pérez-Galán, P.; Ferrer, C.; Echavarren, A.M. Gold(I)-catalyzed intermolecular addition of carbón nucleophiles to 1,5- and 1,6-enynes. J. Org. Chem. 2008, 73, 7721–7730. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, G.; Soto, J.P.; Carrillo, D.; Manzur, C.; Roisnel, T.; Hamon, J.-R. Deacetylation of chiral ferrocenyl containing β-diketones promoted by ethylene diamine: Spectroscopic and structural characterization of the fragmentation products. J. Organomet. Chem. 2014, 770, 14–20. [Google Scholar] [CrossRef][Green Version]
- Pellerite, M.J. Unsual reaction chemistry in thermal decomposition of alkali metal 2-alkoxy-2,3,3,3-tetrafluoropropionate salts. J. Fluor. Chem. 1990, 49, 43–66. [Google Scholar] [CrossRef]
- Hojo, M.; Masuda, R.; Okada, E. A facile and convenient synthetic route to 1-amino-4,4-bis(trifluoroacetyl)- and 1-amino-4-trifluoroacetyl-1,3-butadienes. Synthesis 1990, 425–427. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3 and 4 are not available from the author. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadierno, V. (E)-1,1,1-Trifluoro-6,6-bis(4-methoxyphenyl)hexa-3,5-dien-2-one. Molbank 2020, 2020, M1120. https://doi.org/10.3390/M1120
Cadierno V. (E)-1,1,1-Trifluoro-6,6-bis(4-methoxyphenyl)hexa-3,5-dien-2-one. Molbank. 2020; 2020(1):M1120. https://doi.org/10.3390/M1120
Chicago/Turabian StyleCadierno, Victorio. 2020. "(E)-1,1,1-Trifluoro-6,6-bis(4-methoxyphenyl)hexa-3,5-dien-2-one" Molbank 2020, no. 1: M1120. https://doi.org/10.3390/M1120
APA StyleCadierno, V. (2020). (E)-1,1,1-Trifluoro-6,6-bis(4-methoxyphenyl)hexa-3,5-dien-2-one. Molbank, 2020(1), M1120. https://doi.org/10.3390/M1120




