Abstract
A simple synthetic approach to dimethyl 2-{[2-(2-methoxy-1-methoxycarbonyl-2-oxoethyl)-4,5,7-trimethoxy-3-(2,4,5-trimethoxyphenyl)-2,3-dihydro-1H-inden-1-yl]methyl}malonate has been developed, based on a B(C6F5)3-induced domino dimerization of 2-(2,4,5-trimethoxyphenyl)cyclopropane-1,1-diester.
1. Introduction
Donor–acceptor (D–A) cyclopropanes [1,2,3] have proven themselves as unique substrates containing multiple reaction centers, that allow them to participate in a broad variety of transformations including unusual ones, such as diverse dimerization reactions [4,5,6]. The cyclodimerization reactions of (D–A) cyclopropanes represent an efficient way to significantly increase the structural complexity of a substrate in a single synthetic step and can provide straightforward routes to complex polycyclic compounds. Various cyclodimerizations, reported to date, can be divided into three main groups: (1) (3 + 3)-cyclodimerizations affording cyclohexane, 1,2,3,4-tetrahydronaphthalene or 9,10-dihydroanthracene derivatives and their heterocyclic analogues [7,8,9,10]; (2) (3 + 2)-cyclodimerization leading to arylindanes, diarylcyclopentanes, etc. [9,10,11,12,13,14]; (3) other types of cyclodimerizations [10,15,16,17,18,19,20]. The chemoselectivity of the cyclodimerizations is guided by both the nature of the substituents in the starting D–A cyclopropane and the choice of a suitable Lewis acid. Among the disclosed dimerization processes, one should note the cyclopropane-to-indane transformation as an analogue of styrene- and stilbene-based biosynthesis of indane structures, such as diisoeugenol, pallidol, griffipavixanthone, laetevirenol A, quadrangularin A, parthenocissin A, parvifolol (Figure 1) exhibiting cytotoxic [21,22], antioxidant [21,23,24,25,26], and other [25,26,27] activities.
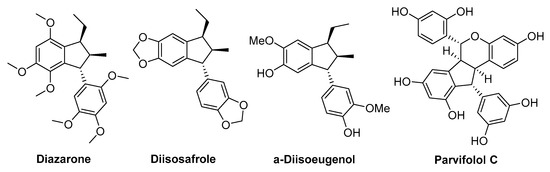
Figure 1.
Examples of bioactive natural indanes.
In line with our ongoing research related to D–A cyclopropane dimerizations [7,8,11,12,13,14,20], herein, we report the synthesis of polyoxygenated indane 1, which is a structural analog of diazarone, via a B(C6F5)3-induced (3 + 2)-cyclodimerization of 2-(2,4,5-trimethoxyphenyl)cyclopropane-1,1-diester 2 in one step in highly chemo-, regio- and stereoselective manner.
2. Results and Discussion
Starting cyclopropane 2 was synthesized from commercial 2,4,5-trimethoxybenzaldehyde 3 via a two-step synthetic sequence which involves the Knövenagel condensation with dimethyl malonate to afford arylidenemalonate 4 followed by the Corey-Chaykovsky reaction (Scheme 1) [28].
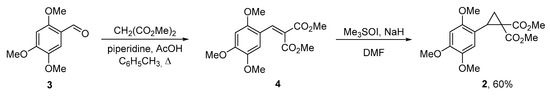
Scheme 1.
Synthesis of cyclopropane 2.
The structure of 2,4,5-trimethoxyphenyl-substituted cyclopropane 2 was unambiguously confirmed by single crystal X-ray analysis (Figure 2) [29]. In general, the structure of cyclopropane 2 closely matches the structure of related 2-arylcyclopropane-1,1-dicarboxylates described previously [30,31,32]. All of them have the same configuration of ester groups. The methoxycarbonyl group in the trans-position with respect to the donor aromatic substituent is located approximately along the bisector of the angle C(2)–C(1)–C(3) with the carbonyl oxygen atom directed towards the cyclopropane fragment, while the alkoxy group, respectively, in the opposite direction. The second ester group is usually arranged so that the carbonyl oxygen atom has an exo-location relative to the three-membered ring and the methoxy group is endo-located, the torsion angle between two ester groups being dependent on the nature of the cis-aromatic substituent.
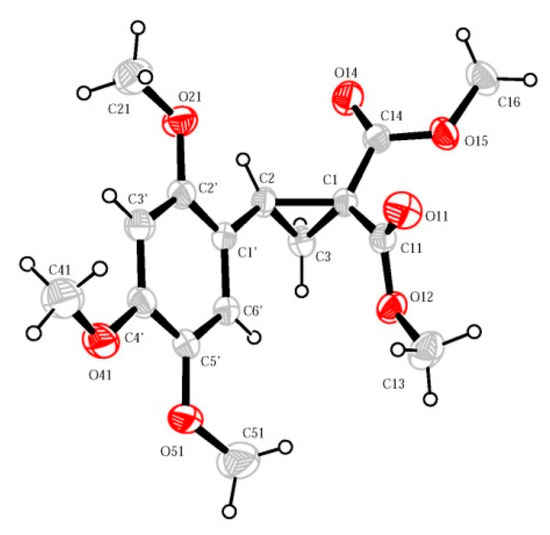
Figure 2.
Molecular structure (ORTEP-3 [33]) from single crystal X-ray study of 2.
In this paper, we studied the possibility of using B(C6F5)3 as a catalyst of cyclopropane 2 dimerization accounting for fact that this reagent is referred to as an ideal boron Lewis acid [34]. Our preliminary studies revealed that the use of conventional Lewis acids (BF3·Et2O, SnCl4) does not lead to satisfactory results as complex mixtures of dimeric products and products of oligomerization were obtained. After a short screening of the reaction conditions, we have found that the best yields are obtained after heating 2 M solution of 2 in 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) in the presence of B(C6F5)3 (10 mol %) under microwave irradiation at 58 °C for 7 h (Scheme 2).
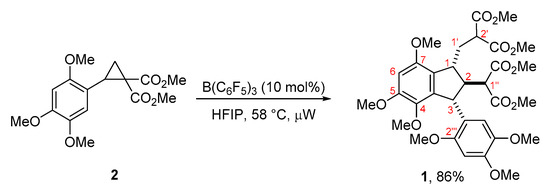
Scheme 2.
Synthesis of dimer 1.
According to nuclear magnetic resonance spectroscopy (NMR) data, the dimerization of cyclopropane 2 produced 1 as a mixture of two isomers in 84:16 ratio. The relative configuration of the major isomer of dimer 1 was assigned on the basis of 2D nuclear Overhauser effect spectroscopy (NOESY) experiments (Scheme 3). For major isomer the trans,trans-arrangement of substituents in five-membered ring was established. The minor isomer of 1 was determined to be the C-1 epimer, and its spectral parameters completely correspond to the literature data for the related dimers [12,13].
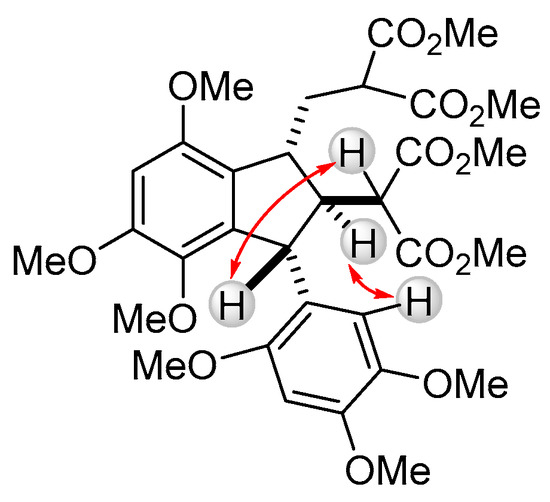
Scheme 3.
Representative NOE responses for the major isomer of 1.
The predominant formation of trans,trans-isomer differs from the previously reported results on the exclusive formation of cis,trans-isomers of the related indanes in the cyclodimerization of the D–A cyclopropanes [12,13]. Taking into account the cis,trans-geometry of the naturally occurring analog, diazarone, we believe that this reversal of stereoselectivity can be explained by the nature of bulky Lewis acid used in this study. Unlike SnCl4 and BF3, B(C6F5)3 coordinates to an acceptor group in cyclopropane 2 in such a way, that attack of isomeric alkene 5, formed by an initial isomerization of 2, on cyclopropane results in an intermediate 6 with anti-arrangement of substituents at carbon atoms that form a new bond (Scheme 4). Cyclization of 6 proceeds under thermodynamic control and affords the most stable trans,trans-1.
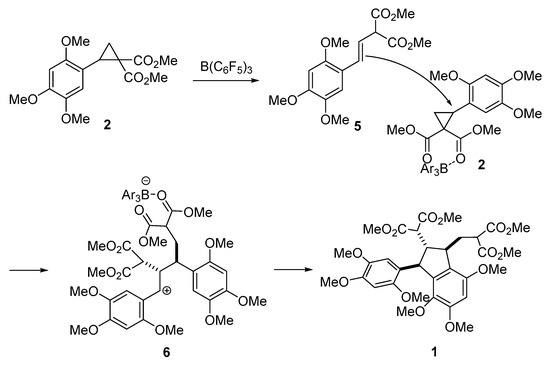
Scheme 4.
The proposed mechanism of dimer 1 formation.
In summary, the B(C6F5)3-induced cyclodimerization of 2-(2,4,5-trimethoxyphenyl)cyclopropane-1,1-diester 2 provides a concise route to polyoxygenated indane 1, which is a structural analogue of diazarone and has a potential for pharmacological studies.
3. Materials and Methods
NMR spectra were acquired on Bruker AM-400 and Bruker Avance 600 spectrometers at room temperature; the chemical shifts δ were measured in ppm with respect to the solvent (1H: CDCl3, δ = 7.27 ppm; 13C: CDCl3, δ = 77.0). The splitting patterns are designated as s, singlet; d, doublet; m, multiplet; dd, double doublet; br., broad. The coupling constants (J) were in Hertz. The 1H-NMR, 13C-NMR, 2D heteronuclear single quantum coherence (HSQC), heteronuclear multiple bond correlation (HMBC), nuclear Overhauser effect (NOESY) NMR spectra for the synthesized compound are available in the Supplementary Material. Infrared spectra were recorded on the Infralum FT-801 spectrometer. High resolution and accurate mass measurements were carried out using a micrOTOF-QTM ESI-TOF (electro spray ionization/time of flight, Bruker, Billerica, MA, USA) and LTQ Orbitrap mass spectrometer (Thermo Fischer Scientific, Waltham, MA, USA). Elemental analyses were performed with an EA-1108 CHNS elemental analyzer instrument (Fisons, Ipswich, UK). X-ray analysis was performed on STOE STADIVARI PILATUS-100K diffractometer (Stoe & Sie, Darmstadt, Germany). The microwave reaction was performed in a Monowave 300–Anton Paar microwave reactor (Anton Paar Gmbh, Graz, Austria) in sealed reaction vessels. The temperature was monitored with the installed IR detector. The melting points (m.p.) were determined using a 9100 capillary melting point apparatus (Electrothermal, Stone, UK). Analytical thin layer chromatography (TLC) was carried out with silica gel plates (silica gel 60, F254, supported on aluminum); the revelation was done by UV lamp (365 nm). Column chromatography was performed on silica gel 60 (230–400 mesh, Merck, Darmstadt, Germany). All reactions were carried out using freshly distilled and dry solvents. Commercial reagents employed in the synthesis were analytical grade, obtained from Aldrich (St. Louis, MI, USA) or Alfa Aesar (Ward Hill, MO, USA). CCDC 1972617 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk).
3.1. Dimethyl 2-(2,4,5-trimethoxybenzylidene)malonate (4)
To a solution of 2,4,5-trimethoxybenzaldehyde (5 g, 25.5 mmol) and dimethyl malonate (2.92 mL, 25.5 mmol) in toluene (8.5 mL), glacial acetic acid (292 µL, 5.1 mmol) and piperidine (252 µL, 2.55 mmol) were added. The mixture was refluxed with the Dean-Stark trap until water separation was finished (1 h). Upon cooling, the organic layer was washed with water (3 × 10 mL), dried with Na2SO4, concentrated in vacuo. The purification by flash chromatography (SiO2) afforded target alkene 4.
1H NMR (CDCl3, 400 MHz): δ = 3.74 (s, 3H, CH3O), 3.77 (s, 6H, 2 × CH3O), 3.79 (s, 3H, CH3O), 3.86 (s, 3H, CH3O), 6.43 (s, 1H, Ar), 6.89 (s, 1H, Ar), 8.03 (s, 1H, CH=). 13C NMR (CDCl3, 100 MHz): δ = 52.2 (2 × CH3O), 55.9 (CH3O), 56.2 (2 × CH3O), 96.5 (CH, Ar), 111.6 (CH, Ar), 113.2 (C, Ar), 122.3 (C, Ar), 137.8 (CH=, Ar), 143.0 (C, Ar), 152.7 (C, Ar), 154.1 (C, Ar), 165.0 (CO2Me), 167.8 (CO2Me). IR (KBr): ν = 3006, 2948, 1726, 1710, 1596, 1599, 1522, 1478, 1469, 1446, 1434, 1257, 1224, 1209, 1189, 1175, 1131, 1069, 1047 cm−1. HRMS ESI-TOF: m/z = 333.0944 [M + Na]+ (333.0945 calcd for C15H18NaO7).
3.2. Dimethyl 2-(2,4,5-trimethoxyphenyl)cyclopropane-1,1-dicarboxylate (2)
To dry DMF (14 mL) NaH (60% suspension in oil, 329 mg, 8.2 mmol) and trimethylsulfoxonium iodide (1.81 g, 8.2 mmol) were successively added in a single portion under argon atmosphere at room temperature. Vigorous evolution of hydrogen lasted ca. 10 min, after which the reaction mixture was stirred for additional 30 min. Dimethyl 2-(2,4,5-trimethoxybenzylidene)malonate (2.22 g, 6.9 mmol) in dry DMF (2 mL) was added in portions. The resulting mixture was stirred for 2 h, poured into ice-cooled aq. solution of NH4Cl (25 mL) and extracted with ethyl acetate (5 × 10 mL). The combined organic fractions were washed with water (5 × 10 mL), dried with Na2SO4 and concentrated in vacuo. The resulting residue was purified by recrystallization from Et2O yielding cyclopropane 2. Yield 1.38 g (60%); white solid; mp 109–110 °C. 1H NMR (CDCl3, 400 MHz): δ = 1.71 (dd, 2J = 5.2 Hz, 3J = 9.4 Hz, 1H, CH2), 2.13 (dd, 2J = 5.2 Hz, 3J = 8.4 Hz, 1H, CH2), 3.26 (dd, 3J = 9.4 Hz, 3J = 8.4 Hz, 1H, CH), 3.37 (s, 3H, CH3O), 3.76 (s, 3H, CH3O), 3.77 (s, 6H, 2 × CH3O), 3.84 (s, 3H, CH3O), 6.45 (s, 1H, Ar), 6.50 (s, 1H, Ar). 13C NMR (CDCl3, 100 MHz): δ = 19.0 (CH2), 28.3 (CH), 36.2 (C), 52.0 (CH3O), 52.5 (CH3O), 55.8 (CH3O), 56.40 (CH3O), 56.44 (CH3O), 97.1 (CH, Ar), 111.9 (CH, Ar), 114.1 (C, Ar), 142.2 (C, Ar), 148.8 (C, Ar), 153.5 (C, Ar), 167.2 (CO2Me), 170.3 (CO2Me). IR (KBr): ν = 2926, 2950, 2841, 1721, 1515, 1471, 1440, 1431, 1401, 1318, 1294, 1276, 1211, 1123, 1030 cm−1. HRMS ESI-TOF: m/z = 347.1098 [M + Na]+ (347.1101 calcd for C16H20NaO7). Anal. calcd for C16H20O7: C, 59.25; H, 6.22. Found: C, 59.16; H, 6.05. Crystal Data for C16H20O7 (M = 324.32 g/mol): triclinic, space group P-1 (no. 2), a = 8.7161(3) Å, b = 9.1767(3) Å, c = 10.0231 (3) Å, α = 90.334(2)°, β = 94.335(2)°, γ = 95.415(2)°, V = 795.77(4) Å3, Z = 2, T = 295 K, μ(CuKα) = 0.090 mm−1, Dcalc = 1.354 g/cm3, 16,914 reflections measured (4.424° ≤ Θ ≤ 73.202°), 3012 unique (Rint = 0.0419, Rsigma = 0.0531) which were used in all calculations. The final R1 was 0.0494 (I > 2σ(I)) and wR2 was 0.1471 (all data).
3.3. Dimethyl 2-{[2-(2-methoxy-1-methoxycarbonyl-2-oxoethyl)-4,5,7-trimethoxy-3-(2,4,5-trimethoxyphenyl)-2,3-dihydro-1H-inden-1-yl]methyl}malonate (1) (as a Mixture of (1RS,2RS,3RS)- and (1RS,2SR,3SR)–isomers in 84:16 Ratio)
A dry reaction microwave tube was charged with cyclopropane 2 (150 mg, 0.46 mmol) and HFIP (0.23 mL) under N2 atmosphere. B(C6F5)3 (24 mg, 0.046 mmol) was added and the reaction mixture was heated in a microwave reactor at 58 °C for 7 h, quenched with conc. aqueous NaHCO3 and extracted with ethyl acetate (3 × 10 mL). The combined organic extracts were washed with NaHCO3 and brine, dried with anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography on a silica gel to afford the desired product. Yield 129 mg (86%); colorless oil; Rf = 0.81 (petroleum ether:ethyl acetate; 1:1); mixture of diastereomers ((1RS,2RS,3RS)-1:(1RS,2SR,3SR)-1 in 84:16 ratio). (1RS,2RS,3RS)-1: 1H NMR (CDCl3, 600 MHz): δ = 2.00–2.07 (m, 2H, C(1′)H2), 2.66 (d, 3J1″,2 = 8.0 Hz, 1H, C(1″)H), 3.16 (t, 3J = 7.6 Hz, 1H, C(1)H), 3.47 (s, 3H, CH3O), 3.51 (s, 3H, CH3O), 3.56 (br. d, 3J2,1″ = 8.0 Hz, 1H, C(2)H), 3.60 (s, 3H, CH3O), 3.61 (s, 3H, CH3O), 3.61–3.63 (m, 1H, C(2′)H), 3.64 (s, 3H, CH3O), 3.745 (s, 3H, CH3O), 3.752 (s, 3H, CH3O), 3.82 (s, 3H, CH3O), 3.847 (s, 3H, CH3O), 3.852 (s, 3H, CH3O), 4.60 (br. s, 1H, C(3)H), 6.28 (s, 1H, C(6‴)H, Ar), 6.40 (br. s, 1H, Ar), 6.50 (s, 1H, Ar). 13C NMR (CDCl3, 125 MHz): δ = 35.0 (C(1′)H2), 44.7 (C(1)H), 45.2 (C(3)H), 50.0 (C(2′)H), 52.22 (CH3O), 52.26 (CH3O), 52.4 (2×CH3O), 52.9 (C(1′′)H), 55.4 (CH3O), 56.1 (C(2)H), 56.17 (CH3O), 56.23 (CH3O), 56.5 (CH3O), 56.8 (CH3O), 60.3 (CH3O), 96.8 (CH, Ar), 97.2 (CH, Ar), 113.3 (C(6‴)H, Ar), 124.3 (C, Ar), 125.4 (C, Ar), 138.0 (C, Ar), 139.7 (C, Ar), 143.1 (C, Ar), 148.3 (C, Ar), 151.1 (C, Ar), 152.2 (C, Ar), 152.8 (C, Ar), 169.2 (CO2Me), 169.3 (CO2Me), 169.9 (CO2Me), 170.3 (CO2Me). (1RS,2SR,3SR)-1: 1H NMR (CDCl3, 600 MHz): δ = 1.62–1.67 (m, 1H, CH2), 2.08–2.14 (m, 1H, CH2), 2.99 (s, 3H, CH3O), 3.03 (s, 3H, CH3O), 3.32–3.34 (m, 1H, CH), 3.38 (d, 3J = 11.4 Hz, 1H, CH), 3.67–3.69 (m, 1H, CH), 3.42 (dd, 3J = 10.4 Hz, 3J = 2.9 Hz, 1H, CH), 3.71 (s, 3H, CH3O), 3.73 (s, 3H, CH3O), 3.768 (s, 3H, CH3O), 3.771 (s, 3H, CH3O), 3.78 (s, 3H, CH3O), 3.84 (s, 3H, CH3O), 3.847 (s, 3H, CH3O), 3.852 (s, 3H, CH3O), 4.76 (d, 3J = 10.2 Hz, 1H, CH), 6.30 (s, 1H, Ar), 6.51 (s, 1H, Ar), 6.76 (s, 1H, Ar). 13C NMR (CDCl3, 150 MHz): δ = 29.3 (CH2), 40.0 (CH), 49.5 (CH), 50.9 (CH), 52.0 (CH), 52.6 (CH), 52.8 (CH3O), 52.9 (CH3O), 53.0 (CH3O), 55.2 (CH3O), 56.1 (CH3O), 56.2 (CH3O), 56.3 (CH3O), 56.8 (CH3O), 56.9 (CH3O), 59.7 (CH3O), 95.9 (CH, Ar), 97.8 (CH, Ar), 113.0 (CH, Ar), 121.8 (C, Ar), 124.6 (C, Ar), 139.8 (C, Ar), 140.0 (C, Ar), 143.1 (C, Ar), 148.1 (C, Ar), 150.9 (C, Ar), 152.6 (C, Ar), 153.1 (C, Ar), 168.1 (CO2Me, Ar), 168.6 (CO2Me), 169.9 (CO2Me, Ar), 170.5 (CO2Me). IR (ZnSe): ν = 2998, 2953, 2843, 1751, 1734, 1608, 1511, 1500, 1464, 1437, 1397, 1330, 1319, 1231, 1208, 1156, 1082, 1036 cm−1. HRMS ESI-TOF: m/z = 648.2420 [M]+ (648.2413 calcd for C32H40O14). Anal. calcd for C32H40O14: C, 59.25; H, 6.22. Found: C, 58.95; H, 6.22.
Supplementary Materials
The following are available online, Figure S1: 1H NMR spectrum of 4; Figure S2: 13C NMR spectrum of 4; Figure S3: 1H NMR spectrum of 2; Figure S4: 13C NMR spectrum of 2; Figure S5: 1H NMR spectrum of 1; Figure S6: 13C NMR spectrum of 1; Figure S7: HSQC 1H-13C spectrum of 1; Figure S8: HMBC 1H-13C spectrum of 1; Figure S9: NOESY 1H-1H spectrum of 1.
Author Contributions
Conceptualization, O.A.I. and I.V.T.; methodology O.A.I.; software, A.O.C., V.B.R., O.A.I.; validation, O.A.I. and I.V.T.; formal analysis, O.A.I.; investigation, M.A.B., A.O.C., V.B.R.; resources, O.A.I., A.O.C.; data curation, O.A.I.; writing—original draft preparation, O.A.I. and I.V.T.; writing—review and editing, A.O.C., O.A.I. and I.V.T.; supervision, O.A.I. and I.V.T.; project administration, A.O.C., O.A.I.; funding acquisition, A.O.C., O.A.I. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the Russian Foundation for Basic Research (grant 18-03-00954) and the Ministry of Education and Science of the Russia Federation for the financial support of the project (grant MK-1567.2018.3). Development Program.
Acknowledgments
The X-ray studies were fulfilled using a STOE STADIVARI PILATUS-100K diffractometer purchased as a part of the MSU.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Werz, D.B.; Biju, A.T. Uncovering the Neglected Similarities of Arynes and Donor–Acceptor Cyclopropanes. Angew. Chem. Int. Ed. 2019. [Google Scholar] [CrossRef]
- Schneider, T.F.; Kaschel, J.; Werz, D.B. A New Golden Age for Donor–Acceptor Cyclopropanes. Angew. Chem. Int. Ed. 2014, 53, 5504–5523. [Google Scholar] [CrossRef]
- Budynina, E.M.; Ivanov, K.L.; Sorokin, I.D.; Melnikov, M.Y. Ring Opening of Donor-Acceptor Cyclopropanes with N-Nucleophiles. Synthesis 2017, 49, 3035–3068. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Trushkov, I.V. Donor–Acceptor Cyclopropanes in the Synthesis of Carbocycles. Chem. Rec. 2019, 19, 2189–2208. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tomilov, Y.V. Dimerization of donor–acceptor cyclopropanes. Mendeleev Commun. 2015, 25, 1–10. [Google Scholar] [CrossRef]
- Mel’nikov, M.Y.; Budynina, E.M.; Ivanova, O.A.; Trushkov, I.V. Recent advances in ring-forming reactions of donor–acceptor cyclopropanes. Mendeleev Commun. 2011, 21, 293–301. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Budynina, E.M.; Khrustalev, V.N.; Skvortsov, D.A.; Trushkov, I.V.; Melnikov, M.Y. A Straightforward Approach to Tetrahydroindolo[3,2-b]carbazoles and 1-Indolyltetrahydrocarbazoles through [3+3] Cyclodimerization of Indole-Derived Cyclopropanes. Chem. Eur. J. 2016, 22, 1223–1227. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Budynina, E.M.; Chagarovskiy, A.O.; Trushkov, I.V.; Melnikov, M.Y. (3 + 3)-Cyclodimerization of Donor–Acceptor Cyclopropanes. Three Routes to Six-Membered Rings. J. Org. Chem. 2011, 76, 8852–8868. [Google Scholar] [CrossRef]
- Novikov, R.A.; Korolev, V.A.; Timofeev, V.P.; Tomilov, Y.V. New dimerization and cascade oligomerization reactions of dimethyl 2-phenylcyclopropan-1,1-dicarboxylate catalyzed by Lewis acids. Tetrahedron Lett. 2011, 52, 4996–4999. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tomilov, Y.V. Dimerization of Dimethyl 2-(Naphthalen-1-yl)cyclopropane-1,1-dicarboxylate in the Presence of GaCl3 to [3+2], [3+3], [3+4], and Spiroannulation Products. Helv. Chim. Acta 2013, 96, 2068–2080. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Budynina, E.M.; Khrustalev, V.N.; Trushkov, I.V.; Melnikov, M.Y. New domino dimerization of cyclopropylindoles: Synthesis of 1,3-bis(indolyl)cyclopentanes. Chem. Heterocycl. Comp. 2015, 51, 936–939. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Budynina, E.M.; Skvortsov, D.A.; Trushkov, I.V.; Melnikov, M.Y. Shortcut Approach to Cyclopenta[b]indoles by [3+2] Cyclodimerization of Indole-Derived Cyclopropanes. Synlett 2014, 25, 2289–2292. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Budynina, E.M.; Skvortsov, D.A.; Limoge, M.; Bakin, A.V.; Chagarovskiy, A.O.; Trushkov, I.V.; Melnikov, M.Y. A bioinspired route to indanes and cyclopentannulated hetarenes via (3+2)-cyclodimerization of donor–acceptor cyclopropanes. Chem. Commun. 2013, 49, 11482–11484. [Google Scholar] [CrossRef] [PubMed]
- Chagarovskiy, A.O.; Ivanova, O.A.; Budynina, E.M.; Trushkov, I.V.; Melnikov, M.Y. [3+2] Cyclodimerization of 2-arylcyclopropane-1,1-diesters. Lewis acid induced reversion of cyclopropane umpolung. Tetrahedron Lett. 2011, 52, 4421–4425. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tarasova, A.V.; Denisov, D.A.; Korolev, V.A.; Tomilov, Y.V. Cascade Dimerization of 2-styryl-1,1-cyclopropanedicarboxylate upon treatment with gallium trichloride. Russ. Chem. Bull. 2016, 65, 2628–2638. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tarasova, A.V.; Korolev, V.A.; Timofeev, V.P.; Tomilov, Y.V. A New Type of Donor–Acceptor Cyclopropane Reactivity: The Generation of Formal 1,2- and 1,4-Dipoles. Angew. Chem. Int. Ed. 2014, 53, 3187–3191. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tarasova, A.V.; Tomilov, Y.V. Synthesis of substituted naphthalenes by GaCl3-mediated cross-dimerization–fragmentation of 2-arylcyclopropane-1,1-dicarboxylates. Russ. Chem. Bull. 2014, 63, 2737–2740. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tarasova, A.V.; Suponitsky, K.Y.; Tomilov, Y.V. Unexpected formation of substituted naphthalenes and phenanthrenes in a GaCl3 mediated dimerization–fragmentation reaction of 2-arylcyclopropane-1,1-dicarboxylates. Mendeleev Commun. 2014, 24, 346–348. [Google Scholar] [CrossRef]
- Novikov, R.A.; Timofeev, V.P.; Tomilov, Y.V. Stereoselective Double Lewis Acid/Organo-Catalyzed Dimerization of Donor–Acceptor Cyclopropanes into Substituted 2-Oxabicyclo[3.3.0]octanes. J. Org. Chem. 2012, 77, 5993–6006. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Budynina, E.M.; Chagarovskiy, A.O.; Rakhmankulov, E.R.; Trushkov, I.V.; Semeykin, A.V.; Shimanovskii, N.L.; Melnikov, M.Y. Domino Cyclodimerization of Indole-Derived Donor–Acceptor Cyclopropanes: One-Step Construction of the Pentaleno[1,6a-b]indole Skeleton. Chem. Eur. J. 2011, 17, 11738–11742. [Google Scholar] [CrossRef]
- Atsumi, T.; Murakami, Y.; Shibuya, K.; Tonosaki, K.; Fujisawa, S. Induction of Cytotoxicity and Apoptosis and Inhibition of Cyclooxygenase-2 Gene Expression, by Curcumin and its Analog, α-Diisoeugenol. Anticancer Res. 2005, 25, 4029–4036. [Google Scholar] [PubMed]
- Ohyama, M.; Tanaka, T.; Ito, T.; Iinuma, M.; Bastow, K.F.; Lee, K.-H. Antitumor agents 200. Cytotoxicity of naturally occurring resveratrol oligomers and their acetate derivatives. Bioorg. Med. Chem. Lett. 1999, 9, 3057–3060. [Google Scholar] [CrossRef]
- Jin, Q.; Han, X.H.; Hong, S.S.; Lee, C.; Choe, S.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Antioxidative oligostilbenes from Caragana sinica. Bioorg. Med. Chem. Lett. 2012, 22, 973–976. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, B.; Pan, Y.; Jiang, L. Stilbene Oligomers from Parthenocissus laetevirens: Isolation, Biomimetic Synthesis, Absolute Configuration, and Implication of Antioxidative Defense System in the Plant. J. Org. Chem. 2008, 73, 5233–5241. [Google Scholar] [CrossRef]
- Bao, L.; Ma, X.; Song, X.; Wang, M.; Liu, H. Two New Resveratrol Tetramers Isolated from Cayratia japonica (Thunb.) Gagn. with Strong Inhibitory Activity on Fatty Acid Synthase and Antioxidant Activity. Chem. Biodivers. 2010, 7, 2931–2940. [Google Scholar] [CrossRef]
- Morikawa, T.; Xu, F.; Matsuda, H.; Yoshikawa, M. Structures of Novel Norstilbene Dimer, Longusone A, and Three New Stilbene Dimers, Longusols A, B, and C, with Antiallergic and Radical Scavenging Activities from Egyptian Natural Medicine Cyperus longus. Chem. Pharm. Bull. 2010, 58, 1379–1385. [Google Scholar] [CrossRef]
- Lavaud, A.; Soleti, R.; Hay, A.-E.; Richomme, P.; Guilet, D.; Andriantsitohaina, R. Paradoxical effects of polyphenolic compounds from Clusiaceae on angiogenesis. Biochem. Pharmacol. 2012, 83, 514–523. [Google Scholar] [CrossRef]
- Buev, E.M.; Moshkin, V.S.; Sosnovskikh, V.Y. Spiroanthraceneoxazolidine as a synthetic equivalent of methanimine in the reaction with donor–acceptor cyclopropanes. Synthesis of diethyl 5-arylpyrrolidine-3,3-dicarboxylates. Tetrahedron Lett. 2016, 57, 3731–3734. [Google Scholar] [CrossRef]
- Boichenko, M.A.; Chagarovskiy, A.O.; Rybakov, V.B.; Trushkov, I.V.; Ivanova, O.A. CCDC 1972617: Experimental Crystal Structure Determination. CSD Commun. 2019. [Google Scholar] [CrossRef]
- Kreft, A.; Lücht, A.; Grunenberg, J.; Jones, P.G.; Werz, D.B. Kinetic Studies of Donor–Acceptor Cyclopropanes: The Influence of Structural and Electronic Properties on the Reactivity. Angew. Chem. Int. Ed. 2019, 58, 1955–1959. [Google Scholar] [CrossRef]
- Xie, M.-S.; Zhao, G.-F.; Qin, T.; Suo, Y.-B.; Qu, G.-R.; Guo, H.-M. Thiourea participation in [3+2] cycloaddition with donor–acceptor cyclopropanes: A domino process to 2-amino-dihydrothiophenes. Chem. Commun. 2019, 55, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, A.A.; Dilman, A.D.; Novikov, R.A.; Khotoshutina, Y.A.; Struchkova, M.I.; Arkhipov, D.E.; Nelyubina, Y.V.; Tabolin, A.A.; Ioffe, S.L. Tandem Pd-catalyzed C–C coupling/recyclization of 2-(2-bromoaryl)cyclopropane-1,1-dicarboxylates with primary nitro alkanes. Tetrahedron Lett. 2016, 57, 11–14. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Piers, W.E.; Chivers, T. Pentafluorophenylboranes: From obscurity to applications. Chem. Soc. Rev. 1997, 26, 345–354. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).