Abstract
2,9-Dimethyl-11-(3-pentadecylphenoxy)dibenzo[c,f][1,2,5]dithiaphosphepine 11-oxide was synthesized (yield 50%) by a two-step procedure. The first step starts with a benzothiadiphosphole and bis-Grignard reagent, and the second step consists of adding the sodium salt of a derivative of cardanol. The structure of newly synthesized compound was elucidated based on 1H-NMR, 13C-NMR, 31P-NMR, IR, Electron Spray Ionization (ESI)–MS, Gas Chromatography-Mass Spectroscopy (GC–MS), and Electron Spray Ionization-High Resolution Mass Spectroscopy (ESI–HRMS).
1. Introduction
The synthesis of heterocyclic systems containing phosphorus is of considerable current interest, principally because they play a central role in coordination chemistry and homogeneous catalysis [1,2,3].
In the past, we reported [4] that benzothiadiphosphole (1) can be easily obtained via a reaction between p-methylthioanisole, PCl3 and AlCl3, and it can be used as efficient phosphorus-donating reagent to obtain a plethora of phosphine derivatives [5,6,7,8]. Subsequently, we found that 1 can be easily transformed in similar structures bearing P–As, P–Sb, or P–Bi bonds that are able to produce arsines, stibines, or bismuthines [9]. Now, we report another application of 1 to obtain a new heterocyclic compound that contains one phosphorus and two sulfur atoms. This compound is of possible interest in coordination chemistry as a bi- or tri-dentate ligand. In addition, the presence of a cardanol moiety not only permits to obtain a product with good lipophilicity but also meets the requirements of the circular economy because the cardanol derivative is obtained as a by-product in cashew nut processing.
2. Results and Discussion
The synthesis of 2,9-dimethyl-11-(3-pentadecylphenoxy)dibenzo[c,f][1,2,5]dithiaphosphepine 11-oxide is shown in Scheme 1. The first step was the reaction between benzothiadiphosphole 1 and a bis-Grignard reagent (e.g., 1,3-bis(bromomagnesium)propane or 1,4-(bromomagnesium)butane). In the second step, the sodium salt of a hydrogenated derivative of cardanol (2) was added to the crude reaction mixture, and, after quenching with water, the final product 3 was obtained. The product was recovered in a 50% yield after purification through chromatography on silica gel.
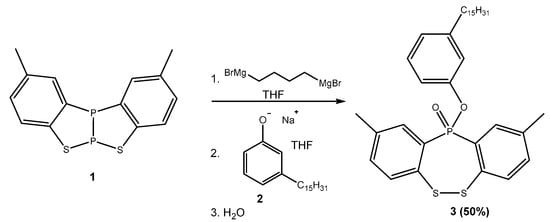
Scheme 1.
Synthesis of compound 3 from benzothiadiphosphole 1 and the cardanol derivative 2.
The structure of the newly synthesized compound was elucidated based on IR, 1H-NMR, 13C-NMR, 31P-NMR, electron spray ionization (ESI)–MS, GC–MS and ESI–HRMS spectroscopy (All spectra are show in Supplementary Materials). Particularly diagnostic was the 31P NMR signal at 29.2 ppm in a region typical of tetracoordinated phosphorus compounds. Additionally to that which was recorded with ESI–HRMS, we recorded Electronic Impact (EI)–MS (with a GC–MS instrument) that showed two signals with 304 and 291 m/z corresponding to the two moieties derived from the breakage of the P–OAr bond.
Based on our previously reported studies [7,10], the mechanistic pathway is likely that which is shown in Scheme 2.
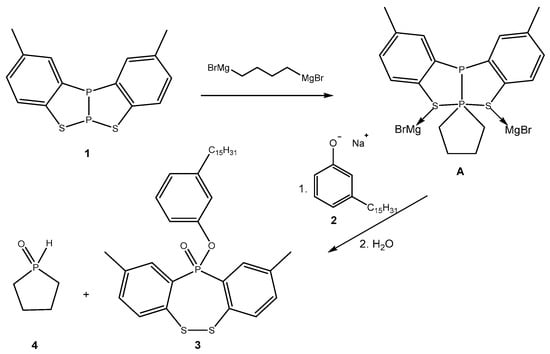
Scheme 2.
Proposed mechanism for the formation of compound 3.
In the first step, the benzothiadiphosphole 1 reacted with the bis-Grignard reagent to give the intermediate A [7], which underwent attack from the sodium phenolate 2 to give, after quenching with water and work-up, the final product 3 and the phosphine oxide 4. In particular, after quenching with water, the phosphine precursor of 4 and the phosphinite precursor of 3 were formed but, after the work-up, compounds 4 and 3 were produced.
3. Materials and Methods
The 1H and 13C spectra were recorded on an Inova 600 (Varian, Palo Alto, CA, USA) spectrometer operating at 600 MHz (for 1H NMR) and at 150.8 MHz (for 13C NMR). The 31P NMR spectrum was recorded on a Mercury 400 (Varian, Palo Alto, CA, USA) spectrometer operating at 161.9 MHz. Chemical shifts were referenced to the solvent for 1H and 13C NMR (δ = 7.26 and 77.0 ppm, respectively for CDCl3) and to an 85% H3PO4 external standard for 31P NMR. Signal multiplicities were established by Distortionless Enhancement by Polarization Transfer (DEPT) experiments. Chemical shifts were measured in δ (ppm). J values are given in hertz. Electron spray ionization mass spectra (ESI–MS) were recorded with a WATERS 2Q 4000 instrument (Waters, Etten-Leur, The Netherlands). GC–MS analyses were performed on a gas chromatograph (Agilent 6890 series, Agilent, Santa Clara, CA, USA) that was equipped with a (5%-phenyl)-methylpolysiloxane column (30 mm length, 0.250 mm depth, and 0.25 μm thickness), that was interfaced to a quadrupole mass detector (Agilent 8973 network). The GC–MS mass spectrum was recorded at an ionization voltage of 70 eV in EI mode. Chromatographic purifications (FC) were carried out on glass columns that were packed with silica gel (Merck grade 9385, 230−400 mesh particle size, and 60 Å pore size) at medium pressure. Thin layer chromatography (TLC) was performed on silica gel 60 F254-coated aluminum foils (Fluka). Benzothiadiphosphole was prepared as reported in the literature [2,3,4,5,6,7,8,9]. Cardanol derivatives were purchase from Sigma-Aldrich.
Synthesis of 2,9-Dimethyl-11-(3-pentadecylphenoxy)dibenzo[c,f][1,2,5]dithiaphosphepine 11-Oxide (3)
A solution of bis-Grignard reagent (1 mmol) in Tetrahydrofuran (THF) was added dropwise, under a dry nitrogen atmosphere, to a solution of 1 (1 mmol, 0.306 g) in THF (15 mL) at room temperature. The mixture was stirred for 120 min at room temperature. A solution of the sodium salt of hydrogenated cardanol (2.0 mmol, 0.652 g) in dry THF (10 mL) was then added. The reaction mixture was allowed to stand overnight at room temperature. Then was quenched with H2O and extracted with CH2Cl2. The organic layer was dried over anhydrous sodium sulphate and concentrated ‘in vacuo’. The final compound was purified by FC on a silica gel column (diethyl ether/light petroleum ether 1:1; r.f. = 0.27), and 0.297 g (50% yield) of 3 were obtained as greasy solid. 1H-NMR (CDCl3, 600 MHz) δ (ppm): 8.24 (dd, J1 = 11.5 Hz, J2 = 1.5 Hz, 2H), 7.39 (dd, J1 = 7.6 Hz, J2 = 5.3 Hz, 2H), 7.23 (d, J = 7.6 Hz, 2H), 7.05 (app t, J = 8.8 Hz, 1H), 6.92 (s, 1H), 6.91 (d, J = 7.2 Hz, 1H), 6.82 (dd, J = 7.7 Hz, 1H), 2.45 (t, J = 7.9 Hz, 2H), 2.38 (s, 6H), 1.43 (quint., J = 7.3 Hz, 2H), 1.32–1.22 (m, 24H), and 0.88 (t, J = 7.0 Hz, 3H); 13C-NMR (CDCl3, 150.8 MHz) δ (ppm): 150.4 (d, J2PC = 8.9 Hz), 144.6, 138.9 (d, J2PC = 11.7 Hz), 138.2 (d, J3PC = 9.9 Hz), 137.4 (d, J3PC = 6.7 Hz), 133.5 (d, J4PC = 2.6 Hz), 132.7, 132.1 (d, J1PC = 135.7 Hz), 130.9 (d, J2PC = 11.6 Hz), 129.0, 124.8, 120.8 (d, J3PC = 4.4 Hz), 118.1 (d, J3PC = 4.7 Hz), 35.6, 31.9, 31.3, 29.69, 29.68, 29.65, 29.57, 29.48, 29.3, 29.1, 22.7, 21.0, and 14.1 (three signals overlapped); 31P-NMR (CDCl3, 161.9 MHz), δ (ppm): 29.2; IR (cm−1): 3024, 2903, 1221 (P=O), 1206 (P–OAr), 787, 727, 675; GC–MS (70 eV) m/z (%): 594 (M+, 13), 304 (18), 291 (46), 259 (20), 244 (12), and 108 (100); UV-vis λmax = 304 nm, log e = 3.34; ESI–MS (m/z): 595 (M + H)+, 617 (M + Na)+, 633 (M + K)+; ESI–HRMS (m/z): calculated for C35H48O2PS2 [M + H]+: 595.2828, found: 595.2833.
4. Conclusions
We have reported an easy, one-pot, two step synthesis of a new heterocyclic compound of possible interest in coordination chemistry.
Supplementary Materials
The following are available online, Figure S1: 1H-NMR spectrum of the compound 3, Figure S2: 13C-NMR spectrum of the compound 3, Figure S3: 31P-NMR spectrum of the compound 3, Figure S4: GC–MS spectrum of the compound 3, Figure S5: ESI–MS spectrum of the compound 3, Figure S6: ESI–HRMS spectrum of compound 3; Figure S7: IR spectrum of compound 3.
Author Contributions
Methodology G.M., D.T., and M.M.; writing—original draft preparation G.M. and C.B.; supervision C.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors have not received financial assistance from any funding agencies.
Acknowledgments
Many thanks to Luca Zuppiroli for mass spectra.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Noyori, R. Asymmetric Catalysis in Organic Synthesis; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Burk, M.J.; Gross, M.F.; Martinez, J.P. Asymmetric catalytic synthesis of beta-branched amino acids via highly enantioselective hydrogenation of alpha-enamides. J. Am. Chem. Soc. 1995, 117, 9375–9376. [Google Scholar] [CrossRef]
- Jiang, Q.; Xiao, D.; Zhang, Z.; Cao, P.; Zang, X. Highly enantioselective hydrogenation of cyclic enol acetates catalyzed by a Rh–PennPhos complex. Angew. Chem. Int. Ed. 1999, 38, 516–518. [Google Scholar] [CrossRef]
- Baccolini, G.; Beghelli, M.; Boga, C. An improved synthesis of fused 1,2,3-benzothiadiphospholes and a proposed reaction pathway. Heteroatom Chem. 1997, 8, 551–556. [Google Scholar] [CrossRef]
- Baccolini, G.; Boga, C.; Mazzacurati, M.; Monari, M. Transformations of benzothiadiphosphole system: General one-pot synthesis of 1,2,5-dithiaphosphepines and their precursor phosphanethiols. Heteroatom Chem. 2005, 16, 339–345. [Google Scholar] [CrossRef]
- Baccolini, G.; Boga, C.; Mazzacurati, M.; Sangirardi, F. High atom-economical one-pot synthesis of secondary phosphines and their borane complexes using recycling phosphorus donor reagent. Org. Lett. 2006, 8, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Baccolini, G.; Micheletti, G.; Boga, C. The role played by phosphorus hexacoordination in driving the stereochemical outcome of a phosphination reaction. J. Org. Chem. 2009, 74, 6812–6818. [Google Scholar] [CrossRef] [PubMed]
- Boga, C.; Micheletti, G.; Delpivo, C.; Mazzacurati, M. A simple route to new cyclic (chloroalkyl)phosphane-, diphosphane-, and aminophosphane derivatives. Heteroatom Chem. 2013, 24, 392–397. [Google Scholar] [CrossRef]
- Baccolini, G.; Boga, C.; Mazzacurati, M.; Micheletti, G. The first flights of a molecular shuttle transporting elements: Easy one-pot formation of organic cyclic arsanes, stibanes and bismutanes. Chem. Eur. J. 2009, 15, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Baccolini, G.; Boga, C.; Guizzardi, G.; Ponzano, S. Simple and general synthesis of new 11H-11λ5-dibenzo[c, f][1,2,5]dithiaphosphepine derivatives. Tetrahedron Lett. 2002, 43, 9299–9302. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).