Abstract
The compound 3-{[(2,3-Dichlorophenyl)amino]methyl}-5-(furan-2-ylmethylidene)-1,3-thiazolidine-2,4-dione has been designed, synthesized, and screened for its in vitro antibreast cancer activity, using human breast adenocarcinoma cell lines (MCF-7) and in vitro anti-inflammatory activity. By hemolysis assay, it showed that it has a nonhemolytic and nontoxic effect on human blood cell. The title compound 5, subjected to in vitro activities, showed that it is cytotoxic with an IC50 of 42.30 µM and a good anti-inflammatory agent. The docking results against cyclin dependent kinase 2 (CDK2) (PDB ID: 3QQK) gave insights on its inhibitory activity.
1. Introduction
Thiazolidinedione derivatives are known in the field of medicinal chemistry due to their pharmacological properties. Wang et al. showed the neuroprotection ability of a thiazolidinedione derivative (ATZD2) to pass the blood–brain barrier (BBB) [1]. Thiazolidinediones are known to possess anticancer [2], antimicrobial [3], antidiabetic [4,5], antioxidant [6], and antiviral [7] properties. In view of the potential activities of thiazolidinedione derivatives, we designed and synthesized the title compound and studied its in vitro anticancer and anti-inflammatory effects.
Cancer is a worldwide burden, and female breast cancer accounts for 11.6% of the total cancer deaths [8]. Cancer prevention can be performed by targeting cyclin A and cyclin dependent kinase 2 (CDK2) [9]. The CDK 2 in complex with its cyclins (cyclin A and cyclin E), are known to regulate cell cycle at G1/S transition. In this study, we have docked the title compound against the Cyclin Dependent Kinase 2 (CDK2), PDB ID: 3QQK using Schrödinger software (version 1.8, Schrodinger, New York, NY, USA) to predict the affinity between the protein and ligand as well as the degree of their inhibition by docking score. Computational technology development is regarded as a new approach in drug discovery. Inaccessibility of docking facility before synthesis has many negative impacts such as time consuming, huge waste of chemicals and wastage of money for in vitro or in vivo activity on non-active compounds.
2. Results
2.1. Chemistry
The synthesis of the title compound is outlined in Scheme 1. The reaction of thiourea 1 on 2-chloroacetic acid 2 in concentrated HCl under a reflux condition of 110 °C for 18 h gives thiazolidine-2,4-dione 3, which is the key intermediate [10]. Product 4 was given by the base-catalyzed condensation of thiazoline-2,4-dione 3 with thiophen-2-carbaldehyde/furfural. End product 5 was synthesized by dissolving compound 4 (0.01 mol) in dimethylformamide (DMF) (20 mL) in the presence of K2CO3 (0.02 mol). The reaction mixture was stirred for 30 min at RT, and then 1.5 mL of formaldehyde (40%) was added. The 2,3-dichloroaniline was added to it under stirring condition. The resulting precipitate obtained by filtration was washed with ice water, dried, and then recrystallized from methanol to get a pure white color compound of 55% yield.
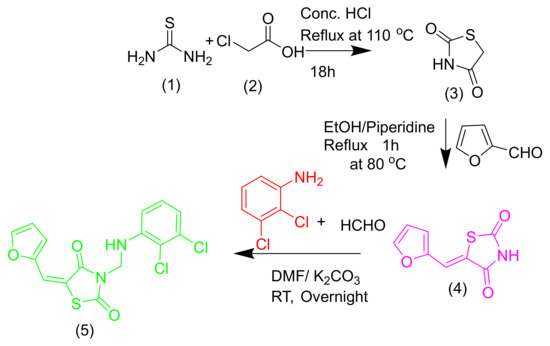
Scheme 1.
The synthesis of 3-{[(2,3-Dichlorophenyl)amino]methyl}-5-(furan-2-ylmethylidene)-1,3-thiazolidine-2,4-dione.
MP: 158–160 °C; wheatish colored powder; FT IR (KBr, νmax, cm−1): 3122 (N-H), 2930 (C-H), 1728, 1620 and 1614 (C=O), respectively, 1544 (C=C), and 769, 736 (C-Cl); 1H NMR (400 MHz, CDCl3, δ ppm): 7.66 (d, 1H, Ar-H J = 1.68 Hz), 7.64 (s, 1H, ethylene-H), 7.09–7.11 (d, 1H, Ar-H, J = 8 Hz), 7.06–7.07 (d, 1H, Ar-H, J = 4 Hz), 6.88–6.89 (dd, 1H, Ar-H, J = 4 Hz, J = 4 Hz), 6.79–6.80 (d, 1H, Ar-H, J = 4 Hz), 6.56–6.57 (d, 1H, Ar-H, J = 4 Hz), 5.68 (s, 1H, N-H), and 5.24–5.26 (d, 2H, methylene-H, J = 8 Hz); 13C NMR (100 MHz, DMSO-d6, δ ppm): 169.3, 165.5, 149.1, 147.9, 143.2, 131.9, 128.4, 119.8, 118.9, 117.4, 116.3, 113.6, 110.3 and 50.5; LCMS (m/z): 369 [M + H]+, 371 [M + H + 2]+, and 373 [M + H + 4]+. Anal. calcd. for C15H10N2O3SCl2: C, 48.80; H, 2.73; N, 7.59; O, 13.0; S, 8.68; Cl, 19.20. Found: C, 48.72; H, 2.70; N, 7.54; O, 12.90; S, 8.65; Cl, 19.17.
The structure of the synthesized compounds emphasized its selectivity to the protein since it forms the π–π interaction on the furan ring and hydrogen bond with Leu83. The structure activity relationship (SAR) of the synthesized compound showed that the presence of the strong bonds with the protein is of crucial importance.
2.2. Biology
2.2.1. Molecular Docking
The inhibitory action of the new compound was shown by the docking results where the molecule was docked against the active site of cyclin dependent kinase 2 (CDK2). Many interactions were displayed, such as hydrogen bonds, π–π stacking, and hydrophobic interactions. The figures below (Figure 1 and Figure 2) show the docking results, and the docking score is 7.299 kCl/mol.
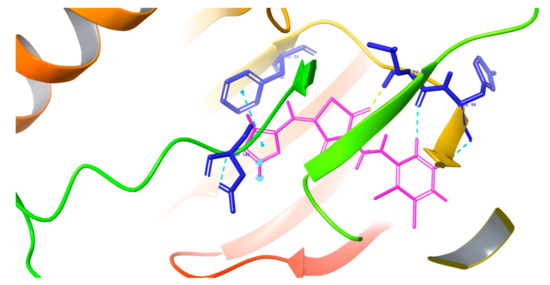
Figure 1.
Docking pose of the title compound against cyclin dependent kinase 2 (CDK2).
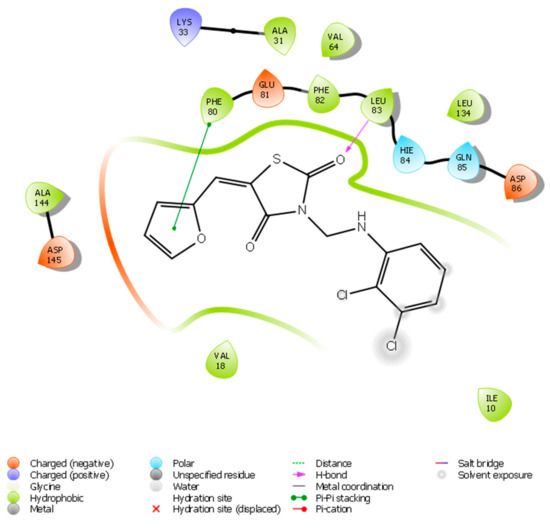
Figure 2.
Ligand interaction diagram of the title compound with CDK2.
The oxygen of the thiazolidinedione ring receives a hydrogen bond from Leu83, whereas the furan ring makes a π–π stacking bond with Phe80. There are also three aromatic hydrogen bonds with Asp145, Leu83, and Hie84. The title compound also makes many hydrophobic interactions with the residues which surround the binding pocket, such as Gln131, 85, Ile10, Asp86, Phe82, Glu81, Val64, Leu134, Ala144, Hie84, Val18, and Lys33. The molecule affinity toward the protein is explained by all of these interactions, and the docking score of the molecule is 7.299 kCal/mol.
2.2.2. Anticancer Activity
The antiproliferative activity of the title compound against MCF-7 is shown in the Table 1.

Table 1.
Antiproliferative activities of the title compound against MCF-7 cell line (IC50 in µMol).
The Table 2 shows the toxicity of the compound on MCF-7 by using MTT assay.

Table 2.
Cytotoxicity of compound 5 on MCF-7 using MTT assay.
From the table above, end product 5 is toxic on MCF-7 cell lines.
2.2.3. Anti-Inflammatory Activity
The results of anti-inflammatory activity are shown in the table below (Table 3.)

Table 3.
Anti-inflammatory results.
The IC50 (µg/mL) of the compound at different concentrations was compared to that of the positive standard diclofenac sodium.
The results above show that compound 5 can be a good anti-inflammatory agent, and its IC50 is almost equivalent to that of the reference drug diclofenac sodium.
2.2.4. Hemolysis Assay
Hemolysis assay showed that the title compound is non hemolytic and nontoxic (Table 4).

Table 4.
Percentage of hemolysis assay.
The figure above (Figure 3) indicates that the title compound is nontoxic and nonhemolytic on human blood.
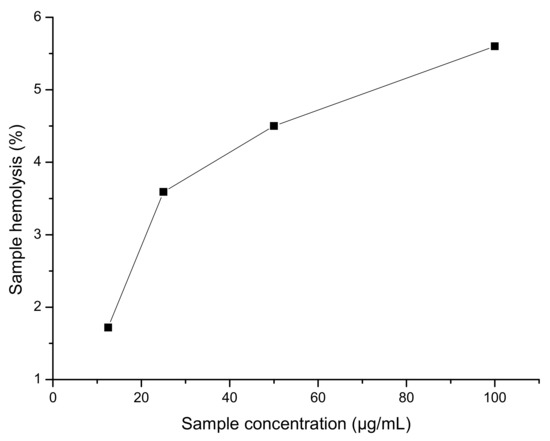
Figure 3.
Hemolysis assay results.
3. Discussion
3.1. Chemistry
General Information of 3-{[(2,3-Dichlorophenyl)amino]methyl}-5-(furan-2-ylmethylidene)-1,3-thiazolidine-2,4-dione
The spectral details of representative compound 5 are as follows: In the IR spectrum of 5 (Supplementary Figure S4), an absorption band found at 3122 cm−1 was attributed to NH stretch. The presence of the –C–H group in the product was evidenced by a band appearing at 2930 cm−1. The absorption bands seen at 1728, 1620, and 1614 cm−1 were due to the stretching frequency of –C=O, respectively (–C=O has three bands because it has three –C=O). The absorption band seen at 1544 cm−1 was due to –C=C stretching. Likewise, an absorption band that appeared at 769 and 736 cm−1 proved the presence of –C–Cl stretching. On recording 1H NMR spectrum (Supplementary Figure S2), the formation of thiazolidinedione derivative 5 was supported by the presence of respective signals for the protons present in the molecule. A singlet seen at δ (ppm) 7.64 could be accounted for ethylene-H. A triplet appeared in the region δ (ppm) 5.67 was accounted for one of the protons of the furan ring. The remaining proton on the furan ring appeared as a doublet in region δ (ppm) 7.10 (J = 8 Hz). The proton on the phenyl ring appeared as a doublet in the region of (ppm) 6.89 (J = 4 Hz and 1.8 Hz) δ (ppm). The remaining protons on the phenyl ring appeared as a doublet at δ (ppm) 6.79–6.80 (J = 4 Hz). The two aliphatic protons appeared at δ (ppm) 5.25 (d, J = 8 Hz). The N–H peak was seen at δ (ppm) 5.68 (1H, N-H). These data confirmed the formation of the title compound 5. Furthermore, it was supported by recording the 13C NMR (100 MHz, DMSO-d6, δ ppm) spectrum (Supplementary Figure S3). The signals that appeared in the spectrum could be assigned to the exact number of carbon atoms, including magnetically equivalent ones. The molecular mass of compound 5 was determined by LCMS (Supplementary Figure S1) and was found to be 369 [M + H]+, 371 [M + H + 2]+, 373 [M + H + 4]+.
3.2. Biology
3.2.1. Anticancer Activity
Cytotoxicity Assessment Using Methyl Thiazolyl Tetrazolium (MTT) Assay
The compound’s cytotoxicity on MCF-7 cells was evaluated using MTT assay [11]. Cells were seeded at a density of 5000 cells/well in 96 well microtiter plates and incubated at 37 °C under 5% CO2 in a humidified atmosphere for 24 h. The test compound was added to the cells in five different concentrations (5, 10, 25, 50, and 100 µM) and incubated for 48 h. Then, 100 µL of MTT solution (1 mg/mL) was added to the wells and incubated for 4 h.
3.2.2. Anti-Inflammatory Activity
Evaluation of Anti-Inflammatory Activity by Inhibition of Albumin Denaturation
Anti-inflammatory activity of the synthesized compound was shown by the albumin denaturation techniques [12,13]. The sample to be tested was incubated at 37 °C for 20 min and then heated to 52 °C for 20 min, and the turbidity was measured at 660 nm. Diclofenac sodium was taken as reference, and each experiment was done in triplicate. The following equation was used to determine anti-inflammatory activity.
3.2.3. Hemolysis Assay
This assay is performed in order to check the in vitro toxicity of the synthesized compound. Human blood was used. According to Sashidhar et al., during the procedure [14], the cells were incubated along with the compound (100 µg/mL), and the absorbance of the supernatant was measured at 540 nm. The hemolysis percentage was obtained using the following formula:
4. Materials and Methods
All materials used were bought from Sigma-Aldrich (Bangalore, India) and Spectrochem Pvt. Ltd. (Mumbai, India). The solvents used were from commercial sellers and were used without any further purification. The open capillary method was used to determine the melting point. A Shimadzu FT-IR spectrometer was used to record the FTIR. The 1H and 13C NMR spectra were measured with a Bruker Avance II 400 MHz spectrometer (Bruker, Punjab, India) operating at 400 MHz for 1H and at 100 MHz for 13C nuclei, respectively. Tetramethylsilane (TMS) was used as an internal standard. The chemical shift (δ) and the coupling constants (J) are in parts per million (ppm) and are taken in Hertz.
The LC mass spectrometer (Agilent Technology, Santa Clara, CA, USA) was used to record mass spectra. A CHNS Elementar Vario EL III was used to do elemental analysis. The compound’s purity was checked by thin layer chromatography (TLC) on a silica-coated aluminum sheet (silica gel 60F254) with the help of ethyl acetate and hexane mixtures (1:3), and the UV was visualized at 254 nm.
The ligand docking to the protein structural model was done using the Glide module in Schrödinger. The grid was generated by the receptor grid generation panel in Glide. The molecular docking was done by a ligand-docking panel in Glide. The 2D sketcher was employed to draw the ligand structure. The 3D sketcher was generated by LigPrep. The pH range of 7.0 ± 2 was respected using Epik module. The cyclin dependent kinase 2 (PDB ID: 3QQK) was preprocessed by Protein Preparation Wizard, and the missing hydrogens and amino acid chains were added. After the ligand preparation was done, it was docked to the protein under the extra precision (XP) mode. After docking, the complex was visualized and analyzed for fitting and interactions.
The human breast adenocarcinoma cells (MCF-7) (ATCC) came from the National Centre for Cell Sciences (NCCS), Pune, India, cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum and 1% antibiotic–antimycotic solution. After being incubated at 37 °C under 5% CO2, they were subcultured upon attaining 70% confluence and used for the experiments after three consecutive passages. Formazan crystals were solubilized in DMSO, and absorbance was recorded at 570 nm by a multimode microplate reader (FluoSTAR Omega, BMG Labtech, Ortenberg, Germany).
5. Conclusions
We have designed and successfully synthesized a new thiazolidinedione derivative. The title compound 5 showed its potency in regard to in vitro antibreast cancer activity against human breast adenocarcinoma cells (MCF-7), and it was nontoxic and nonhemolytic on human blood at a 100 µg/mL concentration. Despite the fact that the title compound is potent on breast cancer cell lines (MCF-7), it is also an anti-inflammatory agent. The docking results of the compound showed the inhibition of CDK2, which is a better target for anticancer activity.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-8599/2019/4/M1083/s1, Figure S1: Mass spectrum of the compound 5, Figure S2a–c: 1H NMR spectrum of the compound 5, Figure S3: 13C NMR spectrum of the compound 5, Figure S4: The FTIR spectrum of the compound 5.
Author Contributions
The conceptualization of the paper was carried out by N.U. and B.K.S. The methodology, software validation, docking studies and synthesis were carried out by N.U. The discussion of results and formatting of the manuscript were supervised by B.K.S.
Funding
This research received no external funding.
Acknowledgments
The authors are thankful to the Directors of SAIF Punjab University and USIC Mangalore University for providing a facility for spectral analysis. We also thank Yenepoya Research Centre, Yenepoya (Deemed to be University), Mangalore, for carrying out in vitro anticancer activity. Last but not least, we acknowledge Vaishali Rai, M Assistant Professor in the Department of Microbiology, St. Aloysius College (Autonomous), Mangalore, for his contribution in hemolysis assay. One of the authors (Nadine Uwabagira) also thanks the Indian Council for Culture Relations (ICCR) for guaranteeing the fellowship.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, X.K.; Sun, T.; Li, Y.J.; Wang, Y.H.; Li, Y.J.; Yang, L.D.; Feng, D.; Zhao, M.G.; Wu, Y.M. A novel thiazolidinediones ATZD2 rescues memorydeficits in a rat model of type 2 diabetes through antioxidant and anti-inflammation. Oncotarget 2017, 8, 107409–107422. [Google Scholar] [PubMed]
- Ozen, C.; Unlusoy, M.C.; Aliary, N.; Ozturk, M.; Bozdag-Dundar, O. Thiazolidinedione or Rhodanine: A study on synthesis and anticancer activity comparison of novel thiazole derivatives. J. Pharm. Sci. 2017, 20, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Valadbeigi, E.; Ghodsi, S. Synthesis and characterization of some new thiazolidinedione derivatives containing a coumarin moiety for their antibacterial and antifungal activities. Med. Chem. 2017, 7, 178–185. [Google Scholar] [CrossRef]
- Naim, M.J.; Alam, M.J.; Nawaz, F.; Naidu, V.G.; Aaghaz, S.; Sahu, M.; Siddiqui, N.; Alam, O. Synthesis, molecular docking and anti-diabetic evaluation of 2,4- thiazolidinedione based amide derivatives. Bioorg. Chem. 2017, 73, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Mello, T.; Ceni, E.; Surrenti, E.; Surrenti, C. The potential of antidiabetic thiazolidinediones for anticancer therapy. Expert Opin. Investig. Drugs 2006, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Marc, G.; Stana, A.; Oniga, S.D.; Pirnau, A.; Vlase, L.; Oniga, O. New phenolic derivatives of thiazolidine-2,4-dione with antioxidant and antiradical properties: Synthesis, characterization, in vitro evaluation and quantum studies. Molecules 2019, 24, 2060. [Google Scholar] [CrossRef] [PubMed]
- Omeragic, A.; Kara-Yacoubian, N.; Kelschenbach, J.; Sahin, C.; Cummins, C.L.; Volsky, D.J.; Bendayan, R. Peroxisome proliferator-activated receptor-gamma agonists exhibit anti-inflammatory and antiviral effects in an EcoHIV mouse model. Sci. Rep. 2019, 9, 9428. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Dachineni, R.; Ai, G.; Kumar, R.D.; Sadhu, S.S.; Tummala, H.; Bhat, J. Cyclin A2 and CDK2 as novel targets of aspirin and salicylic acid: A potential role in cancer prevention. Mol. Cancer Res. 2016, 14, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Raissa, K.C.; Jamerson, F.; Moreira, H.A.; Pinto, O.G.; Camargo, L.T.; Naves, P.L.; Camargo, A.J.; Ribeiro, L.; Ramos, L.M. Synthesis, antimicrobial activity and structure-activity relationship of some5-arylidene-thiazolidine-2,4-dione derivatives. J. Braz. Chem. Soc. 2019, 30, 164–172. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid calorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Mizushima, Y.; Kobayashi, M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharma. Pharmac. 1968, 20, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Sakat, S.; Preeti, T.; Juvekar, A. In vitro anti-inflammatory activity of aqueous and methanol extracts of erythrina indica Lam leave. Pharmacologyonline 2009, 3, 221–229. [Google Scholar]
- Sashidhara, K.V.; Rao, K.B.; Kushwara, P.; Modukuru, R.K.; Singh, P.; Shukla, P.K.; Chopra, S.; Pasupeleti, M. Novel chalcone-thiazole-hybrids as potent inhibitors of drug resistance Staphylococcus aureus. ACS Med. Chem. Lett. 2015, 6, 809–813. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).