Reactions of Polychlorinated Pyrimidines with DABCO
Abstract
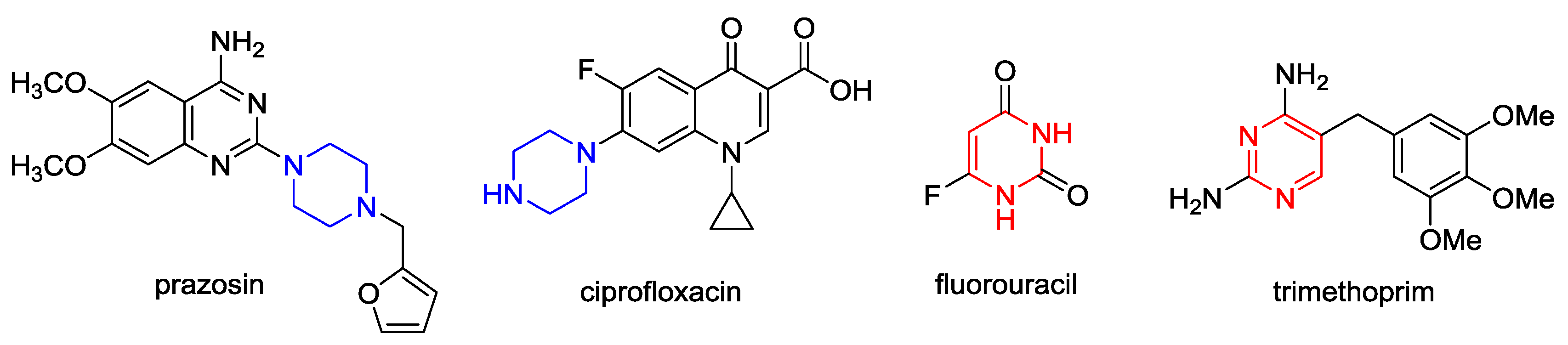
:1. Introduction
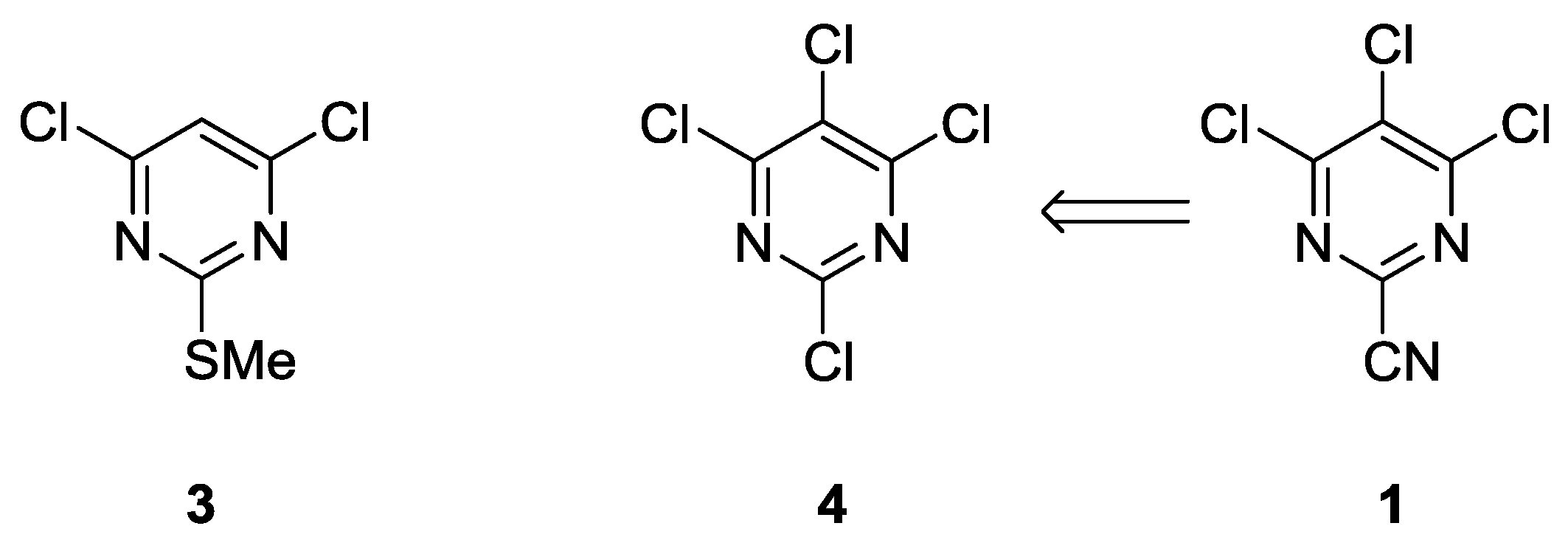
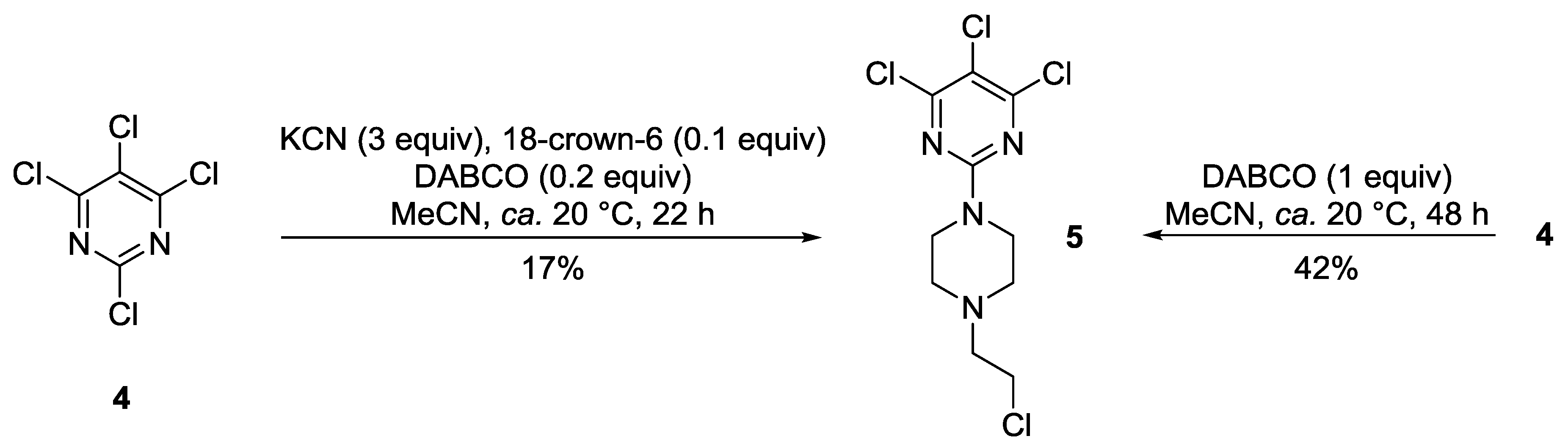
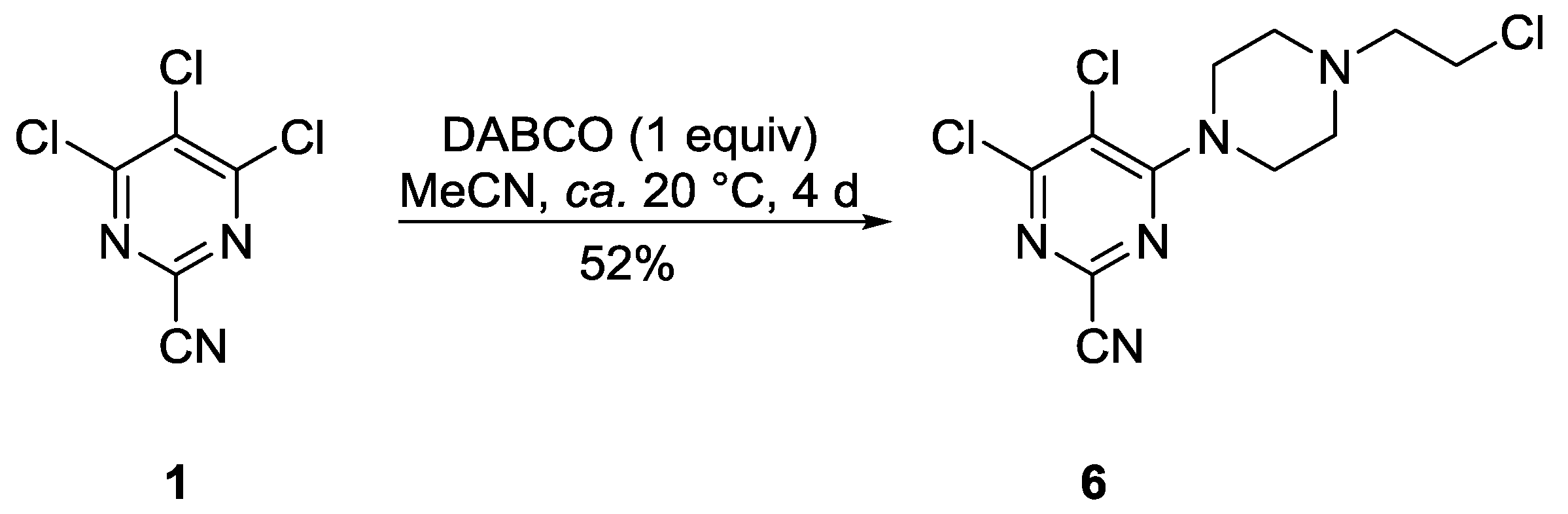
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Gettys, K.E.; Ye, Z.; Dai, M. Recent Advances in Piperazine Synthesis. Synthesis 2017, 49, 2589–2604. [Google Scholar] [CrossRef]
- Bugaenko, D.I. 1,4-Diazabicyclo[2.2.2]octane in the synthesis of piperazine derivatives. Chem. Heterocycl. Com. 2017, 53, 1277–1279. [Google Scholar] [CrossRef]
- Yavari, I.; Bayat, M.J.; Ghazanfarpour-Darjani, M. Synthesis of N-alkyl-N′-aryl-piperazines via copper-catalyzed C–N bond formation. Tetrahedron Lett. 2014, 55, 5595–5596. [Google Scholar] [CrossRef]
- Dong, H.-R.; Chen, Z.-B.; Li, R.-S.; Dong, H.-S.; Xie, Z.-X. Convenient and efficient synthesis of disubstituted piperazine derivatives by catalyst-free, atom-economical and tricomponent domino reactions. RSC Adv. 2015, 5, 10768–10772. [Google Scholar] [CrossRef]
- Wang, H.-J.; Earley, W.G.; Lewis, R.M.; Srivastava, R.R.; Zych, A.J.; Jenkins, D.M.; Fairfax, D.J. An efficient one-pot, two-step synthesis of 4-substituted 1-heteroarylpiperazines under microwave irradiation conditions. Tetrahedron Lett. 2007, 48, 3043–3046. [Google Scholar] [CrossRef]
- Wu, H.-Q.; Yang, K.l.; Chen, X.-Y.; Arulkumar, M.; Wang, N.; Chena, S.-H.; Wang, Z.-Y. A 3,4-dihalo-2(5H)-furanone initiated ringopening reaction of DABCO in the absence of a metal catalyst and additive and its application in a one-pot two-step reaction. Green Chem. 2019, 21, 3782–3788. [Google Scholar] [CrossRef]
- Wang, H.-J.; Wang, Y.; Csakai, A.J.; Earley, W.G.; Herr, R.J. Efficient N-Arylation/Dealkylation of Electron Deficient Heteroaryl Chlorides and Bicyclic Tertiary Amines under Microwave Irradiation. J. Comb. Chem. 2009, 11, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, Q.-S.; Li, Q.-Z.; Li, M.-P.; Shi, C.-Z.; Du, Z. Sulfonylation of 1,4-Diazabicyclo[2.2.2]octane: Charge-Transfer Complex Triggered C–N Bond Cleavage. ChemistryOpen 2019, 8, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Kolesinska, B.; Barszcz, K.; Kaminski, Z.J.; Drozdowska, D.; Wietrzyk, J.; Switalska, M. Synthesis and cytotoxicity studies of bifunctional hybrids of nitrogen mustards with potential enzymes inhibitors based on melamine framework. J. Enzyme Inhib. Med. 2011, 27, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Koyioni, M.; Manoli, M.; Koutentis, P.A. The Reaction of DABCO with 4-Chloro-5H-1,2,3-dithiazoles: Synthesis and Chemistry of 4-[N-(2-Chloroethyl)piperazin-1-yl]-5H-1,2,3-dithiazoles. J. Org. Chem. 2016, 81, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Manoli, M.; Koutentis, P.A. Reaction of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine with benzyltriethylammonium chloride. Tetrahedron Lett. 2018, 59, 3589–3593. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. A qualitative comparison of the reactivities of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine and 4,5-dichloro-1,2,3-dithiazolium chloride. Molecules 2015, 20, 14576–14594. [Google Scholar] [CrossRef] [PubMed]
- Koutentis, P.A.; Rees, C.W.; White, A.J.P.; Williams, D.J. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. Chem. Commun. 2000, 303–304. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Rees, C.W. Reaction of tetracyanoethylene with SCl2; new molecular rearrangements. J. Chem. Soc., Perkin Trans. 1 2000, 1089–1094. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.K. 4-Chloro-6-ethoxy-2-(methylthio)pyrimidine. Molbank 2017, 2017, M923. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.K. Synthesis of 2-cyanopyrimidines. Molbank 2019. submitted. [Google Scholar]
- Childress, S.J.; McKee, R.L. Chloroaminopyrimidines. J. Am. Chem. Soc. 1950, 729, 4271–4272. [Google Scholar] [CrossRef]
- Griffith, D.A. Purine Compounds and Uses Thereof as Cannabinoid Receptor Ligands. WO2004/37823, 6 May 2004. [Google Scholar]
- Schareina, T.; Zapf, A.; Cotté, A.; Müller, N.; Beller, M. A Bio-inspired Copper Catalyst System for Practical Catalytic Cyanation of Aryl Bromides. Synthesis 2008, 20, 3351–3355. [Google Scholar] [CrossRef]
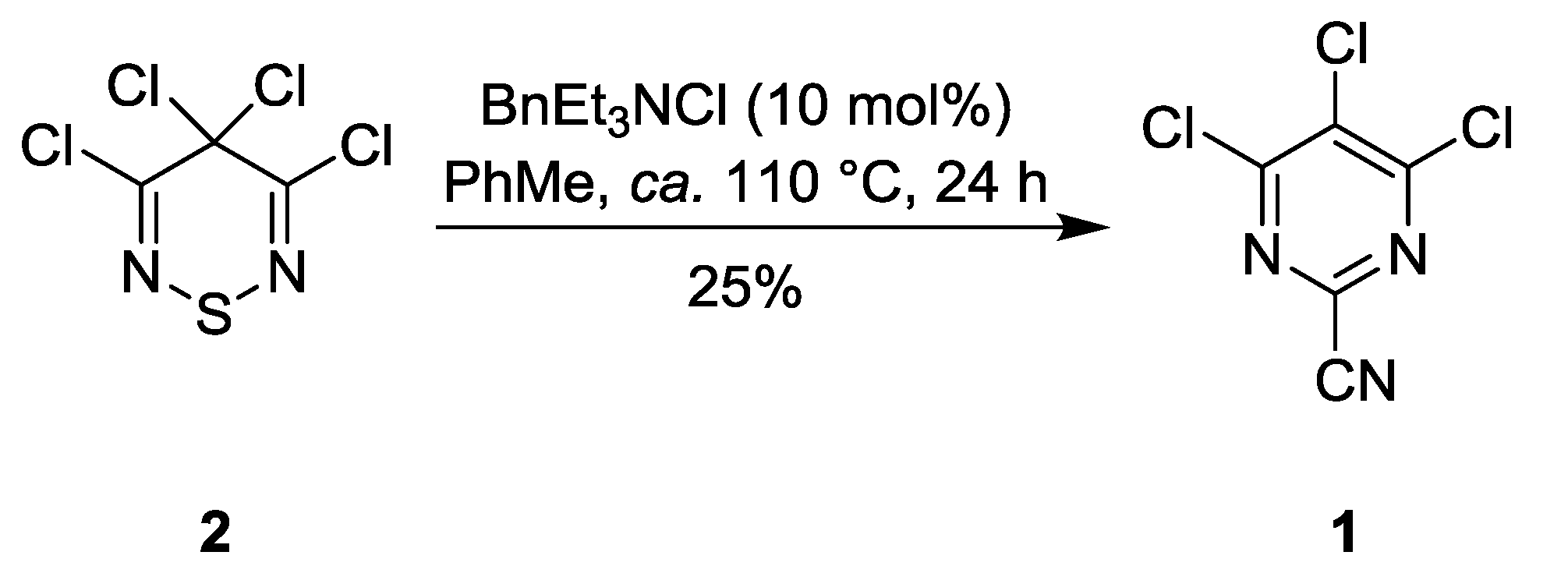
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Koutentis, P.A. Reactions of Polychlorinated Pyrimidines with DABCO. Molbank 2019, 2019, M1084. https://doi.org/10.3390/M1084
Kalogirou AS, Koutentis PA. Reactions of Polychlorinated Pyrimidines with DABCO. Molbank. 2019; 2019(4):M1084. https://doi.org/10.3390/M1084
Chicago/Turabian StyleKalogirou, Andreas S., and Panayiotis A. Koutentis. 2019. "Reactions of Polychlorinated Pyrimidines with DABCO" Molbank 2019, no. 4: M1084. https://doi.org/10.3390/M1084
APA StyleKalogirou, A. S., & Koutentis, P. A. (2019). Reactions of Polychlorinated Pyrimidines with DABCO. Molbank, 2019(4), M1084. https://doi.org/10.3390/M1084





