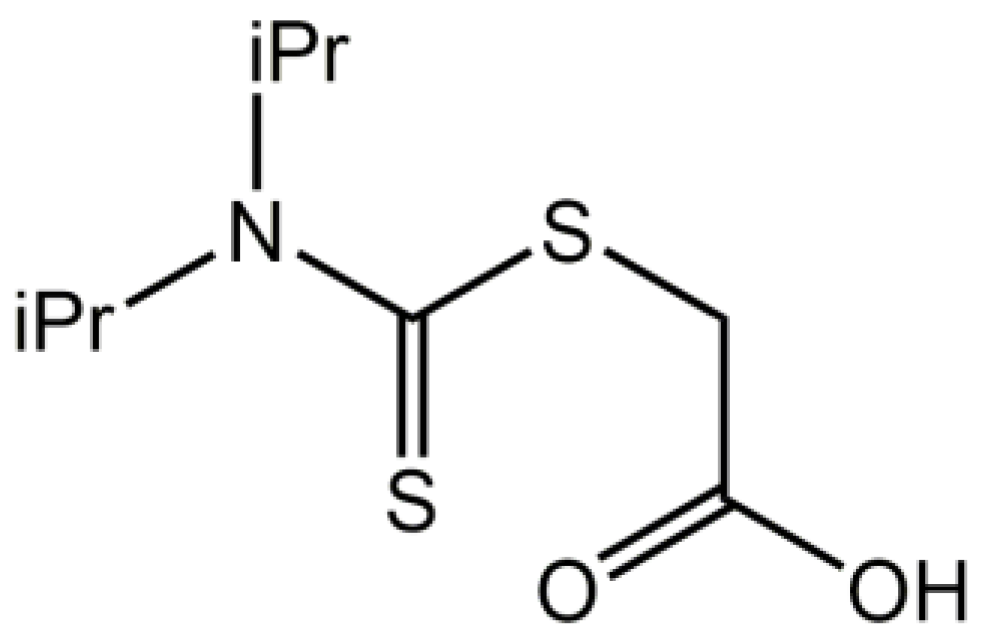
2-{[Bis(propan-2-yl)carbamothioyl]sulfanyl}acetic acid
Abstract
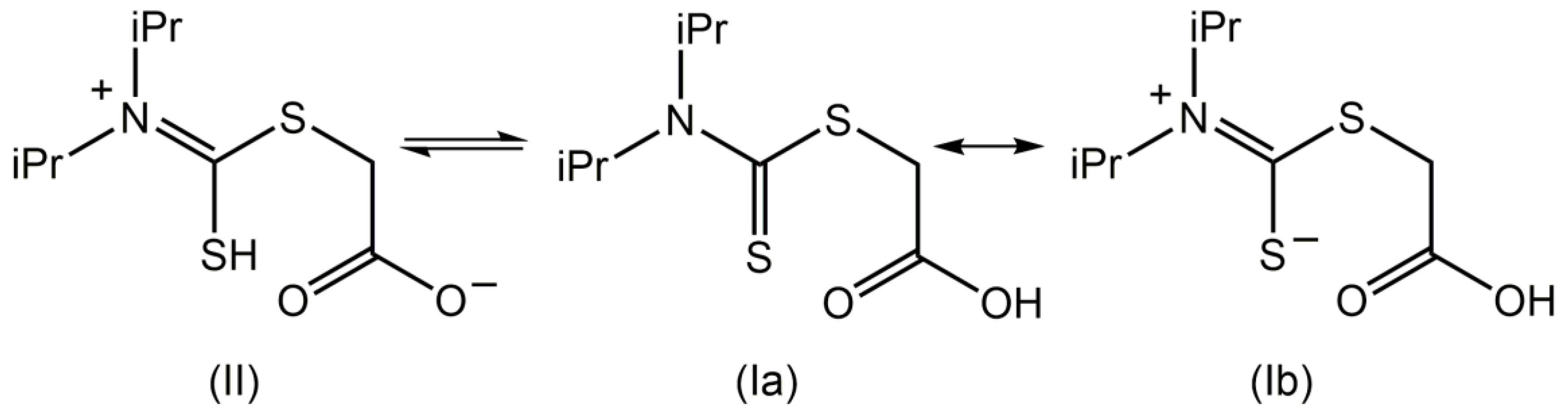
1. Introduction
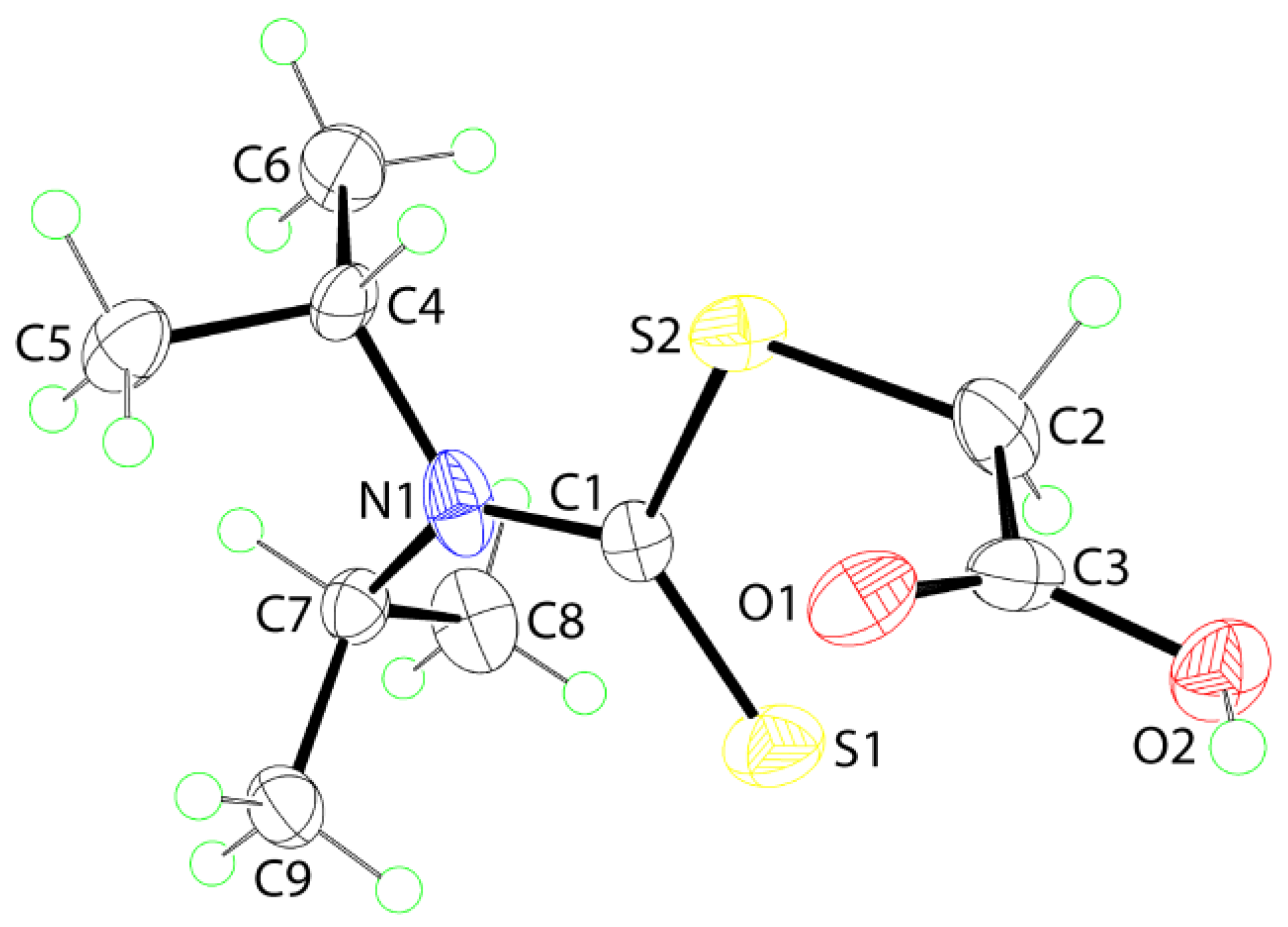
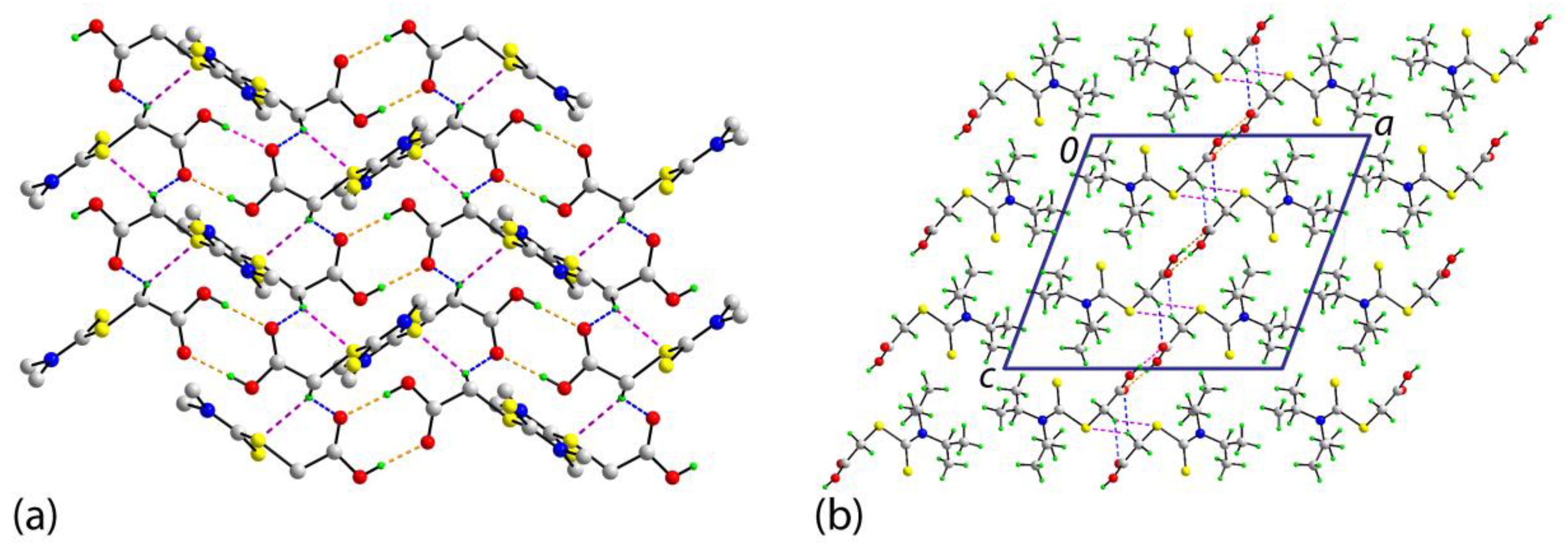
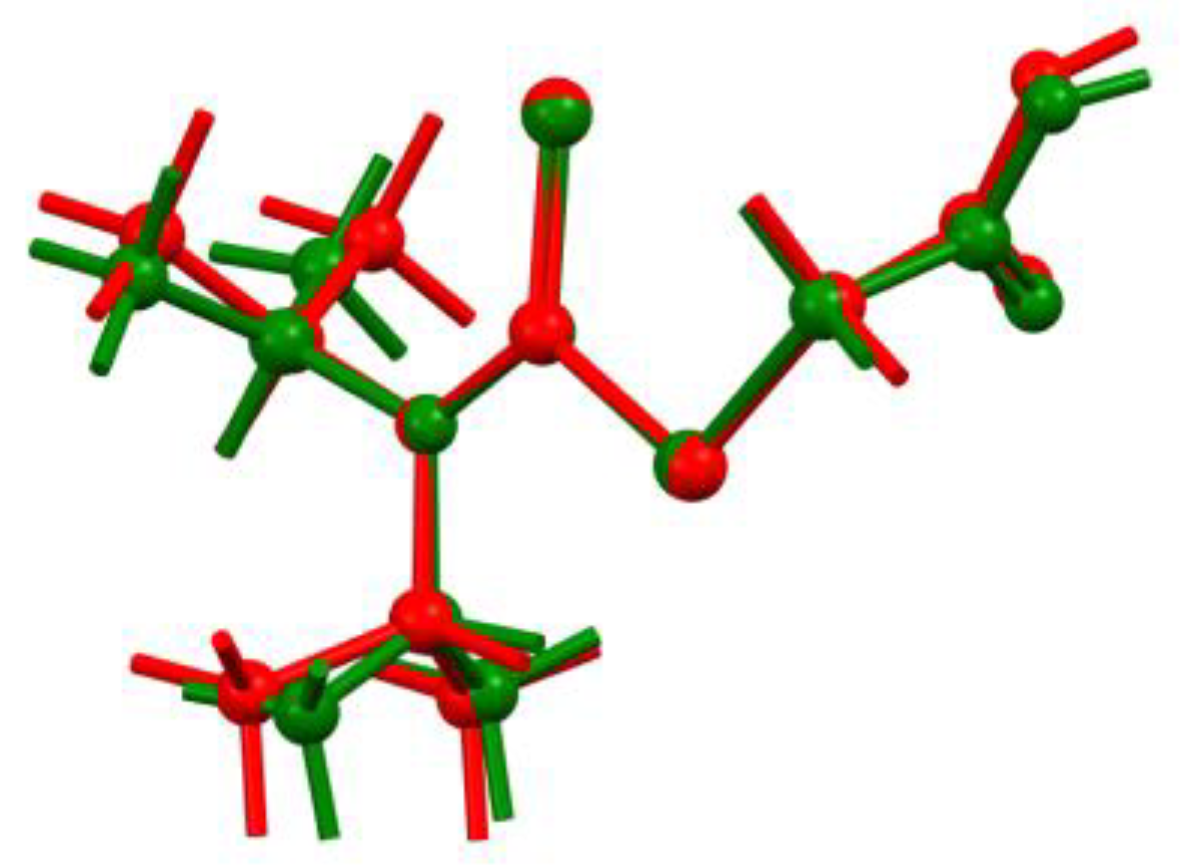
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization
3.3. Crystallography
3.4. Computational Studies
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Anasamy, T.; Thy, C.K.; Lo, K.M.; Chee, C.F.; Yeap, S.K.; Kamalidehghan, B.; Chung, L.Y. Tribenzyltin carboxylates as anticancer drug candidates: Effect on the cytotoxicity, motility and invasivenessof breast cancer cell lines. Eur. J. Med. Chem. 2017, 125, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lo, K.M.; Tiekink, E.R.T. Crystal structure of bis[(μ3-oxido)-(μ2-(N,N-diisopropylthiocarbamoylthio)acetato-κ2O,O’)-((N,N-diisopropylthiocarbamoylthio)acetato-κO)-bis(di-4-methylbenzyl-tin(IV))], C100H136N4O10S8Sn4. Z. Kristallogr. New Cryst. Struct. 2019, 234. in press. [Google Scholar] [CrossRef]
- Gielen, M.; Tiekink, E.R.T. 50Sn Tin compounds and their therapeutic potential. In Metallotherapeutic Drugs and Metal-Based Diagnostic Agents: The Use of Metals in Medicine; Gielen, M., Tiekink, E.R.T., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2005; Chapter 22; pp. 421–439. [Google Scholar]
- Tiekink, E.R.T. Tin dithiocarbamates: Applications and structures. Appl. Organomet. Chem. 2008, 22, 533–550. [Google Scholar] [CrossRef]
- Carter, G.A.; Garraway, J.L.; Spencer, D.M.; Wain, R.L. Fungicides. VI. The antifungal activity of certain dithiocarbamic and hydroxydithioformic acid derivatives. Ann. Appl. Biol. 1963, 51, 135–151. [Google Scholar] [CrossRef]
- Nardi, D.; Massarani, E.; Tajana, A.; Degen, L.; Magistretti, M.J. Antibacterial nitrofuran derivatives. I. 5-Nitro-2-furaldehyde semicarbazones and thiosemicarbazones. J. Med. Chem. 1967, 10, 530–533. [Google Scholar] [CrossRef]
- Jensen, K.A.; Anthoni, U.; Kagi, B.; Larsen, C.; Pedersen, C.T. Studies of thioacids and their derivatives. IX. Thiosemicarbazides. Acta Chem. Scand. 1968, 22, 1–50. [Google Scholar] [CrossRef]
- Wakamori, S.; Yoshida, Y.; Ishii, Y. Syntheses and herbicidal activities of dithiocarbamates. I. Benzyl esters of N-substituted dithiocarbamic acids and related compounds. Agric. Biol. Chem. 1969, 33, 1367–1376. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Bhattacharya, B.; Majhi, K.; Majee, P.; Sarkar, U.; Seikh, M.M. Benzthiazoline-2-thione (BTT) revisited: An experimental and theoretical endeavor to understand UV-spectra. Chem. Phys. Lett. 2017, 686, 88–96. [Google Scholar] [CrossRef]
- Ng, S.W.; Das, V.G.K. Organotin esters of dithiocarbamylacetic acids. J. Organomet. Chem. 1991, 409, 143–156. [Google Scholar] [CrossRef]
- Le-Thanh, H.; Vocelle, D. 1H NMR studies of proton transfer in Schiff base and carboxylic acid systems. Can. J. Chem. 1990, 68, 1909–1916. [Google Scholar] [CrossRef]
- Couperus, P.A.; Clague, A.D.H.; van Dongen, J.P.C.M. Carbon-13 chemical shifts of some model carboxylic acids and esters. Org. Mag. Res. 1978, 11, 1590–1597. [Google Scholar] [CrossRef]
- Takeda, Y.; Tanaka, T. Three rotational isomers of se-methyl N,N-di-isopropyldiselenocarbamate and the sulphur analogue found in low temperature 1H NMR spectra. Org. Mag. Reson. 1975, 7, 107–108. [Google Scholar] [CrossRef]
- Liljefors, T.; Sandström, J. The gear effect. VII†—Conformational analysis of methyl N,N-diisopropylcarbamate, its thiol and diseleno analogues. An experimental proof for a two-step conformational interconversion mechanism. Org. Mag. Reson. 1977, 9, 276–280. [Google Scholar] [CrossRef]
- Vogel, L.; Wonner, P.; Huber, S.M. Chalogen bonding: An overview. Angew. Chem. Int. Ed. 2019, 58, 1880–1891. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; Mckinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer v17.; The University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- Tan, S.L.; Jotani, M.M.; Tiekink, E.R.T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. E 2019, 75, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.M.; Ng, S.W. 2-(4-Morpholine-carbothio-ylsulfan-yl)-acetic acid. Acta Crystallogr. E 2010, 66, o1078. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlis PRO; Rigaku Corporation: Oxford, UK, 2017. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Brandenburg, K.; Putz, H. DIAMOND.; Crystal Impact GbR: Bonn, Germany, 2006. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16; Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView; Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian-basis sets for molecular calculation. 1. 2nd row atoms, Z=11-18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.M.; Azizan, A.H.S.; Lo, K.M.; Tan, S.L.; Tiekink, E.R.T. 2-{[Bis(propan-2-yl)carbamothioyl]sulfanyl}acetic acid. Molbank 2019, 2019, M1082. https://doi.org/10.3390/M1082
Lee SM, Azizan AHS, Lo KM, Tan SL, Tiekink ERT. 2-{[Bis(propan-2-yl)carbamothioyl]sulfanyl}acetic acid. Molbank. 2019; 2019(4):M1082. https://doi.org/10.3390/M1082
Chicago/Turabian StyleLee, See Mun, Ainnul Hamidah Syahadah Azizan, Kong Mun Lo, Sang Loon Tan, and Edward R. T. Tiekink. 2019. "2-{[Bis(propan-2-yl)carbamothioyl]sulfanyl}acetic acid" Molbank 2019, no. 4: M1082. https://doi.org/10.3390/M1082
APA StyleLee, S. M., Azizan, A. H. S., Lo, K. M., Tan, S. L., & Tiekink, E. R. T. (2019). 2-{[Bis(propan-2-yl)carbamothioyl]sulfanyl}acetic acid. Molbank, 2019(4), M1082. https://doi.org/10.3390/M1082







