Abstract
A simple approach to synthesize 4b,5,6,9-tetrahydro-7H-dibenzo[c,e]pyrrolo[1,2-a]azepin- 7-one has been developed, based on a three-step transformation of 2-(2-bromophenyl)cyclopropane-1,1-diester. The key stage in this method is an intramolecular cross-coupling of 1-(2-bromobenzyl)-5-(2-bromophenyl)pyrrolidin-2-one under continuous flow conditions in an H-Сube-Pro using commercially available supported Pd catalysts.
1. Introduction
Dibenz[c,e]azepine derivatives attract the attention of synthetic and medicinal chemists due to their potential use in human and veterinary medicine. This scaffold is present in the vasodilator azapetine [1], as well as in a broad variety of compounds exhibiting anticancer [2,3,4,5,6,7,8], anti-inflammatory [9], hypolipidemic [10,11], and other types of biological activities [6,12,13,14,15,16,17,18]. In addition, dibenz[c,e]azepines have been used as chiral organocatalysts in enantioselective synthesis [19,20]. Owing to these applications, the development of new efficient methodologies for the preparation of dibenz[c,e]azepines is of great interest [21]. One of the methods for the synthesis of this scaffold is based on a palladium-catalyzed intramolecular cross-coupling reaction between two aromatic rings in N,N-dibenzylamine derivatives [22,23,24,25,26,27,28,29].
As a part of our efforts towards the synthesis of N-containing heterocycles of potential pharmacological value based on the donor-acceptor cyclopropane transformations [30,31,32,33,34,35], we have recently described a convenient synthesis of polyoxygenated tetrahydrodibenzo[c,e]pyrrolo[1,2-a]azepines [35]. This protocol included: (1) Cyclopropane ring opening with an azide ion, followed by in situ Krapcho dealkoxycarbonylation; (2) the phosphine-mediated reaction of the obtained 4-aryl-4-azidobutyrate with aromatic aldehydes and subsequent in situ reductive cyclization of the formed imine, yielding 5-aryl-1-benzylpyrrolidin-2-ones; (3) their oxidative cyclization to dibenzo[c,e]pyrrolo[1,2-a]azepine derivatives. The last step, however, can be carried out only for substrates containing two electron-rich aromatic rings, and, therefore, has limited application.
We envisioned that the related dibenzo[c,e]pyrrolo[1,2-a]azepines without electron-donating groups in the aryl moieties could be synthesized by an intramolecular cross-coupling reaction of pyrrolidones bearing halogen(s) in ortho-position(s) of the aromatic groups. Herein, we report the synthesis of 4b,5,6,9-tetrahydro-7H-dibenzo[c,e]pyrrolo[1,2-a]azepin-7-one (1) from donor-acceptor cyclopropane in only three steps, the key one is an intramolecular cross-coupling under continuous flow conditions.
2. Results and Discussion
Title compound 1 was synthesized from readily available dimethyl 2-(2-bromophenyl)-cyclopropane-1,1-dicarboxylate 2. At the first step, cyclopropane 2 was converted into azide 3 by the Kerr’s procedure [36], including partial hydrolysis of 2 followed by cyclopropane ring opening with sodium azide, accompanied by decarboxylation (Scheme 1).
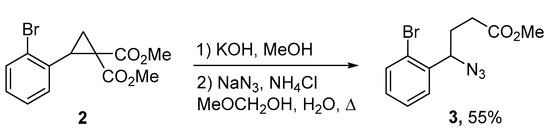
Scheme 1.
Synthesis of azide 3.
Afterward, we transformed 4-aryl-4-azidobutyrate 3 into pyrrolidone 4 using a simple synthetic methodology developed earlier [30]. Namely, azide 3 reacted with 2-bromobenzaldehyde in the presence of triphenylphosphine producing the corresponding imine via a sequence of the Staudinger and aza-Wittig reaction. The treatment of the obtained imine in a one-pot manner with sodium cyanoborohydride induced its reductive cyclization to 5-aryl-1-benzylpyrrolidin-2-one 4. We found that the use of polymer-bound triphenylphosphine instead of the conventional reagent both increased the yield and saved the trouble of imine separation from triphenylphosphine. These advantages outweigh the increase of the reaction time required for the full conversion of azide 3. The formed imine was treated with methanolic NaBH3CN in the presence of acetic acid using a “telescoped” procedure [37]. The resulting amine underwent immediate cyclization affording the desired pyrrolidin-2-one 4 in a reasonable yield (Scheme 2).
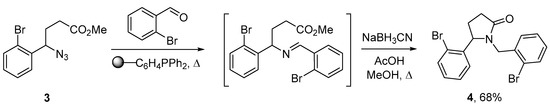
Scheme 2.
Synthesis of pyrrolidin-2-one 4.
The synthesis of tricyclic compound 1 was accomplished by the palladium-catalyzed coupling of two aryl halide functionalities in compound 4. This cross-coupling proceeds with low-to-moderate yield under continuous flow conditions [38] using commercial cartridges, with Pd catalysts FibreCat 1001, FibreCat 1007, FibreCat 1031, FibreCat 1032 (Scheme 3). The best results were achieved with cartridge FibreCat 1007, containing a complex of palladium(II) acetate with polymer-supported phenyldicyclohexylphosphine as a catalyst.
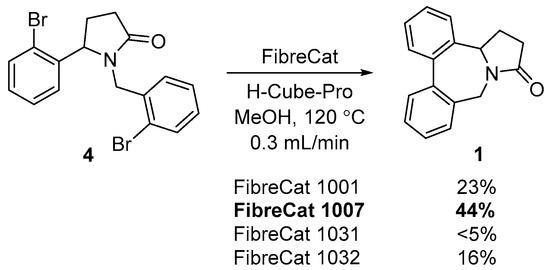
Scheme 3.
Synthesis of tetrahydrodibenzo[c,e]pyrrolo[1,2-a]azepine 1.
In summary, the facile three-step sequence, including a cyclopropane ring opening with an azide ion, accompanied by decarboxylation, a Staudinger/aza-Wittig domino reaction combined with the reductive cyclization, and an intramolecular cross-coupling reaction of 1-(2-bromobenzyl)-5-(2-bromophenyl)pyrrolidin-2-one, provides a concise route to 4b,5,6,9-tetrahydro-7H-dibenzo[c,e]pyrrolo[1,2-a]azepin-7-one.
3. Materials and Methods
NMR spectra were acquired on Bruker Avance 500 spectrometer at room temperature; the chemical shifts δ were measured in ppm with respect to the solvent (1Н: CDCl3, δ = 7.27 ppm; 13C: CDCl3, δ = 77.0). The splitting patterns are designated as s, singlet; d, doublet; m, multiplet; dd, double doublet; br., broad. The coupling constants (J) were in Hertz. The 1H-NMR, 13C-NMR for the synthesized compounds, as well as 2D (HSQC and HMBC) NMR spectra for the selected compounds, are available in the Supplementary Materials. Infrared spectra were recorded on the Infralum FT-801 spectrometer. High resolution and accurate mass measurements were carried out using a micrOTOF-QTM ESI-TOF (Electro Spray Ionization/Time of Flight, Bruker, Billerica, MA, USA) and LTQ Orbitrap mass spectrometer (Thermo Fischer Scientific, Waltham, MA, USA). Elemental analyses were performed with an EA-1108 CHNS elemental analyzer instrument (Fisons, Ipswich, UK). The microwave reaction was performed in a Monowave 300–Anton Paar microwave reactor (Anton Paar Gmbh, Graz, Austria) in sealed reaction vessels. The temperature was monitored with the installed IR detector. The melting points (m.p.) were determined using a 9100 capillary melting point apparatus (Electrothermal, Stone, UK). Analytical thin layer chromatography (TLC) was carried out with silica gel plates (silica gel 60, F254, supported on aluminum); the revelation was done by UV lamp (365 nm). Column chromatography was performed on silica gel 60 (230–400 mesh, Merck, Darmstadt, Germany). All reactions were carried out using freshly distilled and dry solvents. Dimethyl 2-(2-bromophenyl)cyclopropane-1,1-dicarboxylate 2 was synthesized by the published procedure [30]. Commercial reagents employed in the synthesis were analytical grade, obtained from Aldrich (St. Louis, MI, USA) or Alfa Aesar (Ward Hill, MO, USA). The flow reactions were carried out in a ThalesNano H-Cube Pro featuring an HPLC pump to deliver the substrates at flow rates of 0.3 mL/min, the reactor box for CatCarts (FibreCat 1001, FibreCat 1007, FibreCat 1032) employed in this study were purchased in ABCR Gmbh (Karlruhe, Germany).
3.1. Methyl 4-Azido-4-(2-bromophenyl)butanoate (3)
A solution of cyclopropane 2 (225 mg, 0.72 mmol) and KOH (60 mg, 1.07 mmol) in a mixture of methanol (2.9 mL) and water (3.6 mL) was refluxed for 6.5 h. Then, the reaction mixture was quenched with water (2.9 mL) and concentrated to half volume under reduced pressure. The residue was extracted with ethyl acetate (10 mL); aqueous HCl was added to the aqueous layer until pH 1. The obtained suspension was extracted with diethyl ether (3 × 10 mL). The combined organic fractions were dried with Na2SO4 and concentrated, affording 165 mg (76%) of a crude cyclopropane hemimalonate as a colorless oil. This oil was dissolved in the mixture of 2-methoxyethanol (5 mL) and water (0.5 mL), NaN3 (43 mg, 0.66 mmol) and NH4OAc (42 mg, 0.54 mmol) were added. The reaction mixture was heated under reflux for 2 h, quenched with water and extracted with diethyl ether (3 × 10 mL). The combined organic fractions were dried with Na2SO4 and concentrated. Purification by flash chromatography gave 117 mg (72%) of the desired product 3 as a colorless oil, Rf = 0.54 (ethyl acetate:petroleum ether, 1:10).
1H-NMR (CDCl3, 500 MHz) δ = 2.03–2.16 (m, 2H, CH2), 2.43–2.49 (m, 2H, CH2), 3.69 (s, 3H, CH3O), 5.10 (dd, 3J = 8.4 Hz, 3J = 5.4 Hz, 1H, CH), 7.20 (ddd, 3J = 8.0 Hz, 3J = 7.5 Hz, 4J = 1.7 Hz, 1H, Ar), 7.38 (ddd, 3J = 7.8 Hz, 3J = 7.5 Hz, 4J = 1.2 Hz, 1H, Ar), 7.45 (dd, 3J = 7.8 Hz, 4J = 1.7 Hz, 1H, Ar), 7.60 (dd, 3J = 8.0 Hz, 4J = 1.2 Hz, 1H, Ar). 13C-NMR (CDCl3, 125 MHz) δ = 30.5 (CH2), 30.7 (CH2), 51.8 (CH3O), 63.9 (CH), 123.3 (C, Ar), 128.0 (CH, Ar), 128.2 (CH, Ar), 129.8 (CH, Ar), 133.3 (CH, Ar), 138.6 (C, Ar), 173.1 (CO). IR (cm−1) 2995, 2952, 2101, 1739, 1590, 1568, 1469, 1437, 1328, 1251, 1198, 1169, 1120, 1024. HRMS ESI/Q-TOF: m/z = 320.0003 [M + H]+ (320.0005 calculated for C11H12BrN3NaO2). Anal. calculated for С11Н12BrN3O2: C, 44.32; H, 4.06; N, 14.09. Found: C, 44.31; H, 3.89; N, 14.08.
3.2. 1-(2-Bromobenzyl)-5-(2-bromophenyl)pyrrolidin-2-one (4)
A suspension of azide 3 (1.18 g., 3.96 mmol) and polymer-bound triphenylphosphine (Sigma-Aldrich 93093; 1.34 g, ca. 3 mmol/g, 4.02 mmol) in 1,2-dichloroethane (8 mL) was stirred at room temperature for 45 min. After that, 2-bromobenzaldehyde (2.22 g, 12 mmol) was added, the resulting mixture was heated in a microwave reactor at 90 °C for 15 h. The resin was filtered off and washed with dichloroethane; the combined filtrates were concentrated in vacuo. The residue was dissolved in methanol (9.7 mL) and treated with sodium cyanoborohydride (1.51 g, 24 mmol) and glacial acetic acid (2.5 mL, 43.7 mmol). The reaction mixture was refluxed for 4 h, quenched with concentrated aqueous NaHCO3 and extracted with ethyl acetate (3 × 10 mL). The combined organic extracts were washed with brine, dried with anhydrous Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography on a silica gel to afford the desired product. The yield was 1.10 g (68%) as a yellowish solid; mp 112–113 °C; Rf = 0.50 (ethyl acetate:petroleum ether, 1:1). 1Н-NMR (CDCl3, 500 MHz) δ = 1.81–1.86 (m, 1H, C(4)H2), 2.44–2.65 (m, 3H, C(4)H2, C(3)H2), 3.93 (d, 2J = 15.2 Hz, 1H, CH2N), 4.91 (br. s, 1H, C(5)H), 5.13 (d, 2J = 15.2 Hz, 1H, CH2N), 7.11–7.36 (m, 6H, Ar), 7.51 (d, 3J = 8.1 Hz, 1H, Ar), 7.56 (d, 3J = 8.1 Hz, 1H, Ar). 13С-NMR (CDCl3, 125 MHz) δ = 26.8 (CH2), 29.2 (CH2), 44.9 (CH2N), 60.4 (CH), 123.0 (C, Ar), 123.9 (CH, Ar), 126.3 (C, Ar), 127.6 (CH, Ar), 127.8 (CH, Ar), 129.1 (CH, Ar), 129.2 (CH, Ar), 130.4 (CH, Ar), 132.9 (CH, Ar), 133.4 (C, Ar), 135.1 (CH, Ar), 139.4 (C, Ar), 175.8 (C=O). IR (сm−1) 3361, 3086, 3057, 2989, 2969, 2934, 2899, 2852, 2695, 1647, 1587, 1567, 1463, 1447, 1436, 1426, 1410, 1358, 1349, 1323, 1292, 1265, 1239, 1216, 1207, 1115, 1099, 1066, 1045, 1038, 1025. HRMS ESI/Q-TOF: m/z = 386.1605 [M + H]+ (385.1598 calculated for C17H15Br2NO).
Continuous Flow Procedure
In a typical experiment for the reductive coupling, a solution containing pyrrolidin-2-one 4 (1 equivalent) and K2CO3 (3 equivalents) in ultra-pure methanol (0.001 M) was pumped through a catalyst cartridge (FibreCat 1001, FibreCat 1007, FibreCat 1032) heated up to 120 °C at a flow rate of 0.3 mL/min (optimized flow conditions) in the H-Cube Pro A. The solvent was removed under reduced pressure, the product was purified by silica gel flash column chromatography (eluent–petroleum ether:ethylacetate, 10:1 to 1:1).
4b,5,6,9-Tetrahydro-7H-dibenzo[c,e]pyrrolo[1,2-a]azepin-7-one (1). This compund was obtained in a 41% yield (102 mg) using the FiberCat 1007, as a yellow thick oil; Rf = 0.54 (ethyl acetate:petroleum ether; 1:3). 1Н-NMR (CDCl3, 500 MHz) δ = 2.23–2.29 (m, 1H, CH2), 2.51–2.61 (m, 3H, CH2), 3.67 (d, 2J = 13.3 Hz, 1H, CH2N), 4.41 (dd, 3J = 7.6 Hz, 3J = 5.7 Hz, 1H, CH), 4.90 (d, 2J = 13.3 Hz, 1H, CH2N), 7.39–7.54 (m, 8H, Ar). 13С-NMR (CDCl3, 125 MHz) δ = 21.9 (CH2), 31.7 (CH2), 44.2 (CH2N), 57.5 (CH), 124.4 (CH, Ar), 128.3 (CH, Ar), 128.5 (CH, Ar), 128.6 (CH, Ar), 128.7 (CH, Ar), 129.0 (CH, Ar), 129.3 (CH, Ar), 129.5 (CH, Ar), 133.1 (C, Ar), 134.1 (C, Ar), 140.5 (C, Ar), 140.6 (C, Ar), 172.4 (C=O). HRMS ESI-TOF: m/z = 250.1226 [M + H]+ (250.1229 calculated for C17H16NO).
Supplementary Materials
The following are available online, Figure S1: 1H-NMR spectrum of 3; Figure S2: 13C-NMR spectrum of 3; Figure S3: HSQC 1H-13C spectrum of 3; Figure S4: HMBC 1H-13C spectrum of 3; Figure S5: 1H-NMR spectrum of 4; Figure S6: 13C-NMR spectrum of 4; Figure S7: COSY 1H-1H spectrum of 4; Figure S8: HSQC 1H-13C spectrum of 4; Figure S9: HMBC 1H-13C spectrum of 4; Figure S10: 1H-NMR spectrum of 1; Figure S11: 13C-NMR spectrum of 1; Figure S12–S15: GC/MS data of mass spectrometry.
Author Contributions
Conceptualization, O.A.I. and I.V.T.; methodology O.A.I.; software, I.Y.B., O.A.I., I.V.T.; validation, O.A.I. and I.V.T.; formal analysis, O.A.I.; investigation, M.A.B., I.Y.B., S.G.K., A.O.C.; resources, O.A.I., A.O.C. and I.Y.B.; data curation, O.A.I. and I.V.T.; writing—original draft preparation, O.A.I. and I.V.T.; writing—review and editing, O.A.I. and I.V.T.; supervision, O.A.I. and I.V.T.; project administration, A.O.C.; funding acquisition, A.O.C.
Funding
We thank the Russian Science Foundation (grant 17-73-10404) for the financial support of this work.
Acknowledgments
The authors would like to acknowledge Thermo Fisher Scientific Inc., MS Analytica (Moscow, Russia) for providing the equipment for HRMS recording.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Stallworth, J.M.; Jeffords, J.V. Clinical Effects of Azapetine (Ilidar) on Peripheral Arterial Disease. JAMA 1956, 161, 840–843. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, W.; Jiang, Y.; Chen, J.; Zhang, Y.; Gu, X. Assessment of a bifendate derivative bearing a 6,7-dihydro-dibenzo[c,e]azepine scaffold as a potential anti-metastatic agent. Med. Chem. Commun. 2018, 9, 1826–1830. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Jiang, Y.; Qu, Y.; Chen, J.; Feng, D.; Li, C.; Yin, X. Synthesis and biological evaluation of bifendate derivatives bearing 6,7-dihydro-dibenzo[c,e]azepine scaffold as potential P-glucoprotein and tumor metastasis inhibitors. Eur. J. Med. Chem. 2018, 145, 379–388. [Google Scholar] [CrossRef]
- Gu, X.; Tang, X.; Zhao, Q.; Peng, H.; Peng, S.; Zhang, Y. Discovery of alkoxy biphenyl derivatives bearing dibenzo[c,e]azepine scaffold as potential dual inhibitors of P-glycoprotein and breast cancer resistance protein. Bioorg. Med. Chem. Lett. 2014, 24, 3419–3421. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Ren, Z.; Tang, X.; Peng, H.; Zhao, Q.; Lai, Y.; Peng, S.; Zhang, Y. Synthesis and biological evaluation of novel bifendate derivatives bearing 6,7-dihydro-dibenzo[c,e]azepine scaffold as potent P-glycoprotein inhibitors. Eur. J. Med. Chem. 2012, 51, 137–144. [Google Scholar] [CrossRef]
- Mehta, V.P.; Modha, S.G.; Ruijter, E.; Van Hecke, K.; Van Meervelt, L.; Pannecouque, C.; Balzarini, J.; Orru, R.V.A.; Van der Eycken, E. A Microwave-Assisted Diastereoselective Multicomponent Reaction To Access Dibenzo[c,e]azepinones: Synthesis and Biological Evaluation. J. Org. Chem. 2011, 76, 2828–2839. [Google Scholar] [CrossRef]
- Edwards, D.J.; Hadfield, J.A.; Wallace, T.W.; Ducki, S. Tubulin-binding dibenz[c,e]oxepines as colchicinol analogues for targeting tumour vasculature. Org. Biomol. Chem. 2011, 9, 219–231. [Google Scholar] [CrossRef]
- Hall, I.H.; Barnes, B.J.; Ward, E.S.; Wheaton, J.R.; Shaffer, K.A.; Cho, S.E.; Warren, A.E. Targeting of Human Tmolt4 Leukemic Type II IMP Dehydrogenase by Cyclic Imide Related Derivatives. Arch. Pharm. Pharm. Med. Chem. 2001, 334, 229–234. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.M.; ElTahir, K.E.H.; Asiri, Y.A. Synthesis, anti-inflammatory activity and COX-1/COX-2 inhibition of novel substituted cyclic imides. Part 1: Molecular docking study. Eur. J. Med. Chem. 2011, 46, 1648–1655. [Google Scholar] [CrossRef]
- Hall, I.H.; Wong, O.T.; Reynolds, D.J.; Simlot, R. Comparison between 6,7-Dihydro-5H-Dibenz(c,e)-azepine and Lovastatin as Hypolipidemic Agents in Rats. J. Pharm. Sci. 1993, 82, 565–570. [Google Scholar] [CrossRef]
- Hall, I.H.; Murthy, A.R.K.; Wyrick, S.D. Hypolipidemic Activity of 6-Substituted 6,7-Dihydro-5H-Dibenz(c,e)azepine and the Effects of 6,7-Dihydro-5H-Dibenz(c,e)azepine on Lipid Metabolism of Rodents. J. Pharm. Sci. 1986, 75, 622–626. [Google Scholar] [CrossRef]
- De Lera Ruiz, M.; Zheng, J.; Berlin, B.Y.; McCormick, K.D.; Aslanian, R.G.; West, R.; Hwa, J.; Lachowicz, J.; van Heek, M. Bicyclic and tricyclic heterocycle derivatives as histamine H3 receptor antagonists for the treatment of obesity. Bioorg. Med. Chem. Lett. 2013, 23, 6004–6009. [Google Scholar] [CrossRef]
- Tang, X.; Gu, X.; Ren, Z.; Ma, Y.; Lai, Y.; Peng, H.; Peng, S.; Zhang, Y. Synthesis and evaluation of substituted dibenzo[c,e]azepin-5-ones as P-glycoprotein-mediated multidrug resistance reversal agents. Bioorg. Med. Chem. Lett. 2012, 22, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Hadden, M.; Goodman, A.; Guo, C.; Guzzo, P.R.; Henderson, A.J.; Pattamana, K.; Ruenz, M.; Sargent, B.J.; Swenson, B.; Yet, L.; et al. Synthesis and SAR of heterocyclic carboxylic acid isosteres based on 2-biarylethylimidazole as bombesin receptor subtype-3 (BRS-3) agonists for the treatment of obesity. Bioorg. Med. Chem. Lett. 2010, 20, 2912–2915. [Google Scholar] [CrossRef]
- Schmidt, M.; Teitge, M.; Castillo, M.E.; Brandt, T.; Dobner, B.; Langner, A. Synthesis and Biochemical Characterization of New Phenothiazines and Related Drugs as MDR Reversal Agents. Arch. Pharm. Chem. Life Sci. 2008, 341, 624–638. [Google Scholar] [CrossRef]
- Büttner, F.; Bergemann, S.; Guenard, D.; Gust, R.; Seitz, G.; Thoret, S. Two novel series of allocolchicinoids with modified seven membered B-rings: Design, synthesis, inhibition of tubulin assembly and cytotoxicity. Bioorg. Med. Chem. 2005, 13, 3497–3511. [Google Scholar] [CrossRef]
- Reynolds, D.J.; Wong, O.T.; Simlot, R.; Chang, J.J.; Hall, I.H. Acute Toxic and Teratogenic Effects of Cyclic Imides in Rodent. Arch. Pharm. 1994, 327, 237–245. [Google Scholar] [CrossRef]
- Gorshkova, V.K.; Saratikov, A.S.; Tignibidina, L.G. Investigation of the Anticonvulsant and Antihypoxic Activity of New Dibenzazepine Derivatives. Pharm. Chem. J. 1994, 28, 158–162. [Google Scholar] [CrossRef]
- Wang, Y.G.; Ueda, M.; Wang, X.; Han, Z.; Maruoka, K. Convenient preparation of chiral phase-transfer catalysts with conformationally fixed biphenyl core for catalytic asymmetric synthesis of α-alkyl- and α,α-dialkyl-α-amino acids: Application to the short asymmetric synthesis of BIRT-377. Tetrahedron 2007, 63, 6042–6050. [Google Scholar] [CrossRef]
- Kan, S.B.J.; Maruyama, H.; Akakura, M.; Kano, T.; Maruoka, K. Catalyst-Controlled, Enantioselective and Diastereodivergent Conjugate Addition of Aldehydes to Electron-Deficient Olefins. Angew. Chem. Int. Ed. 2017, 56, 9487–9491. [Google Scholar] [CrossRef] [PubMed]
- Boichenko, M.A.; Chagarovskiy, A.O. Recent achievements in the synthesis of dibenz[c,e]azepines. Chem. Heterocycl. Comp. 2017, 53, 1280–1282. [Google Scholar] [CrossRef]
- Balgobin, S.M.C.; Brookes, D.J.; Jiang, J.; Pritchard, R.G.; Wallace, T.W. Axial stereocontrol in tropos dibenz[c,e]azepines: The individual and cooperative effects of alkyl substituents. Org. Biomol. Chem. 2017, 15, 10184–10199. [Google Scholar] [CrossRef]
- Page, P.C.B.; Pearce, C.A.; Chan, Y.; Parker, P.; Buckley, B.R.; Rassias, G.A.; Elsegood, M.R.J. Atropo- and Diastereoselective Construction of Tetracyclic Biphenylazepinium Salts Derived from Aminoalcohols: Use as Catalysts in Enantioselective Asymmetric Epoxidation. J. Org. Chem. 2015, 80, 8036–8045. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, L.; Li, Y.; Soule, J.F.; Doucet, H. Intermolecular versus Intramolecular Palladium-Catalyzed Direct Arylations between 1-(2-Bromoimidazoles) and Aryl Bromides. Adv. Synth. Catal. 2015, 357, 2869–2882. [Google Scholar] [CrossRef]
- Cheetham, C.A.; Massey, R.S.; Pira, S.L.; Pritchard, R.G.; Wallace, T.W. Atroposelective formation of dibenz[c,e]azepines via intramolecular direct arylation with centre-axis chirality transfer. Org. Biomol. Chem. 2011, 9, 1831–1838. [Google Scholar] [CrossRef]
- Yu, M.; Tang, R.Y.; Li, J.H. Synthesis of 6,7-dihydro-5H-dibenzo[c,e]azepines and biaryls by palladium-catalyzed Ullmann reaction. Tetrahedron 2009, 65, 3409–3416. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Mondal, S.; De, N. Synthesis of Polycyclic Sultams by Palladium-Catalyzed Intramolecular Cyclization. Synthesis 2009, 3127–3135. [Google Scholar] [CrossRef]
- Saudan, L.A.; Bernardinelli, G.; Kündig, E.P. Diastereoselective synthesis of (5R,7R)- and (5R,7S)-5,7-Dimethyl-6,7-dihydro-5H-dibenz[c,e]azepines. Synlett 2000, 483–486. [Google Scholar] [CrossRef]
- Hennings, D.D.; Iwama, T.; Rawal, V.H. Palladium-Catalyzed (Ullmann-Type) Homocoupling of Aryl Halides: A Convenient and General Synthesis of Symmetrical Biaryls via Inter- and Intramolecular Coupling Reactions. Org. Lett. 1999, 1, 1205–1208. [Google Scholar] [CrossRef]
- Ivanov, K.L.; Villemson, E.V.; Budynina, E.M.; Ivanova, O.A.; Trushkov, I.V.; Melnikov, M.Y. Ring Opening of Donor-Acceptor Cyclopropanes with the Azide Ion: A Tool for Construction of N-Heterocycles. Chem. Eur. J. 2015, 21, 4975–4987. [Google Scholar] [CrossRef]
- Budynina, E.M.; Ivanov, K.L.; Chagarovskiy, A.O.; Rybakov, V.B.; Trushkov, I.V.; Melnikov, M. Ya. From Umpolung to Alternation: Modified Reactivity of Donor-Acceptor Cyclopropanes Towards Nucleophiles in Reaction with Nitroalkanes. Chem. Eur. J. 2016, 22, 3692–3696. [Google Scholar] [CrossRef]
- Pavlova, A.S.; Ivanova, O.A.; Chagarovskiy, A.O.; Stebunov, N.S.; Orlov, N.V.; Shumsky, A.N.; Budynina, E.M.; Rybakov, V.B.; Trushkov, I.V. Domino Staudinger/aza-Wittig/Mannich Reaction: An Approach to Diversity of Di- and Tetrahydropyrrole Scaffolds. Chem. Eur. J. 2016, 22, 17967–17971. [Google Scholar] [CrossRef] [PubMed]
- Villemson, E.V.; Budynina, E.M.; Ivanova, O.A.; Skvortsov, D.A.; Trushkov, I.V.; Melnikov, M. Ya. Concise Approach to pyrrolizino[1,2-b]indoles from indole-derived donor-acceptor cyclopropanes. RSC Adv. 2016, 6, 62014–62018. [Google Scholar] [CrossRef]
- Chagarovskiy, A.O.; Ivanova, O.A.; Shumsky, A.N.; Trushkov, I.V. Synthesis of hexahydropyridazin-3-ones by reaction between donor-acceptor cyclopropanes and phenylhydrazine. Chem. Heterocyclic Comp. 2017, 53, 1220–1227. [Google Scholar] [CrossRef]
- Boichenko, M.A.; Ivanova, O.A.; Andreev, I.A.; Chagarovskiy, A.O.; Levina, I.I.; Rybakov, V.B.; Skvortsov, D.A.; Trushkov, I.V. Convenient approach to polyoxygenated dibenzo[c,e]pyrrolo[1,2-a]azepines from donor-acceptor cyclopropanes. Org. Chem. Front. 2018, 5, 2829–2834. [Google Scholar] [CrossRef]
- Emmett, M.R.; Grover, H.K.; Kerr, M.A. Tandem Ring-Opening Decarboxylation of Cyclopropane Hemimalonates with Sodium Azide: A Short Route to γ-Aminobutyric Acid Esters. J. Org. Chem. 2012, 77, 6634–6637. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef]
- Feiz, A.; Bazgir, A.; Balu, A.M.; Luque, R. Continuous flow room temperature reductive aqueous homo-coupling of aryl halides using supported Pd catalysts. Sci. Rep. 2016, 6, 32719. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).