Abstract
Exploring the pharmacologically important pyrazolo[1,5-a][1,3,5]triazin-7(6H)-one scaffold for the construction of new bioactive compounds, we developed a synthesis of 4-phenethylthio-2-phenylpyrazolo[1,5-a][1,3,5]triazin-7(6H)-one (4) via S-alkylation of 2-phenyl-4-thioxopyrazolo[1,5-a][1,3,5]triazine-7(6H)-one (3), prepared by the double ring closure of pyrazole and triazine rings upon the treatment of 1-cyanoacetyl-4-benzoylthiosemicarbazide (2) with alkali. The antiproliferative activity of 4 against human lung cancer (A549) and human breast cancer (MDA-MB231) cell lines was investigated. Compound 4 was found to be more active against lung cancer cells than breast cancer cells.
1. Introduction
The pyrazolo[1,5-a][1,3,5]triazine ring is an isostere of the purine heterocyclic system, which is the most functional N-heterocycle in nature. Among purine isosteres, pyrazolo[1,5-a][1,3,5]triazines occupy chemical space of compounds with diverse biological activities. Considering 1,3,5-triazine-based purine isosteres, pyrazolo[1,5-a][1,3,5]triazine is well recognized as the most promising scaffold for the construction of potential therapeutic agents [1]. Numerous effective methods have been developed for the synthesis of pyrazolo[1,5-a][1,3,5]triazine [2] and have been applied for the preparation of new bioactive agents. The interest in applications of this heterocyclic scaffold for drug design has been growing, particularly due to its recent successful applications in the area of kinase inhibitor developments [3,4,5,6,7,8,9,10]. Therapeutically valuable agents aimed at diverse targets have been identified among pyrazolo[1,5-a][1,3,5]triazin-7(6H)-ones, more specifically, S-substituted 2-aryl-4-thiopyrazolo[1,5-a][1,3,5]triazin-7(6H)-ones (Figure 1). Thus, P5SA-4 was found to be a potent activator of protein phosphatase 5 [11], while 1882L04 demonstrated prominent inhibitory activity against bacterial glycosyl transferases [12]. SB-H02 was identified as an effective viral entry inhibitor targeting HIVgp41 [13,14].
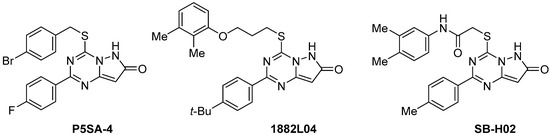
Figure 1.
Bioactive S-substituted 2-aryl-4-thiopyrazolo[1,5-a][1,3,5]triazin-7(6H)-ones.
Our group has been actively working on the development of new efficient methods for the synthesis of pyrazolo[1,5-a][1,3,5]triazines as potential bioactive agents [15,16,17,18,19,20,21,22,23]. Herein, we describe the synthesis of hitherto unreported 4-phenethylthio-2-phenylpyrazolo[1,5-a][1,3,5]triazin-7(6H)-one (4) and the results of its antiproliferative testing against cancer cells.
2. Results and Discussion
The addition of cyanoacetohydrazide to benzoyl isothiocyanate (1) prepared in situ from benzoyl chloride and ammonium thiocyanate produced thiosemicarbazide (2) (Scheme 1). The treatment of 2 with aqueous alkali resulted in its intramolecular cyclization with sequential formation of pyrazole and triazine rings affording pyrazolo[1,5-a][1,3,5]triazine (3). The selective S-alkylation of 3 with 2-phenylethylbromide was successfully achieved in the presence of base at ambient temperature. The signal at 30.6 ppm, observed in the 13C-NMR spectrum (see Supplementary Materials) of 4, suggested that the alkylation occurred at the thiocarbonyl group of 3. In general, the proposed synthetic approach was rather efficient, and compound 4 was prepared from benzoyl chloride in an overall yield of 50%.
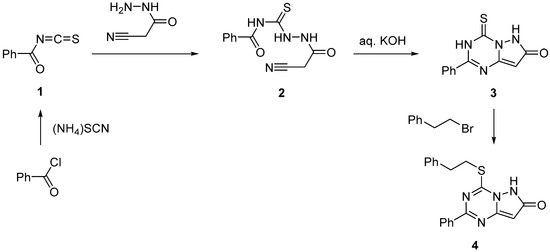
Scheme 1.
Synthesis of 4-phenethylthio-2-phenylpyrazolo[1,5-a][1,3,5]triazin-7(6H)-one (4).
The antiproliferative activity of 4 against human lung cancer (A549) and human breast cancer (MDA-MB231) cell lines was tested using the MTT assay [24]. The initial evaluation of the effect of 4 on cell viability at a 100 µM concentration revealed that the lung cancer cells were more sensitive to treatment with 4. The cell viability was 24 ± 4% and 69 ± 6% for the A549 and MDA-MB231 cells, respectively. Further experiments, which were carried out with 4, estimated the IC50 value for antiproliferative activity of this compound against A549 cells to be 53 ± 3 µM.
It should be noted that the phenethyl group is critical for the antiproliferative effect against A549. Thus, no significant difference from the control was observed when A549 cells were treated with compound 3 at a concentration of 100 µM (cell viability 95 ± 7%).
Floxuridine, used in this study as the reference anticancer drug, was also more active against A549 cells (IC50 = 5.8 ± 1.3 µM) than MDA-MB231 cells (IC50 = 38 ± 9 µM).
3. Experimental
Melting points (uncorrected) were determined on a Gallenkamp melting point apparatus. 1H and 13C-NMR spectra were recorded on a DPX-300 spectrometer (Bruker, Fällanden, Switzerland) at 300 MHz and 75 MHz respectively, using DMSO-d6 as a solvent and TMS as an internal reference.
3.1. Cyanoacetyl-4-benzoylthiosemicarbazide (2)
A solution of N-benzoylisothiocyanate (1), prepared by mixing benzoyl chloride (2.32 mL, 20 mmol) and ammonium thiocyanate (1.44 g, 20 mmol) in acetone (20 mL), was added to the suspension of cyanoacetohydrazide (1.58 g, 16 mmol) in acetone (10 mL) and the reaction mixture was heated under reflux for 3 h. The solvent was evaporated under vacuum, the residue was triturated with cold water (40 mL), and the precipitated product was filtered and recrystallized from methanol. Yield: 68%, m.p. 198 °C [lit. [25] m.p. 198 °C]. 1H-NNR (300 MHz, DMSO-d6): δ 3.88 (2H, s, CH2), 7.54–7.96 (5H, m, Ph), 11.00 (1H, s, NH), 11.77 (1H, s, NH), 12.53 (1H, s, NH) ppm.
3.2. Phenyl-4-thioxopyrazolo[1,5-a][1,3,5]triazine-7(6H)-one (3)
Compound 2 (1.3 g, 5 mmol) was treated with 5% aqueous KOH (12 mL) and the solution obtained was heated under reflux for 3 h. After cooling to room temperature, the reaction mixture was diluted with water (30 mL) and acidified with 3% hydrochloric acid to pH 2. The white precipitate formed was collected by filtration, washed with cold water, and recrystallized from methanol. Yield: 92%, m.p. 300–301 °C [lit. [25] m.p. 302 °C]. 1H-NNR (300 MHz, DMSO-d6): δ 6.04 (1H, s, H-8), 7.55–8.01 (5H, m, Ph), 11.82 (1H, brs, H-6), 13.66 (1H, brs, H-3) ppm. 13C-NNR (75 MHz, DMSO-d6): δ 86.2 (C-8), 128.3, 128.5, 130.8, 131.9 (Ph), 145.1 (C-8a), 149.9 (C-2), 167.0 (C-4), 167.7 (C-7) ppm.
3.3. Phenethylthio-2-phenylpyrazolo[1,5-a][1,3,5]triazin-7(6H)-one (4)
To the solution of compound 3 (0.5 g, 2 mmol) in 1 M aqueous NaOH (2 mL) and ethanol (10 mL), 2-phenylethyl bromide (0.3 mL, 2.2 mmol) was added, and the reaction mixture was stirred at room temperature for 3 days. The precipitate formed was collected by filtration, well washed with water, and recrystallized from acetonitrile. Yield: 80%, m.p. > 250 °C. 1H-NNR (300 MHz, DMSO-d6): δ 3.15 (2H, t, 3J = 7.7 Hz, CH2), 3.69 (2H, t, 3J = 7.7 Hz, CH2), 5.99 (1H, s, H-8), 7.20–7.41 (5H, m, Ph), 7.50–8.50 (5H, m, Ph), 11.74 (1H, brs, H-6) ppm. 13C-NNR (75 MHz, DMSO-d6): δ 30.6 (CH2S), 34.8 (PhCH2), 81.9 (C-8), 126.5, 128.0, 128.4, 128.6, 131.3, 135.8, 139.6 (PhC(2) & PhCH2), 148.3 (C-8a), 155.6 (C-2), 158.2 (C-4), 167.5 (C-7) ppm. Anal. Calcd. for C19H16N4OS: 65.50; H, 4.63; N, 16.08. Found: C, 65.38; H, 4.72; N, 15.93.
3.4. Antiproliferative Assay
The effect of compounds on cell viability was estimated using a standard MTT assay [24]. Floxuridine was used as a positive control. Briefly, an MTT solution (1 mg/mL, 50.0 μL/well) was added after cells were treated with compounds dissolved in DMSO and incubated for 72 h. In order to ensure that the solvent per se had no effect on the cell growth, negative control tests were performed using DMSO at the same concentration. The cells were incubated for another 4 h at 37 °C. The purple formazan crystals that formed were dissolved with DMSO (150.0 μL/well) and the absorbance was measured with a Tecan Genios spectrophotometer at λ = 570 nm. The concentration required for 50% inhibition of cell viability (IC50) was estimated using the median effect plot [26], which was the transformation of sigmoidal dose–response curve into the corresponding linear form: log[fa/(1 − fa)], where fa was the fraction affected or percent viability/100 and (1 − fa) was the fraction unaffected, was plotted against log(compound concentration). The IC50 value was calculated as antilog of the log(compound concentration) intercept, where fa/(1 − fa) = 1 [or log[fa/(1 − fa)] = 0. The results were an average value of three independent experiments.
4. Conclusions
We found that S-alkylation of 2-phenyl-4-thioxopyrazolo[1,5-a][1,3,5]triazine-7(6H)-one (3) with 2-phenylethyl bromide under basic conditions proceeded selectively and resulted in the formation of 4-phenethylthio-2-phenylpyrazolo[1,5-a][1,3,5]triazin-7(6H)-one (4) possessing antiproliferative activity against the A549 human lung cancer cell line.
Supplementary Materials
The following are available online www.mdpi.com/1422-8599/2017/4/M970. Figure S1: 1H-NMR spectrum of compound 4; Figure S2: 13C-NMR spectrum of compound 4; Figure S3: DEPT135 spectrum of compound 4.
Acknowledgments
This work is supported by the Ministry of Higher Education, Malaysia under Fundamental Research Grant Scheme (FRGS).
Author Contributions
S.A.S. performed the synthesis and wrote the paper; E.V.G. and V.R.L. were involved in the synthesis optimization; G.E.T.O. performed preliminary experiments and the MTT assay; W.K.C. and A.V.D. conceived and designed the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lim, F.P.L.; Dolzhenko, A.V. 1,3,5-Triazine-based analogues of purine: From isosteres to privileged scaffolds in medicinal chemistry. Eur. J. Med. Chem. 2014, 85, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Dolzhenko, A.V.; Dolzhenko, A.V.; Chui, W.K. Pyrazolo[1,5-a][1,3,5]triazines (5-aza-9-deazapurines). Synthesis and biological activity. Heterocycles 2008, 75, 1575–1622. [Google Scholar] [CrossRef]
- El Hage, K.; Piquemal, J.-P.; Oumata, N.; Meijer, L.; Galons, H.; Gresh, N. A Simple isomerization of the purine scaffold of a kinase inhibitor, roscovitine, affords a four- to seven-fold enhancement of its affinity for four CDKs. Could this be traced back to conjugation-induced stiffenings/loosenings of rotational barriers? ACS Omega 2017, 2, 3467–3474. [Google Scholar] [CrossRef]
- Ford, D.J.; Horsley, H.T.; Reuberson, J.T. Fused Pyrazole Derivatives as Kinase Inhibitors. WO Patent 2017055305, 6 April 2017. [Google Scholar]
- Laufer, R.; Li, S.-W.; Liu, Y.; Ng, G.; Lang, Y.; Feher, M.; Brokx, R.; Beletskaya, I.; Hodgson, R.; Mao, G.; et al. Discovery of 4-(4-aminopyrazolo[1,5-a][1,3,5]triazin-8-yl)benzamides as novel, highly potent and selective, orally bioavailable inhibitors of Tyrosine Threonine Kinase, TTK. Bioorg. Med. Chem. Lett. 2016, 26, 3562–3566. [Google Scholar] [CrossRef] [PubMed]
- Westman, J. Pyrazolo[1,5-a]triazin-4-amine Derivatives Useful in Therapy. WO Patent 2016206999, 29 December 2016. [Google Scholar]
- Samajdar, S.; Poddutoori, R.; Mukherjee, S.; Goswami, R. Pyrazolo[1,5-a][1,3,5]triazine and Pyrazolo[1,5-a]pyrimidine Derivatives as CDK Inhibitors. WO Patent 2016142855, 15 September 2016. [Google Scholar]
- Horsley, H.T.; Huang, Q.; Neuss, J.C.; Reuberson, J.T.; Vanderhoydonck, B. Fused Bicyclic Heteroaromatic Derivatives as Kinase Inhibitors. WO Patent 2015193169, 23 December 2015. [Google Scholar]
- Marineau, J.J.; Sprott, K.; Schmidt, D. Preparation of Piperidinyloxypyrazolotriazinylamino-Piperidinecarbonylphenyl Derivatives and Analogs for Use as CDK7 Inhibitors. WO Patent 2015154022, 8 October 2015. [Google Scholar]
- Hutterer, C.; Eickhoff, J.; Milbradt, J.; Korn, K.; Zeittraeger, I.; Bahsi, H.; Wagner, S.; Zischinsky, G.; Wolf, A.; Degenhart, C.; et al. A novel CDK7 inhibitor of the pyrazolotriazine class exerts broad-spectrum antiviral activity at nanomolar concentrations. Antimicrob. Agents Chemother. 2015, 59, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Groll, M.; Richter, K.; Haslbeck, V.; Striggow, F.; Martin, H.; Schmidt, W.; Weiwad, M.; Fischer, G. Activators of Protein Phosphatase 5. EP Patent 2878677, 3 June 2015. [Google Scholar]
- Gampe, C.M.; Kahne, D.E.; Kahne, S.W.; Qiao, Y.; East, S.; Parkes, A.L.; Southey, M.; Hunter, J.; Whittaker, M.; Arthuis, M. Preparation of 6H,7H-Pyrazolo[1,5-a][1,3,5]triazin-7-one Derivatives as Inhibitors of Bacterial Glycosyl Transferases. WO Patent 2016191658, 1 December 2016. [Google Scholar]
- Holden, P.M.; Kaur, H.; Goyal, R.; Gochin, M.; Rizzo, R.C. Footprint-based identification of viral entry inhibitors targeting HIVgp41. Bioorg. Med. Chem. Lett. 2012, 22, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Holden, P.M.; Allen, W.J.; Gochin, M.; Rizzo, R.C. Strategies for lead discovery: Application of footprint similarity targeting HIVgp41. Bioorg. Med. Chem. Lett. 2014, 22, 651–661. [Google Scholar]
- Lim, F.P.L.; Luna, G.; Dolzhenko, A.V. A one-pot, three-component, microwave-assisted synthesis of novel 7-amino-substituted 4-aminopyrazolo[1,5-a][1,3,5]triazine-8-carbonitriles. Tetrahedron Lett. 2015, 56, 7016–7019. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Luna, G.; Dolzhenko, A.V. A one-pot, three-component aminotriazine annulation onto 5-aminopyrazole-4-carbonitriles under microwave irradiation. Tetrahedron Lett. 2015, 56, 521–524. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Luna, G.; Dolzhenko, A.V. A new, one-pot, multicomponent synthesis of 5-aza-9-deaza-adenines under microwave irradiation. Tetrahedron Lett. 2014, 55, 5159–5163. [Google Scholar] [CrossRef]
- Kalinin, D.V.; Kalinina, S.A.; Dolzhenko, A.V. A new synthesis of amino substituted azolo[1,3,5]triazines via reaction of N1,N1-dimethyl-N2-azolylformamidines with cyanamide. Heterocycles 2013, 87, 147–154. [Google Scholar] [CrossRef]
- Kalinin, D.V.; Kalinina, S.A.; Dolzhenko, A.V. Synthesis of novel trichloromethyl substituted azolo[1,3,5]triazines. Heterocycles 2012, 85, 2515–2522. [Google Scholar] [CrossRef]
- Bera, H.; Ojha, P.K.; Tan, B.J.; Sun, L.; Dolzhenko, A.V.; Chui, W.K.; Chiu, G.N.C. Discovery of mixed type thymidine phosphorylase inhibitors endowed with antiangiogenic properties: Synthesis, pharmacological evaluation and molecular docking study of 2-thioxo-pyrazolo[1,5-a][1,3,5]triazin-4-ones. Part II. Eur. J. Med. Chem. 2014, 78, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, J.; Bera, H.; Dolzhenko, A.V.; Chiu, G.N.C.; Chui, W.K. Fragment-based approach to the design of 5-chlorouracil-linked-pyrazolo[1,5-a][1,3,5]triazines as thymidine phosphorylase inhibitors. Eur. J. Med. Chem. 2013, 70, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.P.L.; Dolzhenko, A.V. 4-Amino-substituted pyrazolo[1,5-a][1,3,5]triazin-2-amines: A new practical synthesis and biological activity. Tetrahedron Lett. 2014, 55, 6684–6688. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Kow, K.K.; Yeo, E.H.; Chow, S.C.; Dolzhenko, A.V. Synthesis and antileukemic activity of new fluorinated 5-aza-9-deazapurines. Heterocycles 2016, 92, 1121–1131. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Elmoghayar, M.R.H.; Abdalla, S.O.; Yousry, M.; Nasr, A.S. The reaction of isothiocyanates with 2-cyanoethanoic acid hydrazide. A novel synthesis of 1,3,4-thiadiazoles. J. Heterocycl. Chem. 1984, 21, 781–784. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3 and 4 are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).