Abstract
In this note, we report the discovery of a novel pyridinium chlorochroamte-catalyzed process in which an α-bromomethyl-tetrahydrofuran bond was oxidatively cleaved to give a γ-lactone functionality. The title compound was synthesized from a C15 polybrominated acetogenin compound, isolated from the marine sponge Mycale rotalis, by benzoylation followed by pyridinium chlorochromate-catalyzed oxidation. This new degraded derivative was fully characterized by 1H-NMR, 13C-NMR, FTIR (Fourier transform infrared), EIMS (Electron impact mass spectrometry) and HRESIMS (High-resolution electrospray ionisation mass spectrometry).
1. Introduction
Oxidation methods, mediated or catalyzed by transition metal oxo-species, are a class of processes of pivotal importance in organic synthesis [1,2,3,4]. Pyridinium chlorochroamte (PCC) is a well known oxidizing reagent. Although it is mostly used in the oxidation of primary and secondary alcohols to aldehydes and ketones, respectively, it has also been employed in a number of other processes [5], and recently, we [6,7,8,9] and others [10,11,12,13,14] have disclosed its ability to catalyze new synthetically useful reactions. We were now interested in testing some oxidative methods previously developed in our laboratories [8,15,16,17] on a number of structurally diverse molecular frameworks in order to access a selection of variously functionalized substances for structure-activity relationship studies [18]. During these studies, we came across a novel PCC-catalyzed process featuring the oxidative cleavage of an α-bromomethyl-tetrahydrofuran bond to give a γ-lactone functionality. In particular, we report here the synthesis of the title compound through the oxidative degradation of a polybrominated acetogenin substance previously isolated in our laboratories from the marine sponge Mycale rotalis [19].
2. Results and Discussion
The starting compound 1 (Scheme 1) was benzoylated with excess BzCl in pyridine to give benzoate 2. The next oxidation step was carried out by application of a slightly modified oxidative procedure previously developed in our laboratories [8]. In particular, compound 2 was oxidized with a catalytic amount (10 mol %) of PCC in the presence of excess periodic acid (H5IO6, 10 equiv.), as the primary oxidant, to give compound 3 in 50% isolated yield and in a reproducible manner. Contrary to what we observed in our previous conditions [8], the process could proceed at a reasonable rate only at room temperature. Spectral data for 3 (see Supplementary Material) were in full agreement with the reported structure. In particular, FT-IR (υmax 1795 cm−1) (see Supplementary) and 13C-NMR (δ 171.9 ppm) (see Supplementary) data pointed to the presence of a γ-lactone ring in 3. In addition to data from NMR spectra (for the 1H-NMR see Supplementary), the structure of 3 was well secured by HRESIMS (see Supplementary) and EIMS (see Supplementary) data. Particularly informative was the EIMS spectrum (GC-MS mode) of 3 recorded with a high sample pressure [20]. It showed a low-intensity, four-peak MH+ at m/z 567/569/571/573, derived from the ion-molecule reaction typical of ethers [20], indicating the presence of three bromine atoms in the molecule, as well as fragmentation peaks relevant to three stepwise HBr losses, while suggesting the lack of the terminal bromoalkyne moiety. FTIR data well supported the absence of the alkyne function while offering clear evidence for a γ-lactone function.
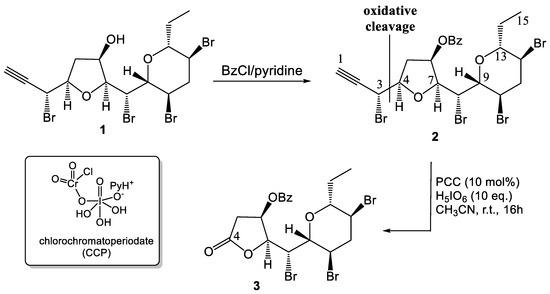
Scheme 1.
Synthesis of (2S,3R)-2-{(R)-Bromo[(2R,3R,5S,6R)-3,5-dibromo-6-ethyltetrahydro-2H-pyran-2-yl]methyl}-5-oxotetrahydrofuran-3-yl Benzoate (3).
According to literature precedents [7,8,21], it is presumable that the active oxidizing species could be chlorochromatoperiodate (CCP, Scheme 1) formed by combination of PCC and periodic acid with water loss. In particular, the α-bromomethyl-tetrahydrofuran bond in the benzoylated species 2 (Scheme 1) underwent a novel type of oxidative cleavage to give the γ-lactone ring present in the final product 3, with the elimination of the three-carbon (C1–C3), bromoalkyne fragment. This process is strongly reminiscent of the pyridinium chlorochromate-mediated oxidative cleavage of α-hydroxymethyl-tetrahydrofurans to γ-lactones previously studied in our group [9,22].
The mechanism of this new process is unknown, but the involvement of a cyclic chlorochromatoperiodate diester (4, Scheme 2) can be hypothesized in agreement with literature precedents [7,8,21] and recent results disclosed by our studies [9].
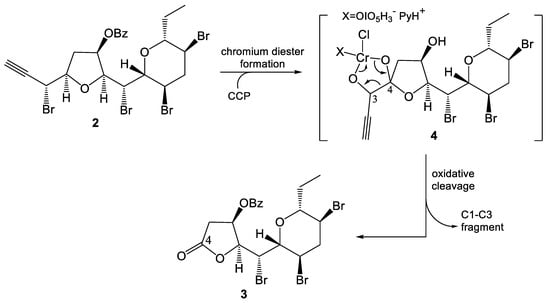
Scheme 2.
Proposed mechanism for the formation of lactone 3.
3. Materials and Methods
3.1. General Information
All reagents were purchased at the highest commercial quality and used without further purification. Reactions were monitored by thin-layer chromatography carried out on precoated silica gel plates (Merck 60, F254, 0.25 mm thick, Merck KGaA, Darmstadt, Germany). NMR experiments were performed with a Varian Unity Inova spectrometer (Varian Inc., Palo Alto, CA, USA) at 400 MHz in CDCl3. Proton chemical shifts were referenced to the residual CHCl3 signal (δ = 7.26 ppm). 13C-NMR chemical shifts were referenced to the solvent (δ = 77.0 ppm). Abbreviations for signal coupling are as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad. Coupling constants are given in Hertz. IR spectra were recorded neat with a Jasco FT-IR 430 spectrophotometer (JASCO, GmbH, Germany) and are reported in cm−1. HRMS spectra were recorded by infusion on Thermo LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with an Electrospray source in the positive mode using MeOH as solvent. Low-resolution EI-MS spectrum of 3 was obtained operating at 70 eV on a GCMS-QP5050A (Shimadzu Corporation, Kyoto, Japan).
3.2. Synthesis of (2S,3R,5R)-2-{(R)-Bromo[(2R,3R,5S,6R)-3,5-dibromo-6-ethyltetrahydro-2H-pyran-2-yl]methyl}-5-[(R)-1-bromoprop-2-yn-1-yl]tetrahydrofuran-3-yl Benzoate (2)
Benzoyl chloride (10 equiv., 0.65 mmol, 75 μL) was added to 1 (36.7 mg, 0.065 mmol) dissolved in pyridine (400 μL), and the mixture was stirred at room temperature for 16 h. Methanol (0.5 mL) was added, and the mixture was stirred for 15 min. and then taken to dryness to give an oily product. Purification by preparative TLC (silica, hexane/EtOAc, 8:2, Rf = 0.54) afforded the benzoyl-derivative 2 (16.2 mg, 37%) as a clear oil.
2: 1H-NMR (400 MHz, CDCl3) (see Supplementary), δ (ppm): 8.07 (2H, d, J = 8.1), 7.60 (1H, t, J = 7.6), 7.45, (2H, t, J = 7.5), 5.43 (1H, bdd, J = 5.4, 2.8), 4.85 (1H, bdd, J = 9.4, 1.1), 4.64 (1H, dd, J = 5.9, 2.3), 4.40 (1H, ddd, J = 8.8, 5.4, 5.4), 4.35 (1H, dd, J = 9.5, 3.1), 4.16 (1H, m), 3.71 (1H, ddd, J = 11.8, 11.8, 4.1), 3.43–3.32 (2H, m), 2.91 (1H, ddd, J = 13.0, 4.0, 4.0), 2.77 (1H, ddd, J = 15.2, 8.7, 6.0), 2.47 (1H, dd, J = 15.4, 4.9), 2.36–2.25 (2H, m), 2.04 (1H, m), 1.56 (1H, m), 0.97 (3H, t, J = 7.3); 13C-NMR (101 MHz, CDCl3) (see Supplementary), δ (ppm) 165.6, 133.7, 129.9, 129.1, 128.5, 84.2, 83.7, 80.2, 79.5, 79.1, 76.8, 73.0, 51.9, 47.2, 46.5, 44.9, 37.2, 36.6, 25.8, 9.6; ESIMS m/z: [M + Na]+ 691/693/695/697/699.
3.3. (2S,3R)-2-{(R)-Bromo[(2R,3R,5S,6R)-3,5-dibromo-6-ethyltetrahydro-2H-pyran-2-yl]methyl}-5-oxotetrahydrofuran-3-yl Benzoate (3)
PCC (10 mol %, 240 μL of a 0.01 M stock solution in acetonitrile) was added at room temperature to a vigorously stirred suspension of H5IO6 (10 equiv., 0.24 mmol, 54.2 mg) in acetonitrile (100 μL). After 5 min., compound 2 (16.0 mg, 0.024 mmol) dissolved in acetonitrile (100 μL + 60 μL rinse) was added. After 16 h, CH2Cl2 (500 μL) was added followed by ethanol (100 μL), and the mixture was taken to dryness. Preparative TLC on silica gel (hexane–EtOAc, 8:2, Rf = 0.40) gave pure 3 (7.3 mg, 50%) as an oil.
3: IR (neat): υmax 1795, 1721, 1265 cm−1; 1H-NMR (CDCl3), δ (ppm): 8.05 (2H, d, J = 7.6), 7.64 (1H, t, J = 7.6), 7.48, (2H, t, J = 7.6), 5.67 (1H, bdd, J = 4.4, 4.4), 4.98 (1H, dd, J = 9.7, 3.2), 4.90 (1H, dd, J = 9.7, 1.6), 4.17 (1H, m), 3.73 (1H, m), 3.45–3.33 (2H, m), 3.07 (1H, dd, J = 18.2, 5.1), 2.93 (1H, ddd, J = 12.9, 4.5, 4.5), 2.84 (1H, d, J = 18.2), 2.31 (1H, ddd, J = 12.7, 12.7, 12.7), 2.05 (1H, m), 1.60 (1H, m), 0.99 (3H, t, J = 7.4); 13C-NMR (CDCl3), δ (ppm): 171.9, 165.3, 134.3, 130.0, 128.7, 128.1, 83.8, 83.4, 79.3, 70.2, 50.2, 46.7, 46.0, 44.7, 38.0, 25.7, 9.6; EIMS m/z: 567/569/571/573 [M + 1]+, 487/489/491 [M + 1]+-HBr, 407/409 [M + 1]+-2HBr, 365/367/369 [M + 1]+-Br-PhCOOH, 327 [M + 1]+-3HBr, 285/287 [M + 1]+-HBr-Br-PhCOOH, 269/271/273 (C7H11Br2, C9–C15 dibromopyrane, fragment), 121 (PhCO2), 105 (PhCO); HRESIMS m/z: calcd for C19H2179Br3NaO5 588.8837 [M + Na]+, found: 588.8826.
4. Conclusions
In conclusion, a new degraded C12 acetogenin–derived substance has been obtained by an unprecedented PCC-catalyzed oxidative cleavage process that adds further insight into our comprehension of the chemistry of this oxidant. Further studies to test the scope of this transformation and its synthetic utility are in progress in our laboratories.
Supplementary Materials
The following are available online, Figure S1: 1H-NMR spectrum of 2, Figure S2: 13C-NMR spectrum of 2, Figure S3: 1H-NMR spectrum of 3, Figure S4: 13C-NMR spectrum of 3, Figure S5: EIMS spectrum of 3, Figure S6: HRESIMS spectrum of 3, Figure S7: FTIR spectrum of 3.
Acknowledgments
The author is grateful to the “Centro di Servizi Interdipartimentale di Analisi Strumentale” (CSIAS) for supplying the NMR facilities.
Conflicts of Interest
The author declares no conflict of interest.
References
- Mijs, W.J.; De Jonge, C.R.H.I. (Eds.) Organic Syntheses by Oxidation with Metal Compounds; Plenum Press: New York, NY, USA, 1986. [Google Scholar]
- Baeckvall, J.-E. (Ed.) Modern Oxidation Methods, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Piccialli, V. Oxidative cyclization of dienes and polyenes mediated by transition metal oxo-species. Synthesis 2007, 2585–2607. [Google Scholar] [CrossRef]
- Piccialli, V. Ruthenium tetroxide and perruthenate chemistry. Recent advances and related transformations mediated by other transition metal oxo-species. Molecules 2014, 19, 6534–6582. [Google Scholar] [CrossRef] [PubMed]
- Piancatelli, G.; Scettri, A.; D’Auria, M. Pyridinium chlorochromate: A versatile oxidant in organic synthesis. Synthesis 1982, 245–258. [Google Scholar] [CrossRef]
- Piccialli, V.; Zaccaria, S.; Borbone, N.; Oliviero, G.; D’Errico, S.; Hemminki, A.; Cerullo, V.; Romano, V.; Tuzi, A.; Centore, R. Discovery of a new PCC-mediated stereoselective oxidative spiroketalization process. An access to a new type of poly-THF spiroketal compound displaying anticancer activity. Org. Biomol. Chem. 2009, 7, 3036–3039. [Google Scholar] [CrossRef]
- Piccialli, V.; Zaccaria, S.; Oliviero, G.; D’Errico, S.; D’Atri, V.; Borbone, N. Insight into pyridinium chlorochromate chemistry: Catalytic oxidation of tetrahydrofuran compounds and synthesis of umbelactone. Eur. J. Org. Chem. 2012, 2012, 4293–4305. [Google Scholar] [CrossRef]
- Piccialli, V.; D’Errico, S.; Borbone, N.; Oliviero, G.; Centore, R.; Zaccaria, S. A general synthesis of bis-α-acyloxy-1,4- and -1,5-diketones through catalytic oxidative opening of acylated THF and THP diols. Eur. J. Org. Chem. 2013, 2013, 1781–1789. [Google Scholar] [CrossRef]
- Zaccaria, S.; Borbone, N.; Oliviero, G.; D’Errico, S.; Piccialli, V. Pyridinium chlorochromate chemistry. New insight into oxidation of tetrahydrofurans. Arkivoc 2017, iv, 273–290. [Google Scholar] [CrossRef]
- Ferraz, H.M.C.; Longo, L.S. Bicyclic β-hydroxytetrahydrofurans as precursors of medium ring keto-lactones. J. Org. Chem. 2007, 72, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, Y.; Tong, R. Cephalosporolide B serving as a versatile synthetic precursor: Asymmetric biomimetic total syntheses of cephalosporolides C, E, F, G, and (4-OMe-)G. Org. Lett. 2013, 15, 5850–5853. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Lee, K.-H.; Lin, Z.; Tong, R. Structural revision of cephalosporolide J and bassianolone. J. Org. Chem. 2014, 79, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tong, R. Total synthesis of purported cephalosporolides H and I, penisporolide B, and their stereoisomers. J. Org. Chem. 2016, 81, 4325–4339. [Google Scholar] [CrossRef] [PubMed]
- Norris, M.D.; Perkins, M.V. Total synthesis of plakilactones C, B and des-hydroxyplakilactone B by the oxidative cleavage of gracilioether furanylidenes. J. Org. Chem. 2016, 81, 6848–6854. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, G.; Caserta, T.; Gomez-Paloma, L.; Piccialli, V. RuO4-promoted syn-oxidative polycyclization of isoprenoid polyenes: A new stereoselective cascade process. Tetrahedron Lett. 2002, 43, 9265–9269. [Google Scholar] [CrossRef]
- Bifulco, G.; Caserta, T.; Gomez-Paloma, L.; Piccialli, V. Corrigendum to “RuO4-promoted syn-oxidative polycyclization of isoprenoid polyenes: A new stereoselective cascade process”: [Tetrahedron Lett. 43 (2002) 9265]. Tetrahedron Lett. 2003, 44, 3429. [Google Scholar] [CrossRef]
- Piccialli, V.; Borbone, N.; Oliviero, G. Ruthenium-catalyzed oxidative cyclization of 1,7-dienes. A novel diasteroselective synthesis of 2,7-disubstituted trans-oxepane diols. Tetrahedron Lett. 2007, 48, 5131–5135. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; D’Alonzo, D.; Piccialli, V.; Mayol, L.; Piccialli, G. A facile synthesis of 5′-fluoro-5′-deoxyacadesine (5′-F-AICAR): A novel non-phosphorylable AICAR analogue. Molecules 2012, 17, 13036–13044. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Mayol, L.; Notaro, G.; Piccialli, V.; Sica, D. Structure and absolute configuration of two new polybrominated C15 acetogenins from the sponge Mycale rotalis. Chem. Commun. 1990, 1559–1561. [Google Scholar] [CrossRef]
- McLafferty, F.W. Mass Spectrometric Analysis... Aliphatic Ethers. Anal. Chem. 1957, 29, 1782–1789. [Google Scholar] [CrossRef]
- Hunsen, M. Pyridinium chlorochromate catalyzed oxidation of alcohols to aldehydes and ketones with periodic acid. Tetrahedron Lett. 2005, 46, 1651–1653. [Google Scholar] [CrossRef]
- Caserta, T.; Piccialli, V.; Gomez-Paloma, L.; Bifulco, G. RuO4-catalyzed oxidative polycyclization of squalene. Determination of the configuration of the penta-tetrahydrofuranyl diol product. Tetrahedron 2005, 61, 927–939. [Google Scholar] [CrossRef]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).