4-Phenethylthio-2-phenylpyrazolo[1,5-a][1,3,5]triazin-7(6H)-one
Abstract
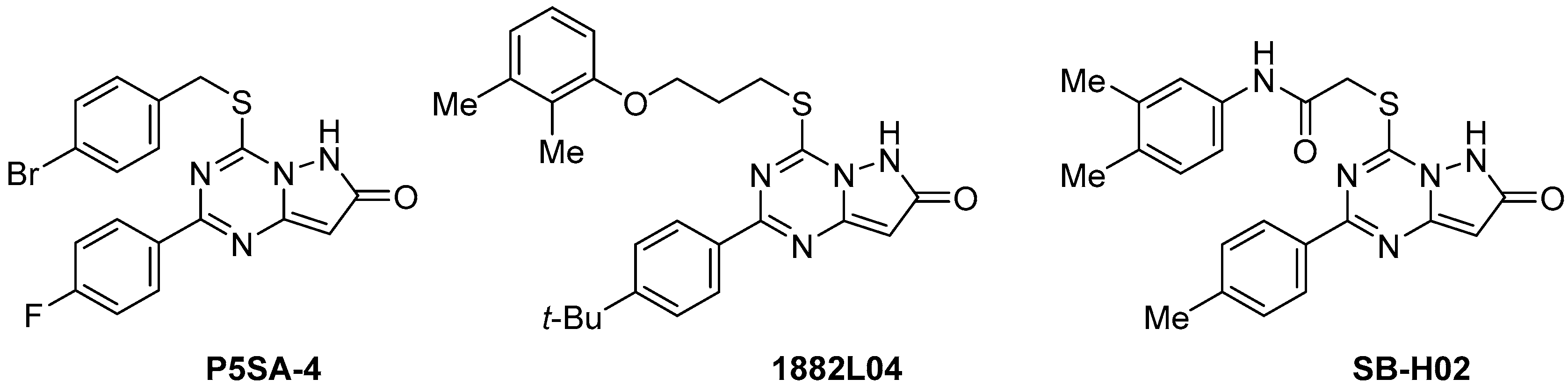
:1. Introduction
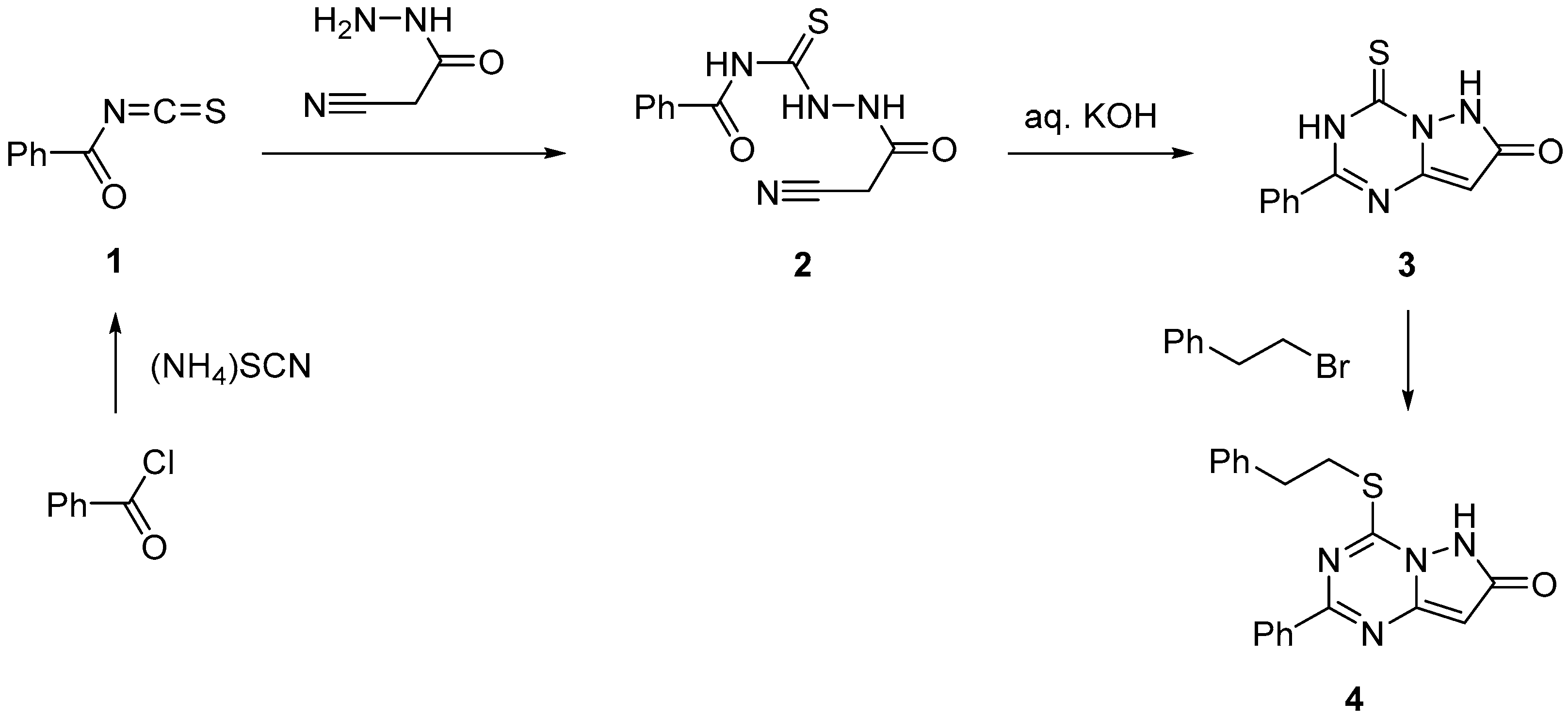
2. Results and Discussion
3. Experimental
3.1. Cyanoacetyl-4-benzoylthiosemicarbazide (2)
3.2. Phenyl-4-thioxopyrazolo[1,5-a][1,3,5]triazine-7(6H)-one (3)
3.3. Phenethylthio-2-phenylpyrazolo[1,5-a][1,3,5]triazin-7(6H)-one (4)
3.4. Antiproliferative Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lim, F.P.L.; Dolzhenko, A.V. 1,3,5-Triazine-based analogues of purine: From isosteres to privileged scaffolds in medicinal chemistry. Eur. J. Med. Chem. 2014, 85, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Dolzhenko, A.V.; Dolzhenko, A.V.; Chui, W.K. Pyrazolo[1,5-a][1,3,5]triazines (5-aza-9-deazapurines). Synthesis and biological activity. Heterocycles 2008, 75, 1575–1622. [Google Scholar] [CrossRef]
- El Hage, K.; Piquemal, J.-P.; Oumata, N.; Meijer, L.; Galons, H.; Gresh, N. A Simple isomerization of the purine scaffold of a kinase inhibitor, roscovitine, affords a four- to seven-fold enhancement of its affinity for four CDKs. Could this be traced back to conjugation-induced stiffenings/loosenings of rotational barriers? ACS Omega 2017, 2, 3467–3474. [Google Scholar] [CrossRef]
- Ford, D.J.; Horsley, H.T.; Reuberson, J.T. Fused Pyrazole Derivatives as Kinase Inhibitors. WO Patent 2017055305, 6 April 2017. [Google Scholar]
- Laufer, R.; Li, S.-W.; Liu, Y.; Ng, G.; Lang, Y.; Feher, M.; Brokx, R.; Beletskaya, I.; Hodgson, R.; Mao, G.; et al. Discovery of 4-(4-aminopyrazolo[1,5-a][1,3,5]triazin-8-yl)benzamides as novel, highly potent and selective, orally bioavailable inhibitors of Tyrosine Threonine Kinase, TTK. Bioorg. Med. Chem. Lett. 2016, 26, 3562–3566. [Google Scholar] [CrossRef] [PubMed]
- Westman, J. Pyrazolo[1,5-a]triazin-4-amine Derivatives Useful in Therapy. WO Patent 2016206999, 29 December 2016. [Google Scholar]
- Samajdar, S.; Poddutoori, R.; Mukherjee, S.; Goswami, R. Pyrazolo[1,5-a][1,3,5]triazine and Pyrazolo[1,5-a]pyrimidine Derivatives as CDK Inhibitors. WO Patent 2016142855, 15 September 2016. [Google Scholar]
- Horsley, H.T.; Huang, Q.; Neuss, J.C.; Reuberson, J.T.; Vanderhoydonck, B. Fused Bicyclic Heteroaromatic Derivatives as Kinase Inhibitors. WO Patent 2015193169, 23 December 2015. [Google Scholar]
- Marineau, J.J.; Sprott, K.; Schmidt, D. Preparation of Piperidinyloxypyrazolotriazinylamino-Piperidinecarbonylphenyl Derivatives and Analogs for Use as CDK7 Inhibitors. WO Patent 2015154022, 8 October 2015. [Google Scholar]
- Hutterer, C.; Eickhoff, J.; Milbradt, J.; Korn, K.; Zeittraeger, I.; Bahsi, H.; Wagner, S.; Zischinsky, G.; Wolf, A.; Degenhart, C.; et al. A novel CDK7 inhibitor of the pyrazolotriazine class exerts broad-spectrum antiviral activity at nanomolar concentrations. Antimicrob. Agents Chemother. 2015, 59, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Groll, M.; Richter, K.; Haslbeck, V.; Striggow, F.; Martin, H.; Schmidt, W.; Weiwad, M.; Fischer, G. Activators of Protein Phosphatase 5. EP Patent 2878677, 3 June 2015. [Google Scholar]
- Gampe, C.M.; Kahne, D.E.; Kahne, S.W.; Qiao, Y.; East, S.; Parkes, A.L.; Southey, M.; Hunter, J.; Whittaker, M.; Arthuis, M. Preparation of 6H,7H-Pyrazolo[1,5-a][1,3,5]triazin-7-one Derivatives as Inhibitors of Bacterial Glycosyl Transferases. WO Patent 2016191658, 1 December 2016. [Google Scholar]
- Holden, P.M.; Kaur, H.; Goyal, R.; Gochin, M.; Rizzo, R.C. Footprint-based identification of viral entry inhibitors targeting HIVgp41. Bioorg. Med. Chem. Lett. 2012, 22, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Holden, P.M.; Allen, W.J.; Gochin, M.; Rizzo, R.C. Strategies for lead discovery: Application of footprint similarity targeting HIVgp41. Bioorg. Med. Chem. Lett. 2014, 22, 651–661. [Google Scholar]
- Lim, F.P.L.; Luna, G.; Dolzhenko, A.V. A one-pot, three-component, microwave-assisted synthesis of novel 7-amino-substituted 4-aminopyrazolo[1,5-a][1,3,5]triazine-8-carbonitriles. Tetrahedron Lett. 2015, 56, 7016–7019. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Luna, G.; Dolzhenko, A.V. A one-pot, three-component aminotriazine annulation onto 5-aminopyrazole-4-carbonitriles under microwave irradiation. Tetrahedron Lett. 2015, 56, 521–524. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Luna, G.; Dolzhenko, A.V. A new, one-pot, multicomponent synthesis of 5-aza-9-deaza-adenines under microwave irradiation. Tetrahedron Lett. 2014, 55, 5159–5163. [Google Scholar] [CrossRef]
- Kalinin, D.V.; Kalinina, S.A.; Dolzhenko, A.V. A new synthesis of amino substituted azolo[1,3,5]triazines via reaction of N1,N1-dimethyl-N2-azolylformamidines with cyanamide. Heterocycles 2013, 87, 147–154. [Google Scholar] [CrossRef]
- Kalinin, D.V.; Kalinina, S.A.; Dolzhenko, A.V. Synthesis of novel trichloromethyl substituted azolo[1,3,5]triazines. Heterocycles 2012, 85, 2515–2522. [Google Scholar] [CrossRef]
- Bera, H.; Ojha, P.K.; Tan, B.J.; Sun, L.; Dolzhenko, A.V.; Chui, W.K.; Chiu, G.N.C. Discovery of mixed type thymidine phosphorylase inhibitors endowed with antiangiogenic properties: Synthesis, pharmacological evaluation and molecular docking study of 2-thioxo-pyrazolo[1,5-a][1,3,5]triazin-4-ones. Part II. Eur. J. Med. Chem. 2014, 78, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, J.; Bera, H.; Dolzhenko, A.V.; Chiu, G.N.C.; Chui, W.K. Fragment-based approach to the design of 5-chlorouracil-linked-pyrazolo[1,5-a][1,3,5]triazines as thymidine phosphorylase inhibitors. Eur. J. Med. Chem. 2013, 70, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.P.L.; Dolzhenko, A.V. 4-Amino-substituted pyrazolo[1,5-a][1,3,5]triazin-2-amines: A new practical synthesis and biological activity. Tetrahedron Lett. 2014, 55, 6684–6688. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Kow, K.K.; Yeo, E.H.; Chow, S.C.; Dolzhenko, A.V. Synthesis and antileukemic activity of new fluorinated 5-aza-9-deazapurines. Heterocycles 2016, 92, 1121–1131. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Elmoghayar, M.R.H.; Abdalla, S.O.; Yousry, M.; Nasr, A.S. The reaction of isothiocyanates with 2-cyanoethanoic acid hydrazide. A novel synthesis of 1,3,4-thiadiazoles. J. Heterocycl. Chem. 1984, 21, 781–784. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3 and 4 are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolnikov, S.A.; Gorgopina, E.V.; Lezhnyova, V.R.; Ong, G.E.-T.; Chui, W.-K.; Dolzhenko, A.V. 4-Phenethylthio-2-phenylpyrazolo[1,5-a][1,3,5]triazin-7(6H)-one. Molbank 2017, 2017, M970. https://doi.org/10.3390/M970
Smolnikov SA, Gorgopina EV, Lezhnyova VR, Ong GE-T, Chui W-K, Dolzhenko AV. 4-Phenethylthio-2-phenylpyrazolo[1,5-a][1,3,5]triazin-7(6H)-one. Molbank. 2017; 2017(4):M970. https://doi.org/10.3390/M970
Chicago/Turabian StyleSmolnikov, Sergey A., Ekaterina V. Gorgopina, Vera R. Lezhnyova, Gwyneth En-Tian Ong, Wai-Keung Chui, and Anton V. Dolzhenko. 2017. "4-Phenethylthio-2-phenylpyrazolo[1,5-a][1,3,5]triazin-7(6H)-one" Molbank 2017, no. 4: M970. https://doi.org/10.3390/M970





