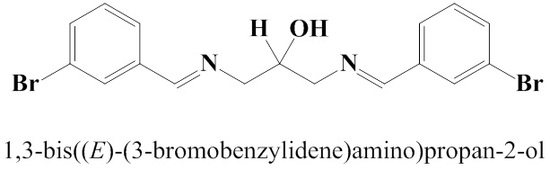
1,3-Bis[(E)-(3-bromobenzylidene)amino]propan-2-ol
Abstract
:1. Introduction
2. Results
2.1. Optimization, MEP, Mulliken, and NPA Analysis
2.2. Electronic Transfer and TD-SCF Analysis
3. Materials and Methods
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alshaheri, A.A.; Tahir, M.I.M.; Rahman, M.B.A.; Begum, T.; Saleh, T.A. Synthesis, characterization and catalytic activity of dithiocarbazate Schiff base complexes in oxidation of cyclohexane. J. Mol. Liq. 2017, 240, 486–496. [Google Scholar] [CrossRef]
- Įiįek, B.; Įalısļır, Ü.; Tavaslı, M.; Tülek, R.; Teke, A. Synthesis and optical characterization of novel carbazole Schiff bases. J. Mol. Struct. 2018, 1153, 42–47. [Google Scholar]
- Locke, J.M.; Griffith, R.; Bailey, T.D.; Crumbie, R.L. Competition between cyclisation and bisimine formation in the reaction of 1,3-diaminopropanes with aromatic aldehydes. Tetrahedron 2009, 65, 10685–10692. [Google Scholar] [CrossRef]
- Halli, M.B.; Sumathi, R.B. Synthesis, spectroscopic, antimicrobial and DNA cleavage studies of new Co(II), Ni(II), Cu(II), Cd(II), Zn(II) and Hg(II) complexes with naphthofuran-2-carbohydrazide Schiff base. J. Mol. Struct. 2012, 1022, 130–138. [Google Scholar] [CrossRef]
- Barone, G.; Terenzi, A.; Lauria, A.; Almerico, A.M.; Leal, J.M.; Busto, N.; García, B. DNA-binding of nickel (II), copper(II) and zinc(II) complexes: Structure–affinity relationships. Chem. Rev. 2013, 257, 2848–2862. [Google Scholar] [CrossRef]
- Elemike, E.E.; Nwankwo, H.U.; Onwudiwe, D.C. Experimental and theoretical studies of (Z)-N-(2-chlorobenzylidene) naphthalen-1-amine and (Z)-N-(3-nitrobenzylidene)naphthalen-1-amine, and their corrosion inhibition properties. J. Mol. Struct. 2018, 1155, 123–132. [Google Scholar] [CrossRef]
- Asadi, Z.; Nasrollahi, N. The effect of metal and substituent on DNA binding, cleavage activity, and cytotoxicity of new synthesized Schiff base ligands and Zn(II) complex. J. Mol. Struct. 2017, 1147, 582–593. [Google Scholar] [CrossRef]
- Resayes, S.; Warad, I.; Choudhary, M.; Wahab, A.; Rasheed, S. Heterocyclic Schiff’s Bases as Novel and new Antiglycation Agents. U.S. Patent 2014/0221429A1, 7 August 2014. [Google Scholar]
- Tadavi, S.K.; Yadav, A.A.; Bendre, R.S. Synthesis and characterization of a novel Schiff base of 1,2-diaminopropane with substituted salicyaldehyde and its transition metal complexes: Single crystal structures and biological activities. J. Mol. Struct. 2018, 1152, 223–231. [Google Scholar] [CrossRef]
- Rivera, A.; Miranda-Carvajal, I.; Ríos-Motta, J.; Bolte, M. Crystal structure of 1,3-bis[(E)-4-methoxybenzylideneamino] propan-2-ol. Acta Cryst. 2016, E72, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Miranda-Carvajal, I.; Ríos-Motta, J.; Bolte, M. Crystal structure of 1,3-bis[(E)-benzylideneamino]propan-2-ol. Acta Cryst. 2017, E73, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Warad, I.; Al-Resayes, S.; Alzaqri, Z.; Khan, M.; Pallepogu, R.; Dwivedi, S.; Musarrat, J.; Shakir, M. Synthesis and structural characterization of Pd(II) complexes derived from perimidine ligand and their in vitro antimicrobial studies. J. Mol. Struct. 2013, 1047, 48–54. [Google Scholar] [CrossRef]
- Warad, I.; Al-Noaimi, M.; Haddad, S.; Al-Demeri, Y.; Hammouti, B.; Ben Hadda, T. Rac-(E,E)-N,N′-Bis(2-chlorobenzylidene)-cyclohexane-1,2-diamine. Acta Cryst. 2013, E69, 1442–1445. [Google Scholar] [CrossRef] [PubMed]
- Abdoh, M.; Warad, I.; Naveen, S.; Lokanath, N.; Salghi, R. Crystal structure of (1E,1′E)-N,N′-(ethane-1,2-diyl)bis[(pyridin-2-yl)-methanimine]. Acta Cryst. 2015, E71, 431–435. [Google Scholar]
- Warad, I.; Khan, A.; Azam, M.; Al-Resayes, S.; Haddad, S. Design and structural studies of diimine/CdX2 (X = Cl, I) complexes based on 2,2-dimethyl-1,3-diaminopropane ligand. J. Mol. Struct. 2014, 1062, 167–173. [Google Scholar] [CrossRef]
- Warad, I.; Khan, A.; Azam, M.; Al-Resayes, S.; Khan, M.; Ahmad, P.; Al-Nuri, M.; Jodeh, S.; Husein, A.; Haddad, S.; et al. Structural studies on Cd(II) complexes incorporating di-2-pyridyl ligand and the X-ray crystal structure of the chloroform solvated DPMNPH/CdI2 complex. Inorg. Chem. Comm. 2014, 43, 155–161. [Google Scholar] [CrossRef]
- Warad, I.; Musameh, S.; Badran, I.; Nassar, N.N.; Brandao, P.; Tavares, C.J.; Barakat, A. Synthesis, solvatochromism and crystal structure of trans-[Cu(Et2NCH2CH2NH2)2.H2O](NO3)2 complex: Experimental with DFT combination. J. Mol. Struct. 2017, 1148, 328–338. [Google Scholar] [CrossRef]
- Warad, I.; Al-Demeri, Y.; Al-Nuri, M.; Suleiman, M.; Al-Ali, A.; Amereih, S. N-[(1E)-(3-Bromophenyl)methylene]-N-(2-piperidin-1-ylethyl)amine. Molbank 2016, 3, M903. [Google Scholar] [CrossRef]





| Bond No. | Bond Type | Å | Angle No. | Angle Type | (o) | |||
|---|---|---|---|---|---|---|---|---|
| 1 | C1 | N2 | 1.47 | 1 | N2 | C1 | C3 | 109.47 |
| 2 | C1 | C3 | 1.54 | 2 | C1 | N2 | C6 | 120 |
| 3 | N2 | C6 | 1.2936 | 3 | C1 | C3 | C4 | 87.9 |
| 4 | C3 | C4 | 1.54 | 4 | C1 | C3 | O7 | 138.96 |
| 5 | C3 | O7 | 1.4299 | 5 | C4 | C3 | O7 | 85.01 |
| 6 | C4 | N5 | 1.4699 | 6 | C3 | C4 | N5 | 109.47 |
| 7 | N5 | C15 | 1.2936 | 7 | C4 | N5 | C15 | 120 |
| 8 | C6 | C27 | 1.54 | 8 | N2 | C6 | C27 | 120 |
| 9 | C15 | C21 | 1.54 | 9 | N5 | C15 | C21 | 120 |
| 10 | C16 | C17 | 1.4014 | 10 | C17 | C16 | C21 | 120 |
| 11 | C16 | C21 | 1.4014 | 11 | C16 | C17 | C18 | 120 |
| 12 | C17 | C18 | 1.4014 | 12 | C16 | C17 | Br37 | 120 |
| 13 | C17 | Br37 | 1.91 | 13 | C18 | C17 | Br37 | 120 |
| 14 | C18 | C19 | 1.4015 | 14 | C17 | C18 | C19 | 120 |
| 15 | C19 | C20 | 1.4014 | 15 | C18 | C19 | C20 | 120 |
| 16 | C20 | C21 | 1.4015 | 16 | C19 | C20 | C21 | 120 |
| 17 | C27 | C28 | 1.4014 | 17 | C15 | C21 | C16 | 120 |
| 18 | C27 | C29 | 1.4014 | 18 | C15 | C21 | C20 | 120 |
| 19 | C28 | C30 | 1.4014 | 19 | C16 | C21 | C20 | 120 |
| 20 | C29 | C32 | 1.4014 | 20 | C6 | C27 | C28 | 120 |
| 21 | C30 | C34 | 1.4014 | 21 | C6 | C27 | C29 | 120 |
| 22 | C30 | Br38 | 1.91 | 22 | C28 | C27 | C29 | 120 |
| 23 | C32 | C34 | 1.4014 | 23 | C27 | C28 | C30 | 120 |
| 24 | C27 | C29 | C32 | 120 | ||||
| 25 | C28 | C30 | C34 | 120 | ||||
| 26 | C28 | C30 | Br38 | 120 | ||||
| 27 | C34 | C30 | Br38 | 120 | ||||
| 28 | C29 | C32 | C34 | 120 | ||||
| 29 | C30 | C34 | C32 | 120 | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warad, I.; Abedalrazeq, H.; Amer, N.; Al-Nuri, M.; Al Ali, A.; Al-Zaqri, N.; Shivalingegowda, N. 1,3-Bis[(E)-(3-bromobenzylidene)amino]propan-2-ol. Molbank 2017, 2017, M971. https://doi.org/10.3390/M971
Warad I, Abedalrazeq H, Amer N, Al-Nuri M, Al Ali A, Al-Zaqri N, Shivalingegowda N. 1,3-Bis[(E)-(3-bromobenzylidene)amino]propan-2-ol. Molbank. 2017; 2017(4):M971. https://doi.org/10.3390/M971
Chicago/Turabian StyleWarad, Ismail, Huda Abedalrazeq, Nisreen Amer, Mohammmed Al-Nuri, Anas Al Ali, Nabil Al-Zaqri, and Naveen Shivalingegowda. 2017. "1,3-Bis[(E)-(3-bromobenzylidene)amino]propan-2-ol" Molbank 2017, no. 4: M971. https://doi.org/10.3390/M971
APA StyleWarad, I., Abedalrazeq, H., Amer, N., Al-Nuri, M., Al Ali, A., Al-Zaqri, N., & Shivalingegowda, N. (2017). 1,3-Bis[(E)-(3-bromobenzylidene)amino]propan-2-ol. Molbank, 2017(4), M971. https://doi.org/10.3390/M971