Abstract
Functional adamantane derivatives are interesting as active pharmaceutical ingredients, building blocks for supramolecular ensembles, and ligands in light of the coordination chemistry of functional materials. In this communication, synthesis of two isomeric 1,3-bis(1,2,4-triazolyl)adamantanes by the reaction of 1,3-dibromoadamantane with 1,2,4-triazole in the absence of solvent is reported. The products, namely 1,3-bis(1,2,4-triazol-1-yl)adamantane and 1-(1,2,4-triazol-1-yl)-3-(1,2,4-triazol-4-yl)adamantane were separated by column chromatography and identified by NMR and mass-spectrometry.
1. Introduction
Derivatives of adamantane with nitrogen-containing groups are known to demonstrate antiviral activity, rimantadine being the most well studied among them [1,2,3,4]. Other types of biological activities of adamantane derivatives have also been reported [5,6,7]. They find use in polymer synthesis [8], the design of nanostructured materials [9,10], ionic liquids [11], and coordination chemistry [12,13]. In addition, arrangement of azoles around a rigid linker makes azolyladamantanes perfect auxiliary ligands for metal-organic framework design; however, only a few examples of such ligands (with 4H-1,2,4-triazolyl and tetrazolyl moieties) have been reported [14,15,16,17,18]. Claramunt et al. reported the monoadamantylation of a number of azoles by their reaction with 1-bromoadamantane at 120–200 °C [4,19,20,21,22]. Recently, Wei et al. reported the synthesis of the title compound by grinding adamantane and 1,2,4-triazole in the presence of aluminum tribromide an tetrabromomethane in the absence of liquid solvents [23]. However, the obvious drawback of this method is the necessity of using highly reactive aluminum tribromide in mechanical activation conditions, so the reaction was carried out leading only on a millimole scale.
2. Results
In previous studies, we used superbasic dimethyl sulfoxide—potassium hydroxide system (DMSO-KOH) for the double alkylation of azoles with high yields [24,25,26,27]. However, our attempt to react 1,3-dibromoadamantane with 1,2,4-triazole in DMSO-KOH was unsuccessful, and only the starting materials were recovered.
Monoadamantylation of 1,2,4-triazole in a superbasic medium was possible only at an elevated temperature (120 °C instead of the typical 60–80 °C) and lead to a mixture of 1-adamantyl-1,2,4-triazole and 1-hydroxyadamantane. We found that the reaction of 1,3-dibromoadamantane with 1,2,4-triazole at an elevated temperature in the absence of solvent can be used to prepare 1,3-bis(1,2,4-triazolyl)adamantanes 1 and 2. At 120 °C, no reaction was observed between 1,2,4-triazole and 1,3-dibromoadamantane, even after 24 h. However, the reaction rate is quite temperature-sensitive, and raising the temperature to 180 °C allowed full conversion in 18 h (Figure 1). Despite the full conversion of starting dibromoderivative, the product yield was about 41%, which is probably due to its incomplete extraction from the aqueous solution during workup. Varying the solvent and pH level did not improve the isolated yield.
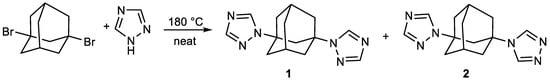
Figure 1.
The scheme of reaction between 1,3-dibromoadamantane and 1,2,4-triazole.
GC/MS analysis of the product isolated from the reaction mixture indicated that two isomers were formed as a result of the reaction. The isomers were separated by column chromatography and identified by their NMR spectra.
3. Materials and Methods
Gas chromatography-mass spectrometry analysis was performed using Agilent 7890A gas chromatograph (Agilent, Santa Clara, CA, USA) equipped with Agilent MSD 5975C mass-selective detector with quadrupole mass-analyzer (electron impact ionization energy 70 eV), software version ChemStation MSD E.02.00.493. The injector temperature was maintained at 300 °C, and the injection volume was 1 μL (split 40:1). The instrument was equipped with an HP-5 ms capillary column of a 30 m length, a 0.25 mm i.d., and a 0.25 μm film thickness. The carrier gas was helium at a constant flow rate of 1 mL/min. The GC oven program started at 79 °C (1.0 min hold) ramped up to 300 °C (heating rate 13 °C/min) for 10 min, and total chromatogram time was 28 min. Transfer line temperature was 300 °C; MS Source—230 °C, and MS Quadrouple—150 °C. The electron energy was 70 eV. From 0 to 4 min, the MS was switched off (solvent delay). Data analysis and instrument control was carried out with MSD 5975C software. NMR spectra were recorded on Bruker DRX400 instrument (Bruker, Billerica, MA, USA) operating at 400 MHz for 1H and 100 MHz for 13C, solvent residual peaks were used as internal standards. Elemental analyses were carried out on a Carlo Erba (Val de Reuil, France) analyzer. IR-spectra of solid samples were recorded on Agilent Cary 630 FT IR (Agilent) spectrophotometer equipped with diamond ATR accessory.
1,3-Dibromoadamantane was prepared according to literature procedure [15], 1,2,4,-triazole (Acros organics) and all other reagents and solvents were used as received.
1,3-Bis(1,2,4-triazol-1-yl)adamantane (1). A powdered mixture of 1,3-dibromoadamantane (2.94 g, 10 mmol) and 1,2,4-triazole (2.76 g, 40 mmol) were heated in a sealed PTFE reaction vessel at 180 °C in an oven for 16 h. After cooling to room temperature, the glassy residue was dissolved in an ethanol–water (1:1) mixture and the solution was neutralized with 2.5 M NaOH. The resulting solution was extracted by chloroform. The solvent was removed on a rotary evaporator, 1.23 g of solid product was obtained. According to GC/MS analysis, it contained 89% Compound 1, 7% Compound 2, and 4% 1-(1,2,4-triazol-1-yl)-3-bromoadamantane (was not isolated). Products 1 and 2 were isolated and separated by column chromatography on silica gel, using MeOH-DCM, 1:6 mixture as a mobile phase (Rf for Compound 1 is 0.72; Rf for Compound 2 is 0.45). Yield of Compound 1: 41% (purity 97%), colorless crystals, m.p. 214–216 °C. 1H-NMR, δ, ppm (CDCl3): 1.82–2.60 m (14H, Adm), 7.96 (2H, H3-Tr), 8.21 (2H, H5-Tr). 13C-NMR, δ, ppm (CDCl3): 29.7 (5,7C-Adm), 34.6 (6C-Adm), 41.2 (4,8,9,10C-Adm), 47.2 (2C-Adm), 59.6 (1,3C-Adm), 139.5 (5C-Tr), 151.5 (3C-Tr). IR bands (cm−1): 3131 (νCH, Tr), 2913 and 2859 (νCH, Adm), 1500 (νTr), 1278 (δCH), 1211 (νTr), 1142 (νTr), 1018, 838 (δCH, Tr), 734 (δCC, Adm). Found: C, 62.12; H, 6.62; N, 30.85. Calculated for C14H18N6: C, 62.20; H, 6.71; N, 31.09.
1-(1,2,4-triazol-1-yl)-3-(1,2,4-triazol-4-yl)adamantane (2). Yield: 3%, colorless crystals, m.p. 262–264 °C. 1H-NMR, δ, ppm (CDCl3): 1.83–2.81 (14H, Adm), 7.95 (1H, 3C-Tr), 8.23 (1H, 5C-Tr), 8.42 (2H, 3,5C-Tr). 13C-NMR, δ, ppm (CDCl3): 29.7 (6C-Adm), 34.2 (5,7C-Adm), 40.9 (4,10C-Adm), 42.3 (8,9C-Adm), 48.2 (2C-Adm), 56.9 (3C-Adm), 59.3 (1C-Adm), 139.6 (5C-Tr), 139.7 (3,5C-Tr), 151.7 (3C-Tr). IR bands (cm−1): 3098 (νCH, Tr), 2938 and 2867 (νCH, Adm), 1499 (νTr), 1276 (δCH), 1197 (νTr), 1143 (νTr), 996, 852 (δCH, Tr), 724 (δCC, Adm), 664. Found: C, 62.42; H, 6.53; N, 30.79. Calculated for C14H18N6: C, 62.20; H, 6.71; N, 31.09.
4. Conclusions
In summary, we propose a simple procedure for the synthesis of the ligand 1,3-bis(1,2,4-triazol-1-yl)adamantane, which is interesting for its coordination chemistry and does not require the use of highly hazardous reagents. In addition, the unsymmetrical ligand 1-(1,2,4-triazol-1-yl)-3-(1,2,4-triazol-4-yl)adamantane can be isolated.
Supplementary Materials
The following are available online. Figure S1: Numbering scheme of 1,3-bis(1,2,4-triazol-1-yl)adamantane (1) and 1-(1,2,4-triazol-1-yl)-3-(1,2,4-triazol-4-yl)adamantane (2); Figure S2: Gas chromatogram of product mixture of the reaction between 1,3-dibromoadamantane and 1,2,4-triazole; Figure S3: Electron-impact mass-spectra of 1,3-bis(1,2,4-triazol-1-yl)adamantane (a), 1-(1,2,4-triazol-1-yl)-3-(1,2,4-triazol-4-yl)adamantane (b) and 1-(1,2,4-triazol-1-yl)-3-bromoadamantane (c); Figure S4: 1H-NMR spectrum of 1,3-bis(1,2,4-triazol-1-yl)adamantane (1); Figure S5: 13C-NMR spectrum of 1,3-bis(1,2,4-triazol-1-yl)adamantane (1); Figure S6: 1H-NMR spectrum of 1-(1,2,4-triazol-1-yl)-3-(1,2,4-triazol-4-yl)adamantane (2); Figure S7: 13C-NMR spectrum of 1-(1,2,4-triazol-1-yl)-3-(1,2,4-triazol-4-yl)adamantane (2).
Acknowledgments
The reported study was funded by the Russian Foundation for Basic Research (RFBR), according to the research project No. 16-33-60149 mol_а_dk.
Author Contributions
A.P. conceived and designed the experiments and wrote the paper; R.M. performed the synthesis, chromatographic separation, and identification of the products.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naik, R.; Jeon, C.-O.; Min, H.-Y.; Choi, H.-K.; Min, K.-H.; Lee, K. Synthesis and bioactivity of novel adamantyl derivatives as potent MDR reversal agents. Bull. Korean Chem. Soc. 2011, 32, 4444–4446. [Google Scholar] [CrossRef]
- Zarubaev, V.V.; Golod, E.L.; Anfimov, P.M.; Shtro, A.A.; Saraev, V.V.; Gavrilov, A.S.; Logvinov, A.V.; Kiselev, O.I. Synthesis and anti-viral activity of azolo-adamantanes against influenza A virus. Bioorg. Med. Chem. 2010, 18, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Xia, Y.; Kim, E.K.; Jin, Y.; Kaur, N.; Kim, E.S.; Kim, D.K.; Jung, H.Y.; Choi, Y.; Park, M.-K.; et al. A novel class of highly potent multidrug resistance reversal agents: Disubstituted adamantyl derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 5376–5379. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.E.; Alarcon, B.; Cabildo, P.; Claramunt, R.M.; Sanz, D.; Elguero, J. Synthesis and in vitro antiviral activity of some N-adamantylazoles and benzazoles. Eur. J. Med. Chem. Ther. 1985, 20, 359–362. [Google Scholar]
- Klimochkin, Y.N.; Shiryaev, V.A.; Leonova, M.V. Antiviral properties of cage compounds. New prospects. Russ. Chem. Bull. 2015, 64, 1473–1496. [Google Scholar] [CrossRef]
- Grillaud, M.; Bianco, A. Multifunctional adamantane derivatives as new scaffolds for the multipresentation of bioactive peptides. J. Pept. Sci. 2015, 21, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Marx, A. An adamantane-based building block for DNA networks. Chem. Asian J. 2011, 6, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-Q.; Guo, J.-W.; Zhu, D.-Y.; Yang, Z.; Yang, C.-F.; Xian, J.-X.; Li, X. Novel halogen-free flame retardants based on adamantane for polycarbonate. RSC Adv. 2015, 5, 67054–67065. [Google Scholar] [CrossRef]
- Singh, R.; Meena, J.S.; Chang, Y.-C.; Wu, C.-S.; Ko, F.-H. Control of active semiconducting layer packing in organic thin film transistors through synthetic tailoring of dielectric materials. RSC Adv. 2014, 4, 29383–29392. [Google Scholar] [CrossRef]
- Zhou, Y.; Brittain, A.D.; Kong, D.; Xiao, M.; Meng, Y.; Sun, L. Derivatization of diamondoids for functional applications. J. Mater. Chem. C 2015, 3, 6947–6961. [Google Scholar] [CrossRef]
- Tanaka, K.; Hiraoka, T.; Ishiguro, F.; Jeon, J.-H.; Chujo, Y. Adamantane ionic liquids. RSC Adv. 2014, 4, 28107–28110. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Liu, X.W.; Chen, L.Q.; Wang, S.Q.; Cheng, Y. Synthesis, structure and superoxide dismutase-like activity of two mixed-ligand Cu(II) complexes with N,N′-bis(2-pyridylmethyl)amantadine. Polyhedron 2016, 117, 788–794. [Google Scholar] [CrossRef]
- Natterer, A.; Adhikari, B.; Fyta, M. Complexes of carbene-functionalized diamondoids and metal atoms: Electronic properties. J. Organomet. Chem. 2016, 815–816, 8–15. [Google Scholar] [CrossRef]
- Boldog, I.; Domasevitch, K.V.; Sanchiz, J.; Mayer, P.; Janiak, C. 1,3,5,7-Tetrakis(tetrazol-5-yl)-adamantane: The smallest tetrahedral tetrazole-functionalized ligand and its complexes formed by reaction with anhydrous M(II)Cl2 (M = Mn, Cu, Zn, Cd). Dalton Trans. 2014, 43, 12590–12605. [Google Scholar] [CrossRef] [PubMed]
- Senchyk, G.A.; Lysenko, A.B.; Boldog, I.; Rusanov, E.B.; Chernega, A.N.; Krautscheid, H.; Domasevitch, K.V. 1,2,4-Triazole functionalized adamantanes: A new library of polydentate tectons for designing structures of coordination polymers. Dalton Trans. 2012, 41, 8675–8689. [Google Scholar] [CrossRef] [PubMed]
- Senchyk, G.A.; Lysenko, A.B.; Krautscheid, H.; Rusanov, E.B.; Chernega, A.N.; Krämer, K.W.; Liu, S.X.; Decurtins, S.; Domasevitch, K.V. Functionalized adamantane tectons used in the design of mixed-ligand copper(II) 1,2,4-triazolyl/carboxylate metal-organic frameworks. Inorg. Chem. 2013, 52, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Senchyk, G.A.; Lysenko, A.B.; Rusanov, E.B.; Chernega, A.N.; Krautscheid, H.; Domasevitch, K.V. Polynuclear and polymeric metal complexes based upon 1,2,4-triazolyl functionalized adamantanes. Inorg. Chim. Acta 2009, 362, 4439–4448. [Google Scholar] [CrossRef]
- Senchyk, G.A.; Lysenko, A.B.; Rusanov, E.B.; Chernega, A.N.; Jezierska, J.; Domasevitch, K.V.; Ozarowski, A. Structure and magnetic behavior of CuII MOFs supported by 1,2,4-triazolyl-bifunctionalized adamantane scaffold. Eur. J. Inorg. Chem. 2012, 2012, 5802–5813. [Google Scholar] [CrossRef]
- Maria Claramunt, R.; Cabildo, P.; Forfar, I.; Foces-Foces, C.; Liamas-Saiz, A.L.; Elguero, J. Adamantylation of N-unsubstituted pyrazole derivatives: Mechanistic and structural studies. Heterocycles 1994, 37, 1623–1636. [Google Scholar] [CrossRef]
- Cabildo, P.; Claramunt, R.M.; Elguero, J. Synthesis and reactivity of new l-(1-adamantyl)pyrazoles. J. Heterocycl. Chem. 1984, 21, 249–251. [Google Scholar] [CrossRef]
- Cabildo, P.; Claramunt, R.M.; Sanz, D.; Foces-Foces, M.C.; Hernandez Cano, F.; Catalan, J.; Elguero, J. Structure of 1-(1-adamantyl)pyrazoles. Tetrahedron 1985, 41, 473–478. [Google Scholar] [CrossRef]
- Cabildo, P.; Claramunt, R.M.; Forfar, I.; Elguero, J. Regioselective adamantylation of N-unsubstituted pyrazole derivatives. Tetrahedron Lett. 1994, 35, 183–184. [Google Scholar] [CrossRef]
- Wei, Z.; Li, J.; Wang, N.; Zhang, Q.; Shi, D.; Sun, K. Solvent-free and direct C(sp3)-H amination of adamantanes by grinding. Tetrahedron 2014, 70, 1395–1400. [Google Scholar] [CrossRef]
- Zatonskaya, L.V.; Schepetkin, I.A.; Petrenko, T.V.; Ogorodnikov, V.D.; Khlebnikov, A.I.; Potapov, A.S. Synthesis and cytotoxicity of bis(pyrazol-1-yl)-alkane derivatives with Polymethylene Linkers and Related Mono- and Dipyrazolium Salts. Chem. Heterocycl. Compd. 2016, 52, 388–401. [Google Scholar] [CrossRef]
- Barsukova, M.O.; Samsonenko, D.G.; Goncharova, T.V.; Potapov, A.S.; Sapchenko, S.A.; Dybtsev, D.N.; Fedin, V.P. Coordination polymers with adjustable dimensionality based on CuII and bis-imidazolyl bridging ligand. Russ. Chem. Bull. 2016, 65, 2914–2919. [Google Scholar] [CrossRef]
- Domina, G.A.; Potapov, A.S.; Khlebnikov, A.I.; Ogorodnikov, V.D. Synthesis of 1,8-di(pyrazol-1-yl)- 3,6-dioxaoctane and its derivatives. Russ. J. Org. Chem. 2009, 45, 1224–1228. [Google Scholar] [CrossRef]
- Potapov, A.S.; Nudnova, E.A.; Khlebnikov, A.I.; Ogorodnikov, V.D.; Petrenko, T.V. Synthesis of new polydentate pyrazolyl-ethene ligands by interaction of 1H-pyrazole and 1,1,2,2-tetrabromoethane in a superbasic medium. J. Heterocycl. Chem. 2011, 48, 645–651. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).