Abstract
The copper complex of 5,10,15,20-tetrakis-(3,4-dibenzyloxyphenyl)porphyrin (CuTDBOPP) was synthesized and characterized with electronic absorption spectroscopy and ESI high-resolution spectrometry. In the electronic spectrum, there was a shift in the Soret band from 424 nm to 420 nm that indicated that the insertion of the metal ion was successful. Additionally, the number of Q bands decreased from four peaks to a single peak at 541 nm. The molar absorptivity of the Soret and Q band are 3.4 × 105 cm−1·M−1 and 1.8 × 105 cm−1·M−1, respectively. The ESI HRMS was in excellent agreement with simulated isotopic distribution spectra. CuTDBOPP was incorporated into a series of Gratzel cells where the open current voltage was recorded.
1. Introduction
It is well established in the literature that porphyrins are an important class of macrocyclic molecules [1,2,3]. They are an integral part of energy production for both plants (photosynthesis) and animals (cellular respiration) [4]. Therefore, porphyrins are of obvious interest when considering ways to produce renewable energy, particularly solar energy. Since the 1980s, porphyrins and phthalocyanins have been utilized in organic thin film photovoltaic cells with some success [5,6]. More recently, porphyrins have been proven to be very successful in a hybrid organic-inorganic solar cell known as the Gratzel cell [7,8,9]. The focus of this research is to report the synthesis and characterization of the copper complex of 5,10,15,20-tetrakis-(3,4-dibenzyloxyphenyl)porphyrin (CuTDBOPP). Electronic absorption spectroscopy confirms the insertion of the copper into the base porphyrin and high-resolution electrospray ionization mass spectrometry is in excellent agreement with the simulated isotopic distribution spectra of CUTDBOPP. The metallic chromophore has been incorporated into a Gratzel cell, and preliminary results are encouraging.
2. Results
2.1. Copper 5,10,15,20-Tetrakis-(3,4-dibenzyloxyphenyl)porphyrin
5,10,15,20-Tetrakis-(3,4-dibenzyloxyphenyl)porphyrin (TDBOPP) was synthesized as previously reported [10]. Copper 5,10,15,20-Tetrakis-(3,4-dibenzyloxyphenyl)porphyrin was prepared using TDBOPP (0.1580 g, 1.079 × 10−4 mol) in 50.0 mL of boiling chloroform. A saturated solution of copper(II) acetate tetrahydrate was prepared in methanol and 3 mL was added to the boiling solution of TDBOPP, as indicated in Figure 1. The reaction was monitored with electronic absorption spectroscopy. After refluxing for 5 min, the absorption spectrum of the mixture was found to have a λmax of 420 nm. The blue shift of the absorption from 424 nm indicated that the reaction was completed. The crude CuTDBOPP was purified using a 400 mesh silica gel column with CHCl3 mobile phase. The eluent was fractionated and spectroscopically tested to isolate the metalated molecule. The CuTDBOPP was visibly different from the other fractions due to the bright reddish-purple color. The eluent was evaporated to dryness and the residue was dried in a vacuum oven at 60 °C. For the 0.1580 g of TDBOPP used, 0.1389 g (9.107 × 10−5 mol) of pure product was obtained, yield 84%. UV-vis (CHCl3) [λ, nm (ε, cm−1·M−1)], 420 (3.4 × 105), 541 (1.8 × 105); HRMS (ESI) m/z calcd for [M]+ CuC100H76N4O8: 1523.4954, Found: 1523.4954. Anal. calcd. for CuC100H76N4O8: C, 78.74; H, 5.02; N, 3.67. Found: C, 78.54; H, 5.45; N, 3.48.
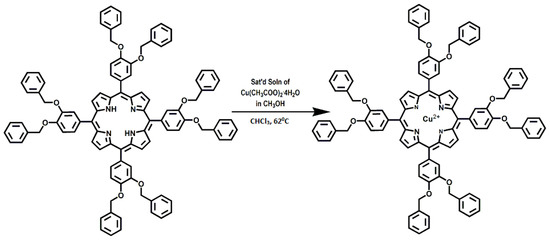
Figure 1.
Synthesis of Copper 5,10,15,20-Tetrakis-(3,4-dibenzyloxyphenyl)porphyrin.
2.2. Construction of Gratzel Cell
A Gratzel cell was constructed to test the suitability of the copper porphyrin for use as a light harvesting dye. The cell construction protocol is well-known [11,12]. A square inch indium tin oxide (ITO) coated conductive glass slide was screen-printed with a commercially formulated TiO2 paste. The TiO2 was sintered at 500 °C for 4 h. The porphyrin was dissolved in DMSO to make an 8.39 × 10−2 mM solution and the ITO glass slide was allowed to soak in the solution in the dark for 24 h. The slide was removed from the solution and washed with ethanol to remove any trace of water. A second ITO glass slide, coated with graphite, was prepared to use as a counter electrode by drilling a small hole to allow for the introduction of the electrolyte solution. The slides were sandwiched together with a piece of 60 micron Surlyn hot melt film between the two. The counter electrode side of the cell was exposed briefly to a 100 °C hot plate to allow the hot melt film to create a secure connection between the two pieces of glass. The electrolyte solution was added to the cell as the final step of the cell construction. Once the cell was fabricated, it was tested without delay using an Abet AM 1.5 calibrated solar simulator (Abet Technologies, Milford, CT, USA). A Keithley 4201 sourcemeter (Tektronix, Beaverton, OR, USA). was used to obtain voltage readings. Four cells were constructed. The following open circuit voltages, Voc, were measured (standard deviation): 461.1 mV (3.8 mV); 462.3 mV (1.9 mV); 454.1 mV (2.3 mV); 447.2 (0.5 mV).
3. Discussion
The electronic absorption spectra of porphyrins are known to be affected by the substituents attached to the macrocycle, the position of those substituents, and, in the case of metalloporphyrins, the identity of the complexed metal ion [13]. In this study, a hypsochromic shift was expected due to the open shell electron configuration of the copper (II) ion. This shift occurs due to the metal-to-ring charge transfer, which increases the energy of the π to π* transition in the porphyrin ligand [4]. This shift was observed as the λmax shifted from 424 nm to 420 nm after the metal was inserted into the porphyrin. Additionally, the increased symmetry of the metalated porphyrin decreased the number of Q bands from four to one [4]. The base porphyrin exhibited fluorescence at 650 nm; however, that was quenched upon insertion of the copper.
The suitability of a dye in a Gratzel cell is determined by many factors. Primarily, the dye must absorb electromagnetic radiation in the range that terrestrial earth receives. How strongly the dye absorbs is also critically important to the overall efficiency of the cell. Porphyrins are well known for their large molar absorptivity in the visible range of the electromagnetic spectrum. The ability to tune the spectral properties of the dye is a plus, which is easy to achieve with porphyrins by changing the substituents on the macrocycle and to some extent by changing the metal ion. These spectral properties make porphyrins ideal candidates for the Gratzel solar cells. Most of the porphyrins currently employed are complex and difficult to synthesize. From a fundamental viewpoint, highly symmetrical porphyrins, such as CuTDBOPP, are more useful because their synthetic ease facilitates the ability to change substituents and make meaningful comparisons between the efficiencies of solar cells constructed with the different porphyrins. The preliminary results with CuTDBOPP are encouraging. Currently, the porphyrin (SM315) solar cell with the highest efficiency (13%) boasts a Voc of 910 mV [9]. While our CuTDBOPP cell Voc is approximately 52% of that value, it is important to point out that a higher Voc does not always translate directly into a higher efficiency. As was seen in that same study, a different porphyrin (SM371) was used that had a Voc of 960 mV but an efficiency of only 12%. The next step is to study current density and the overall cell efficiency for the CuTDBOPP cell. The excellent spectral properties of CuTDBOPP and the Voc measured from the non-optimized cell show great promise for future development.
4. Materials and Methods
4.1. Chemicals and Reagents
Reagents and solvents used in the synthesis of CuTDBOPP and electronic absorption spectroscopy measurements were purchased from Sigma Aldrich Co. (St. Louis, MO, USA) and used as received. Titanium dioxide screen-printing 18NR-AO paste and 50 micron Surlyn hot melt film were purchased from Dyesol, Queanbeyan, Australia. Indium tin oxide conductive glass slides and iodide-tri-iodide electrolyte solution was purchased from ICE, University of Wisconsin-Madison. All other reagents and solvents used in the preparation and construction of the solar cell were purchased from Sigma Aldrich Co. and used as received.
4.2. Instrumentation
UV-visible absorption spectra were recorded on a JASCO V-530 double beam UV-Vis spectrophotometer (JASCO, Easton, MD, USA). Fluorescence spectra were taken on a JASCO FP-6300 spectrofluorometer (JASCO, Easton, MD, USA). The high-resolution mass spectra were taken on a Thermo Orbitrap Elite mass spectrometer (ThermoFisher Scientific, Waltham, MA, USA) which was analyzed at positive ion mode of regular electrospray ionization with a spray voltage of 4.0 kV. An Abet AM 1.5 solar simulator was used to irradiate the solar cell, and the voltage measurements were obtained using a Keithley 2401 sourcemeter.
5. Conclusions
The copper was successfully inserted into the base porphyrin. The expected changes in the spectral properties of the porphyrin were observed. The preliminary solar cell studies show encouraging results, and future work is planned to determine the current density and overall cell efficiency of this and other copper porphyrins.
Supplementary Materials
The following are available online: Figure S1: ESI-HRMS spectra of synthesis product; Figure S2: Comparison of theoretical isotopic distribution to experimental data. Figure S3: Electronic spectra before and after metalation; Table S1: Open circuit voltage of CuTDBOPP solar cells.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
This work was funded by in part by the Research and Special Projects Committee of the University of Montevallo, the Undergraduate Research Program of the University of Montevallo, and the Department of Biology, Chemistry, and Mathematics of the University of Montevallo. We would like to thank Dennis Phillips and Chau-Wen Chou at the Proteomics and Mass Spectrometry Facility at the University of Georgia for the HRMS analysis.
Author Contributions
C.P.T. designed the molecule. C.P.T. and P.B. designed the synthesis of the molecule; C.P.T., P.B., and T.Y.S. worked out the synthesis and purification of the molecule and performed preliminary spectroscopic investigation of the molecule. C.P.T. designed the spectroscopic investigation experiment. L.D.M., G.S., and A.C. repeated the synthesis of the molecule and performed the spectroscopic experiment of the molecule; L.D.M. analyzed the data; C.P.T. contributed reagents and materials; L.D.M. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dolphin, D. The Porphyrins; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Kobayashi, K.; Masuda, T. Tetrapyrrole Biosynthesis in Plant Systems. In Handbook of Porphyrin Science; Kadish, K., Smith, K., Guilard, R., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2012; pp. 141–211. [Google Scholar]
- Drain, C.M.; Singh, S. Combinatorial Libraries of Porphyrins: Chemistry and Applications. In Handbook of Porphyrin Science; Kadish, K., Smith, K., Guilard, R., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2010; pp. 485–537. [Google Scholar]
- Milgrom, L.R. The Colours of Life: An Introduction to the Chemistry of Porphyrins and Related Compounds; Oxford University Press Inc.: New York, NY, USA, 1997. [Google Scholar]
- Hasobe, T. Supramolecular Assemblies of Porphyrin and Phthalocyanine Derivatives for Solar Energy Conversion and Molecular Electronics. In Handbook of Porphyrin Science; Kadish, K., Smith, K., Guilard, R., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2014; pp. 148–194. [Google Scholar]
- Tebo, A.; Herrero, C.; Aukauloo, A. Porphyrins and Metalloporphrins as Components in Artificial Photosythesis Research. In Handbook of Porphyrin Science; Kadish, K., Smith, K., Guilard, R., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2014; pp. 196–233. [Google Scholar]
- Li, L.-L.; Diau, E.W.-G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.M.; Jolley, K.W.; Wagner, P.; Wagner, K.; Walsh, P.J.; Gordon, K.C.; Schmidt-Mende, L.; Nazeeruddin, M.K.; Wang, Q.; Gratzel, M.; et al. Highly efficient porphyrin sensitizers for dye-sensitized solar cells. J. Phys. Chem. C 2007, 111, 11760–11762. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Tidwell, C.P. Synthesis and Characterization of 5,10,15,20-Tetrakis-(3,4-dibenzyloxyphenyl)porphyrin. 2016; Submitted for publication. [Google Scholar]
- Ito, S.; Nazeeruddin, M.K.; Liska, P.; Comte, P.; Charvet, R.; Péchy, P.; Jirousek, M.; Kay, A.; Zakeeruddin, S.M.; Grätzel, M. Photovoltaic characterization of dye-sensitized solar cells: Effect of device masking on conversion efficiency. Prog. Photovoltaics Res. Appl. 2006, 14, 589–601. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A. Transition Metal Complexes of Porphyrins and Porphyrinoids. In Handbook of Porphyrin Science; Kadish, K., Smith, K., Guilard, R., Eds.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2012; pp. 304–414. [Google Scholar]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).