3-{4-[(E)-{4-[(E)-Phenyldiazenyl]phenyl}diazenyl]phenoxy}propane-1,2-diol
Abstract
:1. Introduction
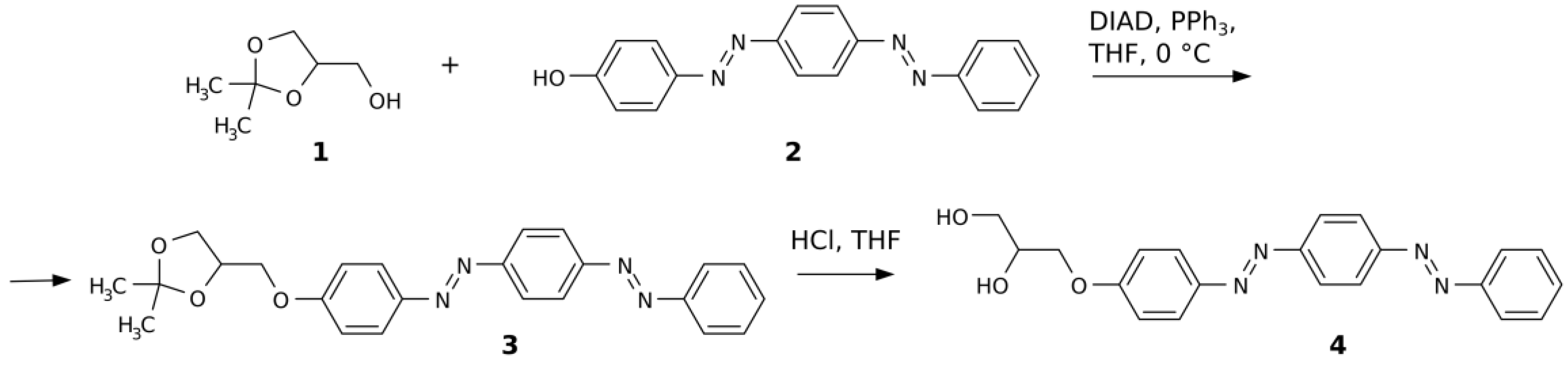
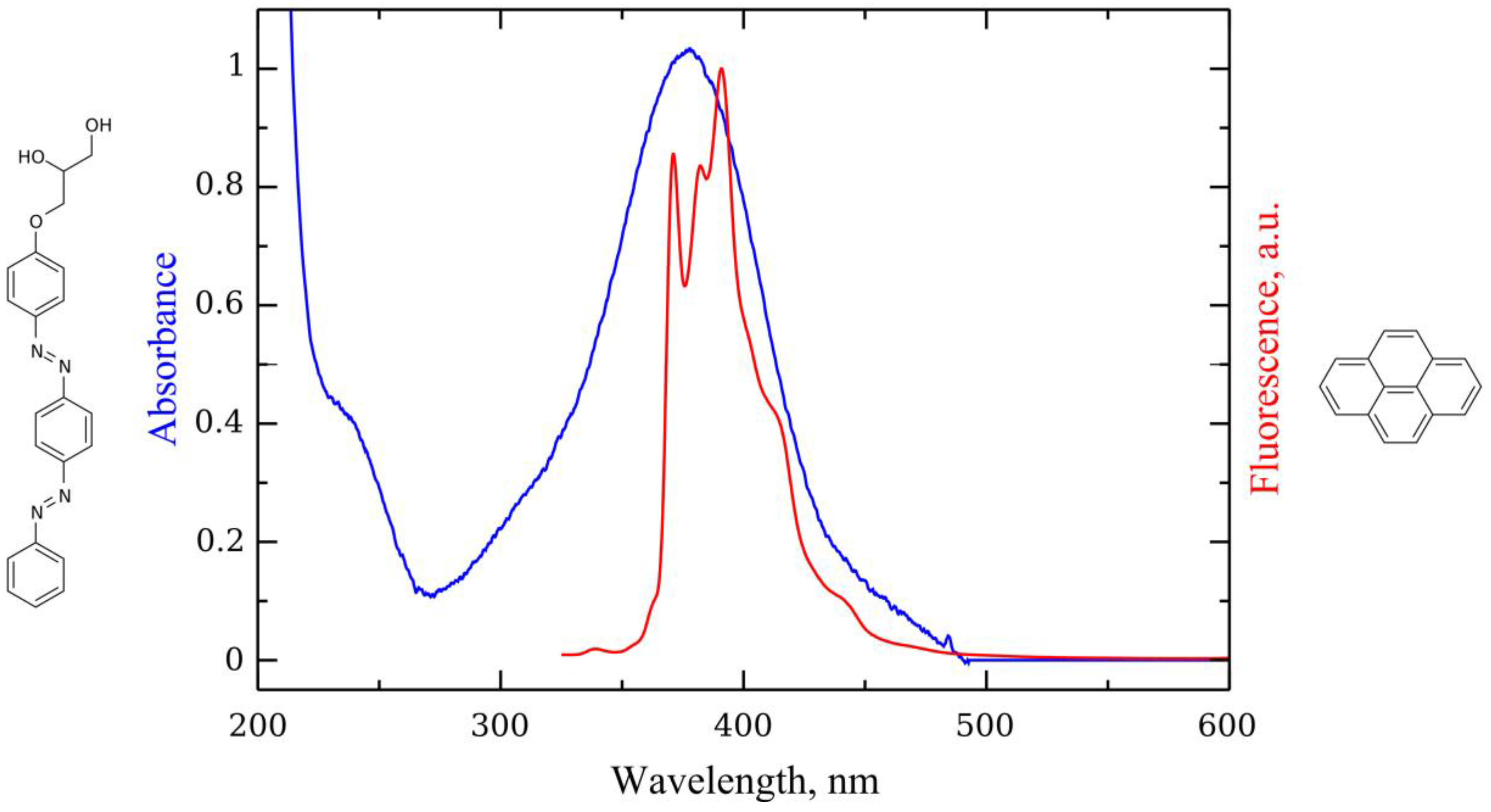
2. Results and Discussion
3. Materials and Methods
3-{4-[(E)-{4-[(E)-Phenyldiazenyl]phenyl}diazenyl]phenoxy} Propane-1,2-diol
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Borrell, J.H.; Montero, M.T.; Morros, A.; Domènech, Ò. Unspecific membrane protein-lipid recognition: Combination of AFM imaging, force spectroscopy, DSC and FRET measurements. J. Mol. Recogn. 2015, 28, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Marquette, A.; Salnikov, E.S.; Glattard, E.; Aisenbrey, C.; Bechinger, B. Magainin 2-PGLa interactions in membranes—Two peptides that exhibit synergistic enhancement of antimicrobial activity. Cur. Top. Med. Chem. 2016, 16, 65–75. [Google Scholar] [CrossRef]
- Ye, X.; McLean, M.A.; Sligar, S.G. Conformational equilibrium of talin is regulated by anionic lipids. Biochim. Biophys. Acta 2016, 1858, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Gorbenko, G.; Trusova, V.; Girych, M.; Adachi, E.; Mizuguchi, C.; Akaji, K.; Saito, H. FRET evidence for untwisting of amyloid fibrils on the surface of model membranes. Soft Matter 2015, 11, 6223–6234. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Coutinho, A.; Prieto, M.; Loura, L.M.S. Electrostatically driven lipid-protein interaction: Answers from FRET. Biochim. Biophys. Acta 2015, 1848, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Bagatolli, L.A. To see or not to see: Lateral organization of biological membranes and fluorescence microscopy. Biochim. Biophys. Acta 2006, 1758, 1541–1556. [Google Scholar] [CrossRef] [PubMed]
- Feigenson, G.W.; Buboltz, J.T. Ternary Phase Diagram of Dipalmitoyl-PC/Dilauroyl-PC/Cholesterol: Nanoscopic Domain Formation Driven by Cholesterol. Biophys. J. 2001, 80, 2775–2788. [Google Scholar] [CrossRef]
- Šachl, R.; Johansson, L.B.-Å.; Hof, M. Förster resonance energy transfer (FRET) between heterogeneously distributed probes: Application to lipid nanodomains and pores. Int. J. Mol. Sci. 2012, 13, 16141–16156. [Google Scholar] [CrossRef] [PubMed]
- Simanshu, D.K.; Kamlekar, R.K.; Wijesinghe, D.S.; Zou, X.; Zhai, X.; Mishra, S.K.; Molotkovsky, J.G.; Malinina, L.; Hinchcliffe, E.H.; et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature 2013, 500, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Malinina, L.; Simanshu, D.K.; Zhai, X.; Samygina, V.R.; Kamlekar, R.; Kenoth, R.; Ochoa-Lizarralde, B.; Malakhova, M.L.; Molotkovsky, J.G.; Patel, D.J.; et al. Sphingolipid transfer proteins defined by the GLTP-fold. Q. Rev. Biophys. 2015, 48, 281–322. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, A.S.; Korotaeva, A.A.; Samoilova, E.V.; Volynsky, P.E.; Vodovozova, E.L.; Boldyrev, I.A. Secretory phospholipase A2 activity in blood serum: The challenge to sense. Biochem. Biophys. Res. Commun. 2014, 454, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, A.S.; Tretiakova, D.S.; Melnikova, D.N.; Molotkovsky, U.G.; Boldyrev, I.A. Novel fluorescent membrane probe 2,3;5,6-bis(cyclohexyl)-BODIPY-labeled phosphatidylcholine. Russ. J. Bioorg. Chem. 2016, 42, 305–307. [Google Scholar] [CrossRef]
- Manhas, M.S.; Hoffman, W.H.; Lal, B.; Bose, A.K. Steroids. Part X. A convenient synthesis of alkyl aryl ethers. J. Chem. Soc. Perkin Trans. 1975, 1, 461–463. [Google Scholar]
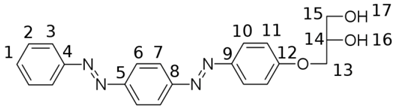 | ||
| Heavy Atom | 1H | 13C |
| 1 | 7.62 | 132.4 |
| 2 | 7.64 | 130.0 |
| 3 | 7.95 | 123.2 |
| 4 | 152.5 | |
| 5 | 153.8 | |
| 6 | 8.06 | 123.9 |
| 7 | 8.09 | 124.2 |
| 8 | 153.1 | |
| 9 | 146.7 | |
| 10 | 7.96 | 125.4 |
| 11 | 7.18 | 115.7 |
| 12 | 162.6 | |
| 13 | 4.02, 4.16 | 70.7 |
| 14 | 3.86 | 70.3 |
| 15 | 3.49 | 63.0 |
| 16, 17 | 5.03, 4.72 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chupin, V.V.; Boldyrev, I.A. 3-{4-[(E)-{4-[(E)-Phenyldiazenyl]phenyl}diazenyl]phenoxy}propane-1,2-diol. Molbank 2017, 2017, M932. https://doi.org/10.3390/M932
Chupin VV, Boldyrev IA. 3-{4-[(E)-{4-[(E)-Phenyldiazenyl]phenyl}diazenyl]phenoxy}propane-1,2-diol. Molbank. 2017; 2017(1):M932. https://doi.org/10.3390/M932
Chicago/Turabian StyleChupin, Vladimir V., and Ivan A. Boldyrev. 2017. "3-{4-[(E)-{4-[(E)-Phenyldiazenyl]phenyl}diazenyl]phenoxy}propane-1,2-diol" Molbank 2017, no. 1: M932. https://doi.org/10.3390/M932
APA StyleChupin, V. V., & Boldyrev, I. A. (2017). 3-{4-[(E)-{4-[(E)-Phenyldiazenyl]phenyl}diazenyl]phenoxy}propane-1,2-diol. Molbank, 2017(1), M932. https://doi.org/10.3390/M932





