Abstract
N-[2-(Cyclohexylamino)-2-oxoethyl]-N-(4-octyloxy)phenyl-prop-2-enamide was prepared in good yield by coupling of 4(octyloxy)aniline, Cyclohexyl isocyanide, paraformaldehyde and acrylic acid by multicomponent Ugi reaction, at room temperature. The structure of the newly synthesized tripeptoid derivative was well characterized using elemental analysis, FTIR, NMR and mass spectral data.
1. Introduction
The field of peptidomimetics has seen an exciting development over the past several years, partly due to the considerable biological importance and putative proteolytic stability of synthetic peptoids over native peptides [1,2,3]. Recently, Ugi coupling [4,5,6,7,8] of various carboxylic acids, amines, isocyanates and aldehydes to a series of tripeptoids has been found to give excellent yields and these have also been reported to exhibit antifungal and antimicrobial activities [9,10,11,12,13]. In addition to such applications, tripeptoids also find application as low molecular weight gelators (LMWGs) [14,15]. The self-assembling nature of low molecular weight organic compounds to form physical gels by non-covalent interactions such as hydrogen bonding, van der Waals interactions, π–π stacking, etc. [16] can be attributed to the presence of flexible solvophilic aliphatic groups and rigid solvophobic functional groups. Also, previous exploration of cyclohexyl moiety in the tripeptoids generally shows effective gelation properties [17]. Quite recently, Biswas et al. reported a new class of peptoid-based low molecular weight organogelators by the aza-Michael addition reaction between glycinamide and substituted alkyl acrylates [18]. In this study, we report the preparation of new tripeptoid with functional acryl and cyclohexyl moiety.
The synthesis of the title compound was achieved by one-pot Ugi multi-component reaction from less expensive raw materials. The synthetic value of the resulting compound can be diverged as a precursor for aza-Michael addition reaction to design varieties of peptoid-based LMWGs. The structure of the title compound was confirmed by 1H, 13C-NMR, FTIR, and mass spectral data.
2. Results and Discussion
N-[2-(Cyclohexylamino)-2-oxoethyl]-N-(4-octyloxy)phenyl-prop-2-enamide was synthesized from Ugi four-component (4C) reaction involving 4(octyloxy)anile, paraformaldehyde, acrylic acid and cyclohexyl isocyanide at ambient conditions for 20 h, as shown in Scheme 1. The physical appearance of the compound is off white solid, insoluble in water, non-polar solvents like n-hexane, and completely soluble in dichloromethane, chloroform, methanol, ethanol and dimethyl sulfoxide.
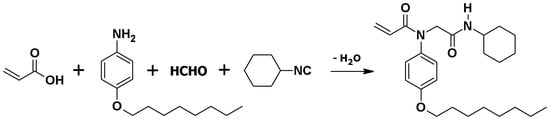
Scheme 1.
Synthesis of N-[2-(cyclohexylamino)-2-oxoethyl]-N-(4-octyloxy)phenyl-prop-2-enamide.
The 1H and 13C-NMR spectrum data of the title compound is in good agreement with the proposed structure. The 1H-NMR spectrum of the compound is depicted in Figure 1. The signals corresponding to aliphatic, aromatic and amide functional groups are assigned to their positions. The spectrum shows multiplet signals at δ 0.88, 1.18, 1.31, and 1.87 ppm due to aliphatic protons of octyl group. The protons of cyclohexyl group was resonated at 3.76 and in the region of 1.28–1.77 ppm. The acryl functional protons are resonated at δ 6.09 and 5.56 ppm.
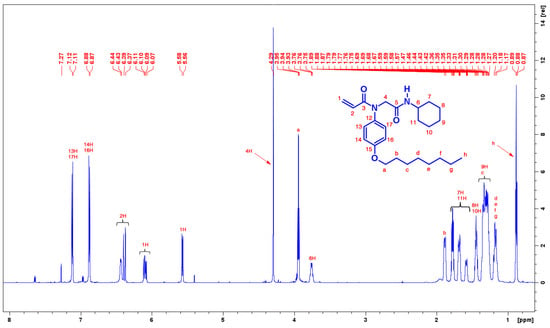
Figure 1.
1H-NMR spectra of desired compound (700 MHz, CDCl3) at room temperature.
13C-NMR spectrum of the compound with assigned numbering is displayed in Figure 2. Several set of signals are cited in the spectrum. The two carbonyl carbons 3 and 5 were cited at 158.8 and 167.8 ppm respectively. The carbon-15 of the benzene attached to the oxygen resonates at 166.7 ppm. The carbon-5 is more deshielded to compare with the carbon-5, as the carbon-5 is attached directly to oxygen and nitrogen, where the combined effect of these electronegative atoms downfield the 13C signal and this effect is diminished in carbon-3. Wherein, the nitrogen is involved in resonance. The carbon 1 and 2 of the acryl moiety resonates at 127.9 and 128.5 respectively.
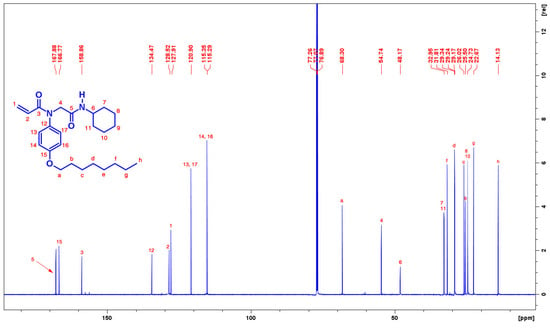
Figure 2.
13C-NMR spectra of desired compound (176 MHz, CDCl3) at room temperature.
3. Experimental Section
3.1. Materials
4(octyloxy)aniline, cyclohexyl isocyanide, paraformaldehyde, acrylic acid, n-hexane and methanol were procured from Sigma Aldrich (Prague, Czech Republic). All the other laboratory chemicals and solvents were of analytical reagent (AR) grade and used without further purification. Double distilled water was used throughout the study.
3.2. Instrumentation
The 1H & 13C-NMR spectra were recorded on a 700 MHz Bruker Avance III HD spectrometer (with a 5 mm dual broad-band probe, 5 mm dual inverse broad-band probe, 1.7 mm triple resonance (1H–13C) probe (Bruker, Leiderdorp, The Netherlands) for multinuclear applications in liquids and solids using tetramethylsilane (TMS) as an internal standard. LCMS measurement was performed on Liquid chromatography system with mass spectrometry detection (Q-TOF)—Agilent ACCURATE MASS 6530 Q TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA). The elemental analysis was carried on a VARIO EL III Elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany) and the results for C, H, and N were within 0.4% of the theoretical values. FTIR analysis was carried out on Nicolet 6700 (ATR, Thermo Scientific, Waltham, MA, USA) in the scanning range 4000–600 cm−1. The resolution was 4 cm−1 with 64 scans and the measurement was done with Germanium crystal.
3.3. Synthesis of N-[2-(Cyclohexylamino)-2-oxoethyl]-N-(4-octyloxy)phenyl-prop-2-enamide
Paraformaldehyde (0.042 g, 1.38 mmol) was placed in a 50 mL of round bottomed flask, and then added solution of 4(octyloxy)aniline (0.305 g, 1.38 mmol in methanol (10 mL). The mixture was stirred at room temperature for 2 h. Later, acrylic acid (0.1 g, 1.38 mmol) was added followed by cyclohexyl isocyanide (0.152 g, 1.38 mmol). The completion of the reaction was monitored using Thin-layer chromatography (TLC) analysis (performed with Merck Kieselgel 60 F 254 plates, Merck Millipore, Bangalore, India). The formation of product was confirmed at 0.36 Rf (1:1 EtOAc:n-hexane) after 20 h. The reaction mixture was diluted with 20 mL ethyl acetate (EtOAc), washed with water (15 mL × 4), followed by saturated solution of brine (20 mL). The collected organic layer was dried over anhydrous sodium sulfate and solvent was removed under reduced pressure to collect the crude product (0.571 g). The crude product was triturated repeatedly using n-hexane to obtain the title compound in pure form as an off white solid (0.48 g) in 84% of yield.
Off White Solid; m.p. 199–201 °C; 1H-NMR (700 MHz, CDCl3): δ 0.88 (t, 3H, J = 7.0 Hz), 1.17–1.20 (m, 8H), 1.27–1.36 (m, 4H), 1.42–1.47 (m, 4H), 1.57–1.69 (m, 4H), 1.75–1.89 (m, 2H), 3.76 (m, 1H), 3.94 (t, 3H, J = 7.0 Hz), 4.29 (s, 2H), 5.57 (d, 1H, J = 14.0 Hz), 6.07–6.11 (m, 1H), 6.37–6.44 (m, 1H), 6.87–6.88 (d, 2H, J = 7.0 Hz), 7.11–7.12 (d, 2H, J = 7.0 Hz), 13C-NMR (176 MHz, CDCl3): (ppm) 167.8, 166.7, 158.8, 134.4, 128.5, 127.9, 120.9, 115.2, 68.3, 54.7, 48.1, 32.9, 31.8, 29.2, 26.0, 25.5, 24.7, 22.6 and 14.1. Anal. calcd. for C25H38N2O3 (414.580): C, 72.43; H, 9.24; N, 6.76. Found: C, 72.77; H, 9.56; N, 7.04. LCMS (ESI): m/z = 415.297 (Found), 415.2961 (Expected for [M + H]+). FTIR (Ge Crystal): 3282, 3083, 2936 cm−1 (-NH Stretch), 2867 cm−1 (C-H Stretch of cyclohexane ring); 1647, 1708 cm−1 (C=O); 1120 cm−1 (C-O-C); 1422 cm−1 (C-H scissoring); 1370 cm−1 (C-H methyl rock); 722 cm−1 (Long chain methyl rock).
Supplementary Materials
LCMS and FTIR spectra for the title compound are available online at www.mdpi.com/1422-8599/2016/4/M921.
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
The work was principally supported by MŠMT ČR-USA Kontakt II (LH14050) and the Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231. This research was also partially supported by the Ministry of Education, Youth and Sports of the Czech Republic - NPU Program I (LO1504).
Author Contributions
S.D.G., designed the synthesis, carryout the experiments for the title compound synthesis and wrote the article. N.S. and P.S., confirmed the data analysis. P.K., done the LCMS measurement. All of the authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Simon, R.J.; Kania, R.S.; Zuckermann, R.N.; Huebner, V.D.; Jewell, D.A.; Banville, S.; Ng, S.; Wang, L.; Rosenberg, S.; Marlowe, C.K.; et al. Peptoids: A modular approach to drug discovery. Proc. Natl. Acad. Sci. USA 1992, 89, 9367–9371. [Google Scholar] [CrossRef]
- Zuckermann, R.N. Peptoid Origins. Biopolymers (PeptSci) 2011, 96, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N.; Kodadek, T. Peptoids as potential therapeutics. Curr. Opin. Mol. Ther. 2009, 11, 299–307. [Google Scholar] [PubMed]
- Váradi, A.; Palmer, T.C.; Dardashti, R.N.; Majumdar, S. Isocyanide-Based Multicomponent Reactions for the Synthesis of Heterocycles. Molecules 2016, 21, 19. [Google Scholar] [CrossRef]
- Koopmanschap, G.; Ruijter, E.; Orru, R.V.A. Isocyanide-based multicomponent reactions towards cyclic constrained peptidomimetics. Beilstein J. Org. Chem. 2014, 10, 544–598. [Google Scholar] [CrossRef] [PubMed]
- El Kaim, L.; Grimaud, L.; Oble, J. Phenol Ugi-Smiles Systems: Strategies for the Multicomponent N-Arylation of Primary Amines with Isocyanides, Aldehydes, and Phenols. Angew. Chem. Int. Ed. 2005, 44, 7961–7964. [Google Scholar] [CrossRef] [PubMed]
- Tye, H.; Whittaker, M. Use of a Design of Experiments approach for the optimisation of a microwave assisted Ugi reaction. Org. Biomol. Chem. 2004, 2, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.; Chen, F.-L.; Huang, P.-C. A Modified U-4CR Reaction with 2-Nitrobenzylamine as an Ammonia Equivalent. Synlett 2006, 2667–2669. [Google Scholar] [CrossRef]
- Galetti, M.D.; Cirigliano, A.M.; Cabrera, G.M.; Ramírez, J.A. Multicomponent synthesis of acylated short peptoids with antifungal activity against plant pathogens. Mol. Divers. 2012, 16, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Liu, J.; Xie, Z.; Luan, S.; Xiao, C.; Tao, Y.; Wang, X. Ugi Reaction of Natural Amino Acids: A General Route toward Facile Synthesis of Polypeptoids for Bioapplications. ACS Macro Lett. 2016, 5, 1049–1054. [Google Scholar] [CrossRef]
- Ghasemi, E.; Shahvelayati, A.S.; Yavari, I. Ugi reaction of thiouridocarboxylic acids: A synthesis of thiourea-peptoids. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 746–750. [Google Scholar] [CrossRef]
- Silva, E.H.B.; Emery, F.S.; Ponte, G.D.; Donate, P.M. Synthesis of Some Functionalized Peptomers via Ugi Four-Component Reaction. Synth. Commun. 2015, 45, 1761–1767. [Google Scholar] [CrossRef]
- Savithri, A.; Thulasi, S.; Varma, R.L. Narrow-rim functionalization of calix[4]arene through Ugi-4CR: Synthesis of a series of calix[4]arene peptoids. J. Org. Chem. 2014, 79, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Mangunuru, H.P.R.; Yang, H.; Wang, G. Synthesis of peptoid based small molecular gelators by a multiple component reaction. Chem. Commun. 2013, 49, 4489–4491. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.C.N.; Neves, R.A.W.; Westermann, B.; Heinke, R.; Wessjohann, L.A. Synthesis of antibacterial 1,3-diyne-linked peptoids from an Ugi-4CR/Glaser coupling approach. Beilstein J. Org. Chem. 2015, 11, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Joo, M.K.; Sohn, Y.S.; Jeong, B. Reverse thermal organogel. Adv. Mater. 2007, 19, 3947–3950. [Google Scholar] [CrossRef]
- Wang, G.; Cheuk, S.; Yang, H.; Goyal, N.; Reddy, P.V.N.; Hopkinson, B. Synthesis and Characterization of Monosaccharide-Derived Carbamates as Low-Molecular-Weight Gelators. Langmuir 2009, 25, 8696–8705. [Google Scholar] [CrossRef] [PubMed]
- Biswas, G.; Moon, H.J.; Boratyński, P.; Jeong, B.; Kwon, Y.-U. Structural sensitivity of peptoid-based low molecular mass organogelator. Mater. Des. 2016, 108, 659–665. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).