Abstract
A novel polyphenolic backbone, hexakis{4-[(4′-hydroxybiphenyl-4-yl)ethynyl]phenyl}benzene, was synthesised using a common synthetic protocol used for the synthesis of similar polyphenolic compounds.
Introduction
Pulsed Electron Paramagnetic Resonance (EPR) [1,2] techniques have become powerful tools for elucidation of structure and conformational flexibility of complex biological systems [3], such as photosynthetic reaction centres [4], integral membrane proteins [5] and nucleic acids [6]. Pulsed EPR measurements give access to accurate distance measurements of 1.2 to 8 nm between two or more paramagnetic centres, which can be endogenous or chemically introduced by Site-Directed Spin-Labelling (SDSL) [3].
Distance measurements in systems containing two spin labels have been proven to be easily accessible and highly accurate and precise [3], however, in multiply labelled complexes these measurements have been proven challenging because of the presence of multi-spin effects. These effects can introduce artefacts hampering data interpretation [7,8]. To quantify and suppress multi-spin effects in pulsed EPR measurements multiply labelled chemical model systems have been synthesised [7,9]. These give access to a better understanding of the phenomenon as they allow measurements to be performed on well-defined systems and under well-defined experimental conditions. Model systems are often easier to simulate.
Results and Discussion
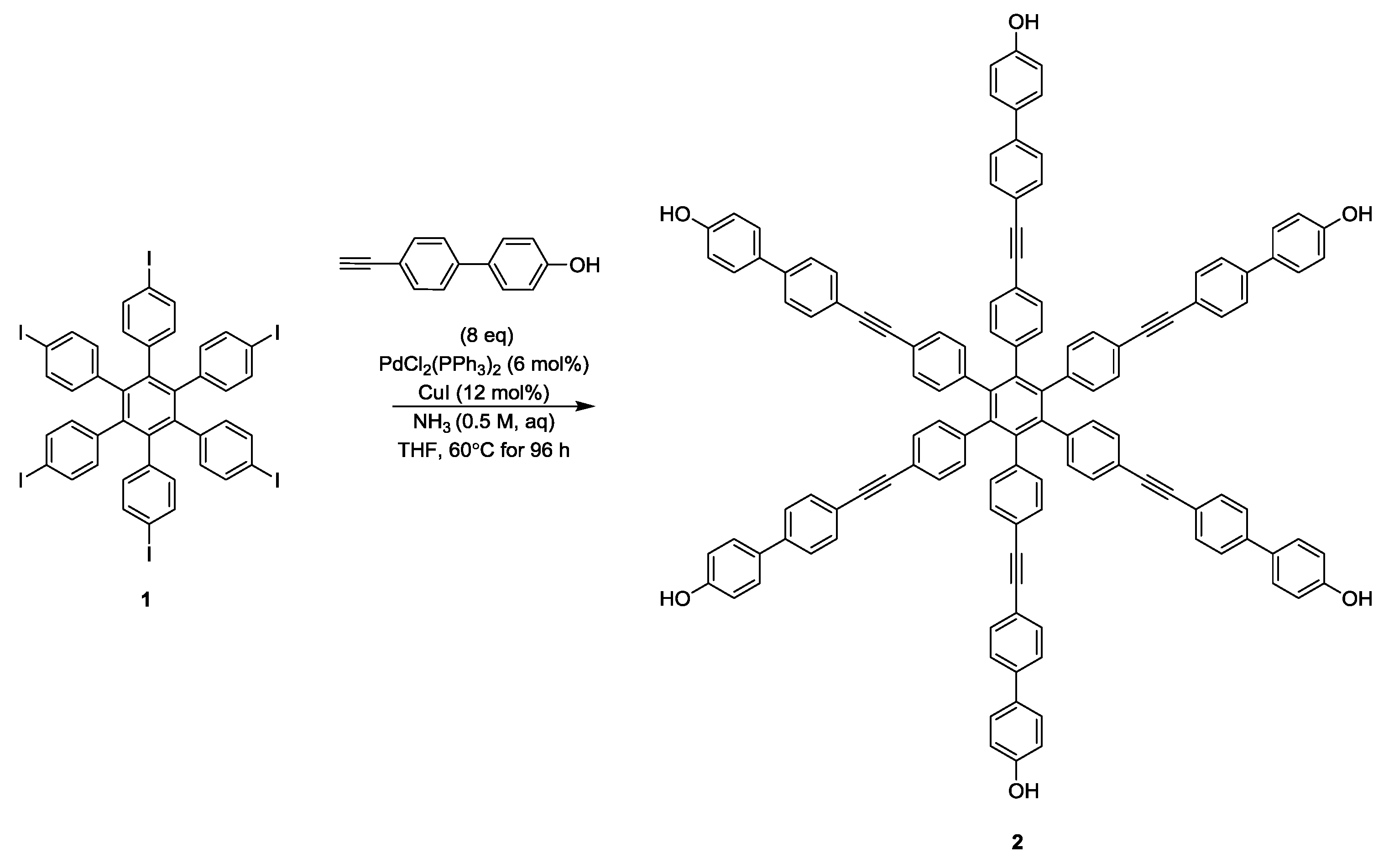
As a precursor to a novel six-fold labelled multi-spin model systems the intermediate hexaphenol hexakis{4-[(4′-hydroxybiphenyl-4-yl)ethynyl]phenyl}benzene has been isolated.
The synthetic process, reported in Scheme 1, involved the iodination of hexaphenylbenzene [10] using a similar protocol designed by Kobayashi et al. [11], followed by Sonogashira cross-coupling, under conditions previously reported by Mohamed Ahmed and Mori [12], and optimised for synthesis of similar polyphenols [9].
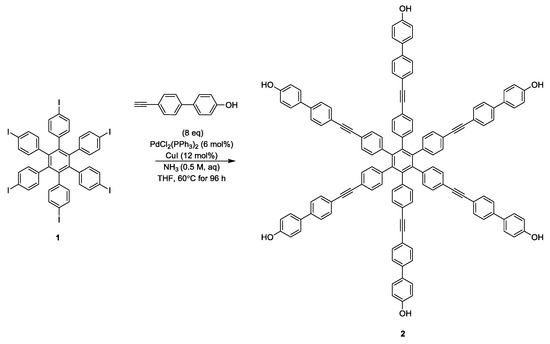
Scheme 1.
Synthesis of hexaphenol hexakis{4-[(4′-hydroxybiphenyl-4-yl)ethynyl]phenyl}benzene 2.
Experimental Section
General Methods
Sonogashira cross-couplings were carried out under nitrogen atmosphere using standard Schlenk techniques and freshly distilled solvents. Dry solvents were obtained anhydrous by a SPS alumina column and collected into flame-dried flasks. Solvents for cross-couplings were degassed via the freeze-pump-thaw technique (×3). 1H and 13C{1H} NMR experiments (Bruker Avance 500, Fällanden, Switzerland) were performed using 500 MHz 1H or 125 MHz 13C NMR at ambient temperature in deuterated solvents. Infrared spectra were recorded with an ATR probe and only characteristics peaks are reported (Shimadzu, Kyoto, Japan). Mass spectrometry data was acquired using matrix-assisted laser desorption/ionisation (MALDI) (Voyager DE-STR, Applied Biosystems, Foster City, CA, USA).
Hexakis{4-[(4′-hydroxybiphenyl-4-yl)ethynyl]phenyl}benzene (2)
Hexakis(4-iodophenyl)benzene (1) was prepared in modified literature procedures [11]. Hexaphenylbenzene (2 g, 3.7 mmol) was dissolved in dry CH2Cl2 (150 mL). To the obtained solution freshly ground iodine (3.8 g, 15 mmol) was added together with [bis-(trifluoroaceteoxy)iodo] benzene (6.4 g, 15 mmol); 3.2 g was added just after addition of iodine and the remaining half was added after 30 min. The obtained mixture was left stirring in the dark under nitrogen atmosphere overnight. Hexane was added to the yellow solution to encourage precipitation. The solids were isolated by filtration. The obtained solids were dissolved in chloroform; the solution was washed with 5% sodium bisulfite aqueous solution followed by water and brine. The organic layer was dried over sodium sulfate and solvents removed under reduced pressure. The obtained solids were recrystallized from chloroform to give 1 as a white solid (0.35 g, 51 %) [11,13].
Hexakis(4-iodophenyl)benzene (0.1 g, 0.08 mmol) was dissolved in dry THF (10 mL) together with PdCl2(PPh3)2 (6% mol., 0.003 g, 0.005 mmol). 4ʹ-Ethynyl-[1,1ʹ-biphenyl]-4-ol (0.12 g, 0.62 mmol) was dissolved in a separate flask in dry THF (10 mL). Both solutions were degassed before drop-wise addition of the alkyne solution to the first solution. The new mixture was degassed once more before addition of CuI (12%, 0.01 g, 0.08 mmol). A 0.5 M aqueous ammonia solution (2.5 mL, 1.3 mmol) was added drop-wise to the new mixture, which was left stirring at 65 °C for 4 days. The mixture was taken up in EtOAc and washed with 10% aqueous HCl solution, water and brine. The organic phase was dried over sodium sulfate and solvents removed under vacuum. The obtained solids (0.2 g) were purified via silica column chromatography (10% EtOAc in CH2Cl2, Rf 0.1). The target molecule was isolated as a brown solid (0.07 g, 55%).
FT-IR (ATR): 2916 (m), 2848 (m), 1662 (m), 1589 (m), 1492 (m), 1274 (m), 1172 (m), 1047 (s), 1022 (s), 997 (s), 821 (s).
1H NMR (500 MHz, DMSO-d6) δ 9.66 (s, 6H), 7.56 (dd, J = 37.2, 7.2 Hz, 36H), 7.18 (d, J = 7.9 Hz, 12H), 7.01 (d, J = 8.0 Hz, 12H), 6.84 (d, J = 8.3 Hz, 12H).
13C NMR (126 MHz, DMSO-d6) δ 157.62, 140.27, 132.07, 131.89, 131.54, 131.46, 130.06, 128.83, 128.73, 127.77, 125.93, 119.96, 115.85, 89.81, 89.55.
MS [MALDI] [M + H]+ calcd for C126H78O6 1687.6, found 1687.6.
1H and 13C NMR spectra are reported in the supplementary materials as Figures S1 and S2 together with MALDI mass spectra as Figures S3 to S5.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
We acknowledge Melanja Smith and Tomas Lebl for NMR experiments, the EPSRC UK National Mass Spectrometry Service Centre at Swansea University and the BSRC Mass Spectrometry and Proteomics Facility, St Andrews. SV is supported by the EPSRC, UK; BEB is grateful for an EaStCHEM Hirst Academic Fellowship by the School of Chemistry, St Andrews and funding from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (REA 334496). There are no competing financial interests.
Author Contributions
SV and BEB planned the research, SV performed the research and analysed the data, SV and BEB wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Milov, A.D.; Salikhov, K.M.; Shirov, M.D. Application of the double resonance method to electron spin echo in a study of the spatial distribution of paramagnetic centres in solids. Sov. Phys. Solid State 1981, 23, 565–569. [Google Scholar]
- Pannier, M.; Veit, S.; Godt, A.; Jeschke, G.; Spiess, H.W. Dead-time free measurement of dipole–dipole interactions between electron spins. J. Magn. Reson. 2000, 142, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, G. DEER distance measurements on proteins. Annu. Rev. Phys. Chem. 2012, 63, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, K.; Hara, H.; Kawamori, A.; Akabori, K. Determination of distances from tyrosine D to QA and chlorophyllZ in photosystem II studied by `2+1' pulsed EPR. BBA-Bioenergetics 1998, 1363, 187–198. [Google Scholar] [CrossRef]
- Ward, R.; Pliotas, C.; Branigan, E.; Hacker, C.; Rasmussen, A.; Hagelueken, G.; Booth, I.R.; Miller, S.; Lucocq, J.; Naismith, J.H.; et al. Probing the structure of the mechanosensitive channel of small conductance in lipid bilayers with pulsed electron-electron double resonance. Biophys. J. 2014, 106, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Krstić, I.; Endeward, B.; Margraf, D.; Marko, A.; Prisner, T. Structure and dynamics of nucleic acids. In EPR Spectroscopy; Drescher, M., Jeschke, G., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 2012; Volume 321, pp. 159–198. [Google Scholar]
- Jeschke, G.; Sajid, M.; Schulte, M.; Godt, A. Three-spin correlations in double electron-electron resonance. Phys. Chem. Chem. Phys. 2009, 11, 6580–6591. [Google Scholar] [CrossRef] [PubMed]
- Giannoulis, A.; Ward, R.; Branigan, E.; Naismith, J.H.; Bode, B.E. PELDOR in rotationally symmetric homo-oligomers. Mol. Phys. 2013, 111, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Valera, S.; Taylor, J.E.; Daniels, D.S.B.; Dawson, D.M.; Athukorala Arachchige, K.S.; Ashbrook, S.E.; Slawin, A.M.Z.; Bode, B.E. A modular approach for the synthesis of nanometer-sized polynitroxide multi-spin systems. J. Org. Chem. 2014, 79, 8313–8323. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, J.A. Synthesis of a hexaalkynylhexaphenylbenzene. Org. Prep. Proced. Int. 1991, 23, 460–463. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kobayashi, N.; Ikuta, M.; Therrien, B.; Sakamoto, S.; Yamaguchi, K. Syntheses of hexakis(4-functionalized-phenyl)benzenes and hexakis[4-(4ʹ-functionalized- phenylethynyl)phenyl]benzenes directed to host molecules for guest-inclusion networks. J. Org. Chem. 2005, 70, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Ahmed, M.S.; Mori, A. Sonogashira coupling with aqueous ammonia directed to the synthesis of azotolane derivatives. Tetrahedron 2004, 60, 9977–9982. [Google Scholar] [CrossRef]
- FT-IR (ATR): 1487 (m), 1138 (m), 1058 (m), 1003 (s), 823 (m). 1H NMR (300 MHz, Chloroform-d) δ 7.23 (s, 12H), 6.54–6.39 (m, 12H).
- ESI and EI mass spectrometry methods did not give the wanted ions. Low resolution MALDI techniques gave the wanted mass peak, however high resolution analysis using MALDI could not be achieved due to the lack of an appropriate standard. Microanalysis for 2 did not give acceptable results. We attribute this to the hygroscopic nature of the compound. Similar behaviour with respect to microanalysis and mass spectrometry was found for similar compounds bearing several phenol groups (see Ref. 9).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).