Abstract
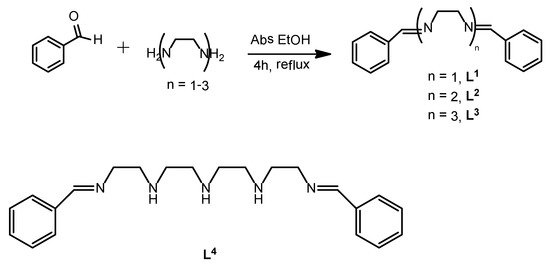
A tetraethylene pentamine-diamine (L4), the biggest compound in the family of dibenzylated diimine-polyamines (L1–L4) has been synthesized by classical Schiff-base reaction between benzaldehyde and the diamine tetraethylenepentamine, and the structure was confirmed by elemental analysis, ESI-MS spectrometry and by IR and 1H-NMR spectroscopy.
Improved understanding of the role of polyamines in metabolism [1,2], and the differences in polyamine biology between normal cells and tumor cells [3], have increased current interest in this type of compounds in the field of drug development [4,5]. The activity of polyamines is very much dependent on their charge and the charge density they display at physiological pH [6].
During the last ten years, some of us have been involved in the studies of many different water-soluble bis-chromophoric polyamines as fluorescent chemosensors [7,8,9,10]. However, more recently studies in new active MALDI-TOF-MS matrices reveals that the introduction of imine groups into the polyamine chain increases the energy absorbed in the UV region, and consequently, the potential application as a MALDI matrix increase [11,12].
Following the method previously reported by Bernardo et al. for polyamine systems [13], in this paper we describe the synthesis and characterization of the tetraethylene pentamine-diamine (L4), derived from benzaldehyde and the diamine tetraethylenepentamine. The broader applicability of this method was demonstrated by the synthesis of a few related compounds (L1–L3) [14] (See scheme 1).
Experimental
A solution of benzaldehyde (0.129 g, 1.225 mmol) in absolute ethanol (20 mL) was added dropwise to a refluxing solution of tetraethylenepentamine (0.115 g, 0.612 mmol) in the same solvent (15 mL). The resulting solution was gently refluxed with magnetic stirring for 4 h. The colour changed from colourless to yellow. The solution was concentrated under vacuum to 1/3 of its volume. Diethyl ether was added to the solution and then cooled at 0 °C during 24 h. The yellow crystals formed were filtered off and dried under vacuum. At room temperature the crystals were not stable and a yellow oil was obtained.
L4: N1-Benzylidene-N2-(2-((2-((2-(benzylideneamino)ethyl)amino)ethyl)amino)ethyl)ethane-1,2-diamine
Yield: 125 mg (56%).
ESI-MS: m/z (rel.int%): 366.26 (100) ([M+H]+).
1H-NMR (CDCl3): δ = 8.3 (s, 2H, N=C–H); 7.5–7.7 (m, 4H, C-Har); 7.4–7.1 (m, 6H, C-Har); 3.8–3.2 (m, 4H, CH2); 2.9–2.1 (m, 12H, CH2) ppm.
IR (cm−1): 1658 (C=N, Imine), 1589, 1492 (C=C, Ar).
Elemental analysis: Calcd for C22H31N5: C, 72.29; H, 8.55; N, 19.16. Found: C, 72.26; H, 7.99; N, 19.65.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
We are grateful to Xunta de Galicia (Spain) for grant 09CSA043383PR (Biomedicine) and to the Scientific Association ProteoMass for financial support. C.N. thanks the Fundação para a Ciência e a Tecnologia/FEDER (Portugal/EU) program postdoctoral contract SFRH/BPD/65367/2009. J.F.L. thanks Xunta de Galicia (Spain) for a research contract by project 09CSA043383PR in Biomedicine. J.L.C. and C.L. thank Xunta de Galicia for the Isidro Parga Pondal Research program.
References and Notes
- Wallace, H.M.; Fraser, A.V.; Hughes, A. A perspective of polyamine metabolism. Biochem J. 2003, 376, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vujcic, S.; Diegelman, P.; Bacchi, C.J.; Kramer, D.L.; Porter, C.W. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem. J. 2002, 367, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.M.; Fraser, A.V. Polyamine analogues as anticancer drugs. Biochem. Soc. Trans. 2003, 31, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, E.; Bachrach, U. (Eds.) Special issue: Polyamines and their Analogs in Cancer and other Diseases. Amino Acids 2007, 33, 175–187.
- Sonawane, N.D.; Szoka, F.C., Jr.; Verkman, A.S. Chloride Accumulation and Swelling in Endosomes Enhances DNA Transfer by Polyamine-DNA Polyplexes. J. Biol. Chem. 2003, 278, 44826–44831. [Google Scholar] [CrossRef] [PubMed]
- Albelda, M.T.; Díaz, P.; García-España, E.; Lima, J.C.; Lodeiro, C.; de Melo, J.S.; Parola, A.J.; Pina, F.; Soriano, C. Switching from intramolecular energy transfer to intramolecular electron transfer by the action of pH and Zn(II) coordination. Chem. Phys. Lett. 2002, 353, 63–68. [Google Scholar] [CrossRef]
- De Melo, J.S.; Pina, J.; Pina, F.; Lodeiro, C.; Parola, A.J.; Albelda, M.T.; Clares, M.P.; García-España, E.; Soriano, C. Energetics and dynamics of naphathalene polyaminic derivatives. Influence of structural design in the balance static vs dynamic excimer formation. J. Phys. Chem. A 2003, 107, 11307–11318. [Google Scholar] [CrossRef]
- Alarcón, J.; Aucejo, R.; Albelda, M.T.; Alves, S.; Clares, P.; García-España, E.; Lodeiro, C.; Marchin, K.L.; Parola, A.J.; Pina, F.; et al. Fluorescent Type II Materials from Naphthylmethyl Polyamine Precursors. Supramol. Chem. 2004, 16, 573–580. [Google Scholar] [CrossRef]
- Bazzicalupi, C.; Bencini, A.; Bianchi, A.; Danesi, A.; Faggi, E.; Giorgi, C.; Lodeiro, C.; Oliveira, E.; Pina, F.; Valtancoli, B. Interaction of polyamine macrocycles with Zn(II) and ATP in aqueos solution. Binary and Ternary systems. A potentiometric, NMR and fluorescence emission studies. Inorg. Chim. Acta 2008, 361, 3410–3419. [Google Scholar] [CrossRef]
- Fernandez, L.; Boucher, M.; Fernández-Lodeiro, J.; Oliveira, E.; Núñez, C.; Santos, H.M.; Capelo, J.L.; Nieto-Faza, O.; Bértolo, E.; Lodeiro, C. Exploiting anionic and cationic interactions with a new emissive imine-based β-naphtol molecular probe. Inorg. Chem. Commun. 2009, 12, 905–912. [Google Scholar] [CrossRef]
- Fernández-Lodeiro, J.; Núñez, C.; Carreira, R.; Santos, H.M.; Silva-López, C.; Mejuto, J.C.; Capelo, J.L.; Lodeiro, C. Novel versatile imine-enamine chemosensor based on 6-nitro-4-oxo-4H-chromene for ion detection in solution, solid and gas-phase: Synthesis, emission, computational and MALDI-TOF-Ms studies. Tetrahedron 2011, 67, 326–333. [Google Scholar] [CrossRef]
- Bernardo, M.A.; Gurrero, J.A.; García-España, E.; Luis, S.V.; Llinares, J.M.; Pina, F.; Ramírez, J.A.; Soriano, C. Thermodynamic, NMR and photochemical study on the acid-base behaviour or N,N'-dibenzylated polyamines and on their interactions with hexacyanocobaltate(III). J. Chem. Soc. Perkin Trans 2 1996, 2335–2342. [Google Scholar] [CrossRef]
- The smaller parent compounds derived from 1,2-ethanediamine (L1), diethylenetriamine (L2), and triethylenetetramine (L3) were obtained by a similar methodology, using 0.038, 0.063 and 0.089 g of diamine, respectively. Compound L1: N1,N2-Dibenzylideneethane-1,2-diamine; Yield: 121 mg (84%); ESI-MS: m/z (rel. int%): 237.13 (100) ([M+H]+); 1H NMR (CDCl3): δ = 8.1 (s, 2H, N=C–H); 7.8 (m, 4H, C-Har); 7.2 (m, 6H, C-Har); 3.8 (s, 4H, CH2) ppm; IR (cm−1): 1647 (C=N, Imine), 1599, 1498 (C=C, Ar); Elemental analysis: Calcd for C16H16N2: C, 81.32; H, 6.82; N, 11.85. Found: C, 80.87; H, 7.02; N,12.05. Compound L2: N1-Benzylidene-N2-(2-(benzylideneamino)¬ethyl)ethane-1,2-diamine; Yield: 103 mg (71%); ESI-MS: m/z (rel. int%): 279.17 (100) ([M+H]+); 1H-NMR (CDCl3): δ = 8.2 (s, 2H, N=C–H); 7.8–7.6 (m, 4H, C-Har); 7.4–7.2 (m, 6H, C-Har); 3.8 (m, 4H, CH2); 2.9 (m, 4H, CH2) ppm; IR (cm−1): 1649 (C=N, Imine), 1586, 1491 (C=C, Ar); Elemental analysis: Calcd for C18H21N3: C, 77.38; H, 7.58; N, 15.04. Found: C, 77.16; H, 8.03; N, 15.34. Compound L3: N1,N1′-(Ethane-1,2-diyl)bis(N2-benzylideneethane-1,2-diamine); Yield: 132 mg (89%); ESI-MS: m/z (rel. int%): 323.22 (100) ([M+H]+); 1H-NMR (CDCl3): δ = 8.1 (s, 2H, N=C–H); 7.7–7.5 (m, 4H, C-Har); 7.4–7.1 (m, 6H, C-Har); 3.7–3.4 (m, 2H, CH2); 2.9–2.1 (m, 8H, CH2) ppm; IR (cm−1): 1656 (C=N, Imine), 1576, 1499 (C=C, Ar); Elemental analysis: Calcd for C20H26N4: C, 74.50; H, 8.13; N, 17.38. Found: C, 74.78; H, 8.16; N, 17.49.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).