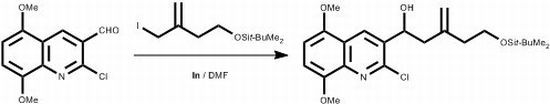
5-(tert-Butyldimethylsilyloxy)-1-(2-chloro-5,8-dimethoxyquinolin-3-yl)-3-methylenepentan-1-ol
Abstract
:Introduction
Result and Discussion

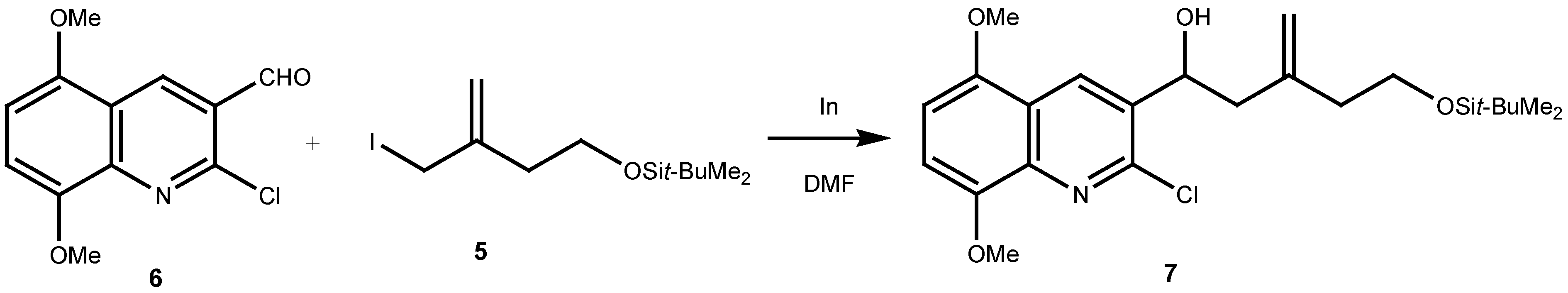
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Musiol, R.; Serda, M.; Hensel-Bielowka, S.; Polanski, J. Quinoline-Based Antifungals. Curr. Med. Chem. 2010, 17, 1960–1973. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bawa, S.; Gupta, H. Biological Activities of Quinoline Derivatives. Mini Rev. Med. Chem. 2009, 9, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Parolin, C.; Palumbo, M.; Palu, G. Antiviral Properties of Quinolone-based Drugs. Curr. Drug Targets Infect. Disord. 2004, 4, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Segev, S.; Rubinstein, E. Future aspects [of quinoline antibacterials]. In Handbook of Experimental Pharmacology; Kuhlmann, J., Dalhoff, A., Zeiler, H.-J., Eds.; Springer: Berlin, Germany, 1998; Volume 127, pp. 454–475. [Google Scholar]
- Rosen, T. 6 The fluoroquinolone antibacterial agents. Prog. Med. Chem. 1990, 27, 235–295. [Google Scholar] [PubMed]
- Lontie, M. Les nouvelles quinolones. J. Pharm. Belg. 1989, 44, 292–301. [Google Scholar] [PubMed]
- Fernandes, P.B.; Chu, D.T.W. Quinolone antibacterial agents. Annu. Rep. Med. Chem. 1988, 23, 133–140. [Google Scholar]
- Kaushik, S.; Gupta, S.P.; Sharma, P.K. Design and development of anti-hepatitis B virus agents. Curr. Med. Chem. 2010, 17, 3377–3392. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Krishnaiah, M.; Venkateswarlu, K.; Das, R. Camptothecins: Review on the bioactive natural products. Part XVIII. Some recent chemical studies. Nat. Prod. Commun. 2006, 1, 255–263. [Google Scholar]
- Okamoto, H.; Cujec, T.P.; Okamoto, M.; Peterlin, B.M.; Baba, M.; Okamoto, T. Inhibition of the RNA-Dependent Transactivation and Replication of Human Immunodeficiency Virus Type 1 by a Fluoroquinoline Derivative K-37. Virology 2000, 272, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Okamoto, M.; Makino, M.; Kimura, Y.; Ikeuchi, T.; Sakaguchi, T.; Okamoto, T. Potent and selective inhibition of human immunodeficiency virus type 1 transcription by piperazinyloxoquinoline derivatives. Antimicrob. Agents Chemother. 1997, 41, 1250–1255. [Google Scholar] [PubMed]
- Wentland, M.P.; Perni, R.B.; Dorff, P.H.; Brundage, P.; Castaldi, M.J.; Carlson, J.A.; Bailey, T.R.; Aldous, S.C.; Carabateas, P.M.; Bacon, E.R.; et al. Antiviral properties of 3-quinolinecarboxamides: A series of novel non-nucleoside antiherpetic agents. Drug Des. Discov. 1997, 15, 25–38. [Google Scholar] [PubMed]
- Solomon, V.R.; Lee, H. Quinoline as a privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.H. Quinoline analogs as antiangiogenic agents and telomerase inhibitors. Top. Heterocycl. Chem. 2007, 11, 213–229. [Google Scholar]
- Joseph, B.; Darro, F.; Behard, A.; Lesur, B.; Collignon, F.; Decaestecker, C.; Frydman, A.; Guillaumet, G.; Kiss, R. 3-Aryl-2-Quinolone Derivatives: Synthesis and Characterization of In Vitro and In Vivo Antitumor Effects with Emphasis on a New Therapeutical Target Connected with Cell Migration. J. Med. Chem. 2002, 45, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Bergh, J.C.S.; Toetterman, T.H.; Termander, B.C.; Strandgarden, K.A.-M.P.; Gunnarsson, P.O.G.; Nilsson, B.I. The first clinical pilot study of roquinimex (Linomide) in cancer patients with special focus on immunological effects. Cancer Invest. 1997, 15, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Jain, M.; Reddy, R.P.; Jain, R. Quinolines and structurally related heterocycles as antimalarials. Eur. J. Med. Chem. 2010, 45, 3245–3264. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.M.; Strobeck, J.E. Quinolinone derivatives in the management of congestive heart failure. Coronary Artery Dis. 1994, 5, 107–111. [Google Scholar] [CrossRef]
- Javed, T.; Baker, N.R. Synthesis and biological activity of quinoline analogues in cardiovascular disease. In Pharmacological Aspects of Cardiovascular Medicine; Javed, T., Ed.; Research Signpost: Trivandrum, India, 2002; pp. 55–83. [Google Scholar]
- Araki, S.; Ito, H.; Butsugan, Y. Indium in organic synthesis: Indium-mediated allylation of carbonyl compounds. J. Org. Chem. 1988, 53, 1831–1833. [Google Scholar] [CrossRef]
- Hughes, R.C.; Dvorak, C.A.; Meyers, A.I. An Asymmetric Approach to Spirocylic Systems: A Formal Synthesis of Zizaene. J. Org. Chem. 2001, 66, 5545–5551. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bouarata, L.; Tebbani, D.; Mosset, P. 5-(tert-Butyldimethylsilyloxy)-1-(2-chloro-5,8-dimethoxyquinolin-3-yl)-3-methylenepentan-1-ol. Molbank 2012, 2012, M790. https://doi.org/10.3390/M790
Bouarata L, Tebbani D, Mosset P. 5-(tert-Butyldimethylsilyloxy)-1-(2-chloro-5,8-dimethoxyquinolin-3-yl)-3-methylenepentan-1-ol. Molbank. 2012; 2012(4):M790. https://doi.org/10.3390/M790
Chicago/Turabian StyleBouarata, Linda, Dahmane Tebbani, and Paul Mosset. 2012. "5-(tert-Butyldimethylsilyloxy)-1-(2-chloro-5,8-dimethoxyquinolin-3-yl)-3-methylenepentan-1-ol" Molbank 2012, no. 4: M790. https://doi.org/10.3390/M790
APA StyleBouarata, L., Tebbani, D., & Mosset, P. (2012). 5-(tert-Butyldimethylsilyloxy)-1-(2-chloro-5,8-dimethoxyquinolin-3-yl)-3-methylenepentan-1-ol. Molbank, 2012(4), M790. https://doi.org/10.3390/M790