Abstract
As a result of three-component one-pot reaction of trans-2-benzoyl-3-(4-nitrophenyl)aziridine with 4-acetamidobenzaldehyde and ammonium acetate, N-(4-(6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene-2-yl)phenyl)acetamide was obtained in good yield. The newly synthesized compound exhibit interesting photochromic behavior in the solid and solution state. The structure of the synthesized compound was confirmed by elemental analysis, 1H-NMR, 13C-NMR and UV-Visible spectral data.
Introduction
Photochromism is a light-induced reversible molecular transition between two forms, with different absorption spectra. Many types of organic photochromic systems have been developed so far [1,2,3,4,5,6,7]. Among different groups of materials, the polyaromatic bicyclic aziridine compounds, very interesting categories of organic photochromic compounds, have individual photochromic properties. These compounds display significant photochromic performance even in the crystalline phase. This property lets us to study them as nominees in the search for intelligent photochromic materials [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Depending on the particular structure, 1,3-diazabicyclo[3.1.0]hex-3-ene derivative crystals showed different colors upon UV irradiation [6,7,8,9]. These photochroms and other photochromic compounds such as diarylethenes are promising candidates for application in optical devices [1,2]. The photochromic behavior of bicyclic aziridine systems is based on a reversible ring opening of the three-membered ring at the C–C bond, stimulated by UV light, which transform a colorless form (closed ring) to the colored azomethine ylide form (open ring) [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]. The closed-ring and open-ring forms of bicyclic aziridines exhibited absorption spectra in solution state. Both photoisomers are stable and able to give photochromic reactions in the crystalline state. The title compound 4a was prepared in good yield from the corresponding ketoaziridine compound 3 [6,7] and the available 4-acetamidobenzaldehyde 1, in the presence of ammonium acetate in one-pot reaction (scheme 1).
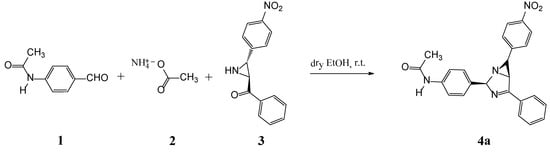
Scheme 1.
Synthesis of N-(4-(6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene-2-yl)phenyl)acetamide.
Results and Discussion
The 1H-NMR spectrum of 4a in CDCl3 showed three singlets at δ 6.80, 2.58 and 3.82 ppm that were assigned to H-2, H-6, and H-5 protons, respectively. No proton-proton coupling was observed for H-5 and H-6 protons, which possibly is owing to the dihedral angle between them (according to Karplus equation probably dihedral angle approximately 90°; nonetheless, direct evaluation of the angle from the amount of the J value is uncertain [16]). Also, the signals of other protons can be seen well within the 1H-NMR spectrum. The singlet signal at δ 2.20 ppm was assigned to the methyl protons of the acetamide group. Relatively broad peak observed at δ 7.24 ppm confirmed the presence of NH proton of the acetamide group. The signal doublet at δ 8.20 ppm belongs to two protons in the ortho position relative to the nitro group. Protons meta position relative to the nitro group appeared as a doublet at δ 7.43 ppm. The resonances of the two protons belong to two protons in the ortho position of the phenyl ring located in bottom right-hand, appeared at δ 8.02 ppm as a doublet. A triplet signal appeared at δ 7.60 ppm, related to proton para position of the same ring. All the other aromatic protons were observed as multiplets in the region δ 7.56–7.51 ppm, integrating for six protons. It can be mentioned that this reaction led to exo- and endo-isomers with orientation of p-acetamidophenyl ring in C-2 position [6,7,8,9,10,11,12,13,14,15,16,19,20,21,22,23,24]. The 1H-NMR spectrum of purified product showed signals belonging to one of the isomers. Based on chemical shifts of the H-2, H-5, and H-6 protons and compared them with those obtained by previously reported in the literature [19,20,21,22,23,24] suggests that endo-isomer of 4 is formed. Also, 13C-NMR spectrum is in good agreement with the title compound. Signal appears at δ 95.3 ppm indicating the presence of carbon at C-2 position. Signals appeared at δ 57.4 and 41.1 ppm indicated the presence of aziridine ring carbons at 5- and 6-positions, respectively. Furthermore, the 13C-NMR spectrum shows signals for carbonyl carbon at δ 168.7 ppm and C=N at 171.2 ppm. The methyl carbon acetamide group was visible at δ 24.4 ppm. The aromatic carbons in the structure of the desired compound were visible at δ 147.1–119.2 ppm.
Suggested photochromic color change of 4a based on reversible aziridine ring-opening and ring-closing is showed in scheme 2. Photochromic reaction of 4a is established by the changes in the UV/Vis absorption spectra when a dichloromethane (DCM) solution of the compound 4a is irradiated with 365 nm light (Figure 1) and is accompanied by a change in color of the solution from colorless (4a) to yellow (4b). This change color occurs in solution state, whereas change color in solid state occurs from colorless crystals (4a) to blue crystals (4b). Upon irradiation with UV light, compound 4a underwent a photochromic reaction (Scheme 2); this goes along with noticeable changes in the absorption spectra as shown in Figure 1.
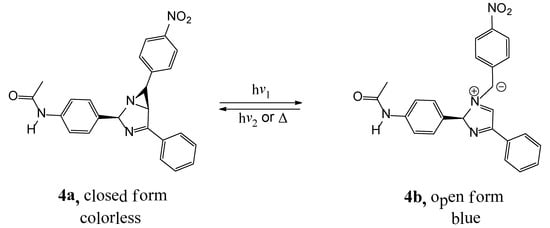
Scheme 2.
Suggested photochromic color change of 4a.
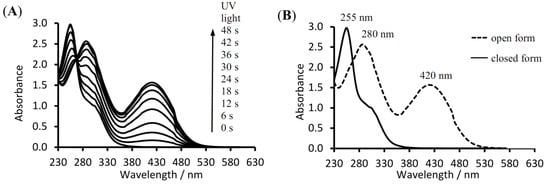
Figure 1.
(A) Overlay spectra of 4a in dichloromethane (DCM) (3.0 × 10–4 M) under 365 nm irradiation at ambient temperature; (B) UV/Vis spectra of 4a at the same conditions [the solid line (—) before and the dashed line (- - -) after irradiation for 48 sec at 365 nm].
UV irradiation of this solution resulted in the appearance of new absorption bands at 280 and 420 nm. The appearance of these new bands is ascribable to the formation of the azomethine ylide form 4b. As expected, with increased irradiation time, intensity of the absorption band in the visible region is gradually increased, which states that the ring opening reaction occurs. These spectral changes showed that 4a exhibit photochromic behavior, similar to known triaryl-1,3-diazabicyclo[3.1.0]hex-3-enes [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Also, an isosbestic point at 270 nm showed the presence of two species 4a and 4b. Color change in solid and solution state was observed by eye-naked, when compound is exposed to light (UV light from mercury, xenon lamp, fluorescent lamp or sunlight) at ambient temperature. Moreover, 4b was converted to 4a when the 4b is kept in the dark for overnight or after putting it in the oven for 15 min at 80 °C.
Experimental
Similar to the recently published synthesis [23], a mixture of 4-acetamidobenzaldehyde 1 (0.163 g, 1 mmol), NH4OAc 2 (0.78 g, 10 mmol) and trans-2-benzoyl-3-(4-nitrophenyl)aziridine 3 (0.268 g, 1 mmol) in absolute ethanol (8 mL) was stirred at room temperature for 26 h. The reaction mixture was filtered, washed with absolute ethanol, dried under reduced pressure, and the resulting solid was recovered, and recrystallized from absolute ethanol (15 mL) to give the target compound 4a as a colorless solid that changed to the blue (4b).
Yield: 84%; m.p. 221–222 °C.
IR (KBr) vmax cm−1: 3420, 3380, 1675, 1683, 1610, 1588, 1500.
1H-NMR (400 MHz, CDCl3) (δ/ppm): 8.20 (d, J = 8.4 Hz, 2H, Ar-H), 8.06 (d, J = 7.2 Hz, 2H, Ar-H), 7.60 (t, J = 7.2 Hz, 1H, Ar-H), 7.56–7.51 (m, 6H, Ar-H), 7.43 (d, J = 8.8 Hz, 2H, Ar-H), 7.24 (br, 1H, NH), 6.80 (s, 1H, H-2), 3.82 (s, 1H, H-5), 2.58 (s, 1H, H-6), 2.20 (s, 3H, CH3).
13C-NMR (100 MHz, DMSO-d6) δ: 171.2 (C=N), 168.7 (C=O), 147.1, 146.6, 139.1, 133.7, 132.2, 131.8, 129.4, 128.9, 128.3, 128.1, 123.9, 119.2, 95.3 (C-2), 57.4 (C-5), 41.1 (C-6), 24.4 (NHCOCH3).
Anal. Calcd. for C24H20N4O3: C, 69.89; H, 4.89; N, 13.58. Found: C, 69.81; H, 4.84; N, 13.54.
UV-Vis (DCM, λmax/nm): 255, 295 (shoulder) before irradiation, and 280, 420 nm after irradiation.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgment
The authors are thankful to Research Council of the Damghan University.
References
- Ishibashi, Y.; Fujiwara, M.; Umesato, T.; Saito, H.; Kobatake, S.; Irie, M.; Miyasaka, H. Cyclization Reaction Dynamics of a Photochromic Diarylethene Derivative as Revealed by Femtosecond to Microsecond Time-Resolved Spectroscopy. J. Phys. Chem. C 2011, 115, 4265–4272. [Google Scholar] [CrossRef]
- Patel, P.D.; Masunov, A.E. Theoretical Study of Photochromic Compounds: Part 3. Prediction of Thermal Stability. J. Phys. Chem. C 2011, 115, 10292–10297. [Google Scholar] [CrossRef]
- Patel, P.D.; Masunov, A.E. Theoretical Study of Photochromic Compounds. 1. Bond Length Alternation and Absorption Spectra for the Open and Closed Forms of 29 Diarylethene Derivatives. J. Phys. Chem. A 2009, 113, 8409–8414. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, D.; Perpete, E.A.; Maurel, F.; Perrier, A. Doubly Closing or Not? Theoretical Analysis for Coupled Photochromes. J. Phys. Chem. C 2010, 114, 9489–9497. [Google Scholar] [CrossRef]
- Akita, M. Photochromic Organometallics, A Stimuli-Responsive System: An Approach to Smart Chemical Systems. Organometallics 2011, 30, 43–51. [Google Scholar] [CrossRef]
- Kiyani, H.; Mahmoodi, N.O.; Tabatabaeian, K.; Zanjanchi, M.A. Photochromic Behavior of Several New Synthesized Bis-1,3-Diazabicyclo[3.1.0]hex-3-enes. J. Phys. Org. Chem. 2009, 22, 559–567. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Kiyani, H.; Yazdanbakhsh, M.R.; Sharifzadeh, B. Synthesis and Photochromic Properties of New Heterocyclic Derivatives of 1,3-diazabicyclo[3.1.0]hex-3-ene. J. Chin. Chem. Soc. 2007, 54, 635–641. [Google Scholar] [CrossRef]
- Kiyani, H.; Mahmoodi, N.O.; Tabatabaeian, K.; Zanjanchi, M.A. Synthesis and Photochromism of 1,3-Diazabicyclo[3.1.0]hex-3-ene Phenol Rings. Mendeleev Commun. 2009, 19, 203–205. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Tabatabaeian, K.; Kiyani, H. Two 1,3-Diazabicyclo[3.1.0]hex-3-enes With a ‘Tripod’ Core. Helv. Chim. Acta 2012, 95, 536–542. [Google Scholar] [CrossRef]
- Dyakonenko, V.V.; Maleev, A.V.; Zbruyev, A.I.; Chebanov, V.A.; Desenko, S.M.; Shishkin, O.V. Layered Crystal Structure of Bicyclic Aziridines as Revealed by Analysis of Intermolecular Interactions Energy. CrystEngComm 2010, 12, 1816–1823. [Google Scholar] [CrossRef]
- Bruno, G.; Nicol, F.; Rotondo, A.; Risitano, F.; Grassi, G.; Foti, F. Structure Investigation of Bridgehead Aziridine: Synthesis, Theoretical, and Crystallographic Study of 2,4,6-Triphenyl-1,3-diazabicyclo[3.1.0]hex-3-ene. Helv. Chim. Acta 2006, 89, 190–200. [Google Scholar] [CrossRef]
- Risitano, F.; Grassi, G.; Foti, F.; Moraci, S. A Novel Efficient Three-Component One-Pot Synthesis of 1,3-Diazabicyclo[3.1.0]hex-3-ene System Under Microwave Irradiation. Synlett 2005, 1633–1635. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Tabatabaeian, K.; Ghavidast, A. Synthesis and Photochromic Behavior of Mono, and Biphotochromic System Linked by p-Phenylene Bridge. Chin. Chem. Lett. 2010, 21, 1199–1202. [Google Scholar] [CrossRef]
- Chebanov, V.A.; Desenko, S.M.; Gurley, T.W. Azaheterocycles Based on α,β-Unsaturated Carbonyls; Springer-Verlag: Berlin&Heidelberg, Germany, 2008; Chapter 1; pp. 17–32. [Google Scholar]
- Trozolo, A.M.; Leslie, T.M.; Sarportdar, A.S.; Small, R.D.; Ferraudi, G.J.; DoMinh, T.; Hartless, R.L. Photochemistry of Some Three-Membered Heterocycles. Pure Appl. Chem. 1979, 51, 261–270. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Kiyani, H. Synthesis of Thiophene Derivatives of 1,3-Diazabicyclo[3,1,0]hex-3-ene. Bull. Korean Chem. Soc. 2004, 25, 1417–1420. [Google Scholar]
- Dürr, H. Perspectives in Photochromism: A Novel System Based on the 1,5-Electrocyclization of Heteroanalogous Pentadienyl Anions. Angew. Chem. Int. Ed. Engl. 1989, 28, 413–431. [Google Scholar] [CrossRef]
- Hadjoudis, E.; Marvidis, I.M. Photochromism and Thermochromism of Schiff Bases in the Solid State: Structural Aspects. Chem. Soc. Rev. 2004, 33, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Hiene, H.W.; Weese, R.H.; Cooper, R.A. Aziridines. XV. The Synthesis and Reactions of 1,3-Diazabicyclo[3.1.0]hex-3-enes. J. Org. Chem. 1967, 32, 2708–2711. [Google Scholar] [CrossRef]
- Padwa, A.; Glazer, E. Photochemical Reorganizations in the 1,3-Diazabicyclo[3.1.0]hex-3-ene System. J. Am. Chem. Soc. 1972, 94, 7788–7797. [Google Scholar] [CrossRef]
- Padwa, A.; Clough, S.; Glazer, E. Photochemical transformations of small-ring heterocyclic compounds. XXIV. Photoisomerization of the Triphenyl-1,3-Diazabicyclo[3.1.0]hex-3-ene System. J. Am. Chem. Soc. 1970, 92, 1778–1779. [Google Scholar] [CrossRef]
- Dyakonenko, V.V.; Shishkin, O.V.; Zbruev, A.V.; Desenko, S.M. 2,2-Dimethyl-6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene. Acta Crystallogr. Sect. E: Struct. Rep. Online 2005, E61, o667–o668. [Google Scholar] [CrossRef]
- Kiyani, H. 2-(4-Diethoxymethylphenyl)-6-(4-nitrophenyl)-4-phenyl-1,3-diazabicyclo[3.1.0]hex-3-ene. Molbank 2012, 2012, M780. [Google Scholar] [CrossRef]
- Zbruyev, A.I.; Vashchenko, V.V.; Andryushchenko, A.A.; Desenko, S.M.; Musatov, V.I.; Knyazeva, I.V.; Chebanov, V.A. Synthesis of polyarene derivatives of fused aziridines by Suzuki-Miyaura cross-coupling. Tetrahedron 2007, 63, 4297–4303. [Google Scholar] [CrossRef]
- Khlebnikov, A.F.; Novikov, M.S. Fused Aziridines As Sources of Azomethine ylides. Chem. Heterocyl. Compd. 2012, 48, 179–190. [Google Scholar] [CrossRef]
- D’yakonenko, V.V.; Zbruev, A.V.; Chebanov, V.A.; Desenko, S.M.; Shishkin, O.V. Molecular and Crystal Structure of 3,3-Dimethyl-5-(2-naphthyl)-1-(4-nitrophenyl)-3,5a-dihydro-1H-azireno[1,2-c]imidazole. J. Struct. Chem. 2005, 46, 1110–1113. [Google Scholar] [CrossRef]
- Zbruev, A.I.; Panikarskaya, V.D.; Kasyan, N.A.; Zavora, L.N.; Lisetskii, L.N.; Desenko, S.M.; Chebanov, V.A. The Photoinduced Transformations of Aziridine Derivatives in Liquid Crystalline Matrices. Russ. J. Phys. Chem. A 2009, 83, 1350–1354. [Google Scholar] [CrossRef]
- Zbruev, A.I.; Yarmenko, F.G.; Chebanov, V.A.; Desenko, S.M.; Shishkin, O.V.; Lukinova, E.V.; Knyazeva, I.V. Synthesis and study of new 2-aryl-1-(4-nitrophenyl)-1,1a-dihydroazireno[1,2-a]quinoxaline derivatives. Russ. Chem. Bull. 2006, 55, 362–368. [Google Scholar] [CrossRef]
- Kaluski, Z.; Figas, E.; Vorobyeva, N.P.; Orlov, V.D. Crystal and Molecular Structure of 2-(4-Methoxyphenyl)-4-phenyl-6-(4-nitrophenyl)-1,3-Diazabicylo[3.1.0]hex-3-ene. J. Struct. Chem. 1994, 35, 134–137. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).