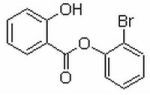
2-Bromophenyl Salicylate
Abstract
:Experimental
General
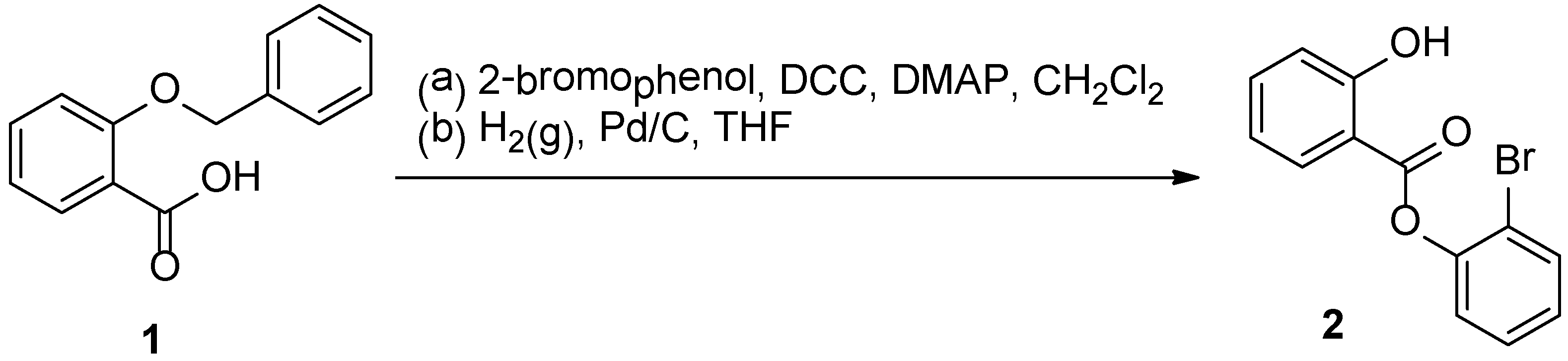
2-Bromophenyl Salicylate (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
References and Notes
- Delgado-Rivera, R.L.; Rosario-Melendez, R.; Uhrich, K.E. Salicylate-based poly(anhydride-esters): A dual system delivery of fibroblast growth factor-2 and the suppression of inflammation. Polymer Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 2010, 51, 598–599. [Google Scholar]
- Osman, A.; Al-Ashmawi, M.I.; Shalaby, M.A. Synthesis and Biological Activity of certain salicylic acid esters. Egypt. J. Pharm. Sci. 1986, 25, 189–194. [Google Scholar]
- Zavodnik, I.B.; Lapshina, E.; Sudnikovich, E.; Boncler, M.; Luzak, B.; Rozalski, M.; Helinska, M.; Watala, C. Structure, stability and antiplatelet activity of O-acyl derivatives of salicylic acid and lipophilic esters of acetylsalicylate. Pharmacol. Rep. 2009, 61, 476–489. [Google Scholar] [CrossRef]
- Kim, C.; Nam, S.-W.; Choi, D.-Y.; Choi, J.-H.; Park, E.-S.; Jhoo, W.-K.; Kim, H.-C. A new antithrombotic agent, aspalatone, attenuated cardiotoxicity induced by doxorubicin in the mouse; possible involvement of antioxidant mechanism. Life Sci. 1996, 60, 75–82. [Google Scholar] [CrossRef]
- Fritzson, I.; Bedingfield, P.T.P.; Sundin, A.P.; McConkey, G.; Nilsson, U.J. N-Substituted salicylamide as selective malaria parasite dihydroorotate dehydrogenase inhibitors. MedChemComm 2011, 2, 895–898. [Google Scholar] [CrossRef]
- Bassoli, A.; Borgonovo, G.; Caimi, S.; Farina, G.; Moretti, M. Oleoylsalicylate derivatives: Synthesis and antifungal activity. Open Nat. Prod. J. 2008, 1, 14–19. [Google Scholar] [CrossRef]
- Silverman, F.P.; Petracek, P.D.; Heiman, D.F.; Ju, Z.; Fledderman, C.M.; Warrior, P. Salicylate activity. 2. Potentiation of atrazine. J. Agric. Food Chem. 2005, 53, 9769–9774. [Google Scholar] [CrossRef] [PubMed]
- Nezu, Y.; Miyazaki, M.; Sugiyama, K.; Wada, N.; Kajiwara, I.; Miyazawa, T. Dimethoxypyrimidines as novel herbicides. Part 2. Synthesis and herbicidal activity of O-pyrimidinylsalicylates and analogues. Pestic. Sci. 1996, 47, 115–124. [Google Scholar] [CrossRef]
- Beber, K.; Jarosch, B.; Langen, G.; Kogel, K.-H. Expression analysis of genes induced in barley after chemical activation reveals distinct disease resistance pathways. Mol. Plant Path. 2000, 1, 277–286. [Google Scholar]
- Hause, B.; Voros, K.; Kogel, K.-H.; Besser, K.; Wasternack, C. A jasmonate-responsive lipoxygenase of barley leaves is induced by plant activators but not by pathogens. J. Plant Physiol. 1999, 154, 459–462. [Google Scholar] [CrossRef]
- The product from (a) is 2-bromophenyl 2-benzyloxybenzoate: FT-IR (ZnSe): 1749, 1470, 1204, 1034, 1018, 749, 737, 695 cm−1; 1H-NMR δ 8.21 (dd, 1H, J = 1.6, 8.1 Hz), 7.69 (d, 1H, J = 7.9 Hz), 7.54–7.61 (m, 3H), 7.27–7.42 (m, 5H), 7.20 (d, 1H, J = 7.8 Hz), 7.22–7.09 (m, 2H), 5.28 (s, 2H); 13C-NMR δ 163.0, 158.9, 148.4, 136.4, 134.4, 133.3, 132.5, 128.5, 128.4, 127.7, 127.2, 126.8, 124.1, 120.5, 118.9, 116.4, 113.7, 70.5; HRMS (ESI, positive ion mode): [M+Na]+ m/z calculated: 405.0102, found: 405.0094.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thompson, D.; Mitchell, S.; Clarke, K.; Sarden, K.; Aiken, K.S. 2-Bromophenyl Salicylate. Molbank 2012, 2012, M789. https://doi.org/10.3390/M789
Thompson D, Mitchell S, Clarke K, Sarden K, Aiken KS. 2-Bromophenyl Salicylate. Molbank. 2012; 2012(4):M789. https://doi.org/10.3390/M789
Chicago/Turabian StyleThompson, Donovan, Sierra Mitchell, Kevin Clarke, Kerry Sarden, and Karelle S. Aiken. 2012. "2-Bromophenyl Salicylate" Molbank 2012, no. 4: M789. https://doi.org/10.3390/M789
APA StyleThompson, D., Mitchell, S., Clarke, K., Sarden, K., & Aiken, K. S. (2012). 2-Bromophenyl Salicylate. Molbank, 2012(4), M789. https://doi.org/10.3390/M789