Abstract
2-Bromophenyl salicylate is synthesized from 2-benzyloxybenzoic acid in two steps. The final compound has been characterized by IR, 1H-NMR, 13C-NMR and HRMS. The melting point for 2-bromophenyl salicylate is provided.
Derivatives of salicylic acids can be of great biological importance. Salicylate derivatives have been probed for their potential to mimic or exceed the effects of salicylic acid in its anti-inflammatory [1,2] and anti-thrombotic activities [3,4]. In addition, researchers have studied the derivatives’ anti-malarial [5], antifungal [6] and herbicidal activities [7,8] and the ability of salicylates to induce immunity to disease in plants [9,10]. Here in we report the synthesis of a new salicylate ester, 2-bromophenyl salicylate 2, in two steps beginning with commercially available 2-benzyloxybenzoic acid 1 (Scheme 1). The first step of the synthesis is a Steglich esterification with 1 and 2-bromophenol. This is followed by debenzylation of the resulting product with hydrogen gas and palladium catalyst to provide 2.
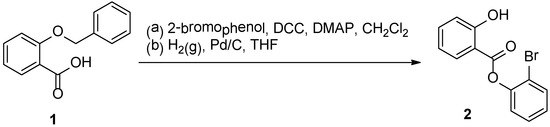
Scheme 1.
Synthesis of 2-bromophenyl salicylate 2.
Experimental
General
Unless noted, all reactions were performed under an atmosphere of argon in oven-dried glassware. Solvents for the reaction were obtained from commercial sources and purified with MBraun Manual Solvent Purification System prior to use. All other chemicals were obtained from commercial sources without further purification. Chromatography was performed with Selecto Scientific Si-gel (particle size 100–200 microns) and the chromatography solvents were purchased from commercial sources and used without further purification. All IR spectra were recorded on a Thermo Nicolet Avatar 370-FTIR. NMR spectra were recorded on a Bruker Multi-Nuclear NMR instrument, 1H-NMR: 250 MHz and 13C-NMR: 63MHz; the solvent was CDCl3. The NMR signals are reported in parts per million (ppm) relative to the residual CHCl3 in the solvent. Signals are described with multiplicity, singlet (s), doublet (d), triplet (t), triplet of doublet (td) and multiplet (m); coupling constants (Hz) and integration. The melting point was measured with the Vernier Melt Station using Vernier LabQuest 2 and is uncorrected. High Resolution Mass Spectrometry was performed using Waters Micromass Q-Tof micro Mass Spectrometer, ESI, positive ion mode.
2-Bromophenyl Salicylate (2)
(a) An oven dried, round-bottom flask equipped with a stir bar and an argon inlet was charged with dichloromethane (50 mL), 2-bromophenol (0.59 mL, 5.05 mmol), N,N′-dicyclohexylcarbodiimide (DCC) (1.14 g, 5.50 mmol), 4-(N,N-dimethylamino)pyridine (DMAP) (122 mg, 1.00 mmol) and 2-benzyloxybenzoic acid (1.14 g, 5.00 mmol). After stirring for 12 hours at room temperature, the resulting white precipitate was removed by vaccum filtration. The clear filtrate was concentrated under vacuum and the crude, yellow oil was purified by flash column chromatography (5% ethyl acetate in petroleum ether) to provide a colorless oil (1.86 g, 97%) [11].
(b) An oven dried, round-bottom flask equipped with a stir bar and an argon inlet was charged with the product from (a) (766.5 mg, 2.00 mmol) in tetrahydrofuran (THF) (20 mL) and 10% Pd/C catalyst (77 mg). The argon inlet was replaced with a hydrogen inlet and the black suspension was stirred under an atmosphere of hydrogen. After 24 h, the hydrogen inlet was removed and the suspension was filtered through a silica-plug (50% ethyl acetate in hexanes). The filtrate was concentrated under vacuum and resulting crude, a yellow solid, was purified by flash column chromatography (2% ethyl acetate in petroleum ether) to provide 2, a white solid (356 mg, 61%). M.p. 63.0–64.7 °C; FT-IR (ZnSe): 3210 (broad, OH), 1686, 1301, 1251, 1208, 1155, 1069, 755, 760, 697 cm−1; 1H-NMR δ 10.34 (s, 1H, OH), 8.18 (dd, 1H, J = 1.5, 7.9 Hz), 7.71 (dd, 1H, J = 1.2, 7.9 Hz) 7.57 (td, 1H, J = 1.4, 7.1 Hz), 7.41 (td, 1H, J = 1.4, 7.4 Hz), 7.21-7.33 (m, 2H), 7.09 (d, 1H, J = 8.8 Hz), 7.02 (d, 1H, J = 7.2 Hz); 13C-NMR δ 167.8, 162.1, 147.6, 136.7, 133.5, 130.5, 128.5, 127.7, 123.7, 119.6, 117.8, 116.1, 111.3; HRMS (ESI, positive ion mode): [M+Na]+ m/z calculated: 314.9633, found: 314.9622.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
Support for this research was provided by the Georgia Southern University Chemistry Department. We thank Jeff Orvis and Marion Welch for their assistance with instrumentation.
References and Notes
- Delgado-Rivera, R.L.; Rosario-Melendez, R.; Uhrich, K.E. Salicylate-based poly(anhydride-esters): A dual system delivery of fibroblast growth factor-2 and the suppression of inflammation. Polymer Prepr. (Am. Chem. Soc., Div. Polym. Chem.) 2010, 51, 598–599. [Google Scholar]
- Osman, A.; Al-Ashmawi, M.I.; Shalaby, M.A. Synthesis and Biological Activity of certain salicylic acid esters. Egypt. J. Pharm. Sci. 1986, 25, 189–194. [Google Scholar]
- Zavodnik, I.B.; Lapshina, E.; Sudnikovich, E.; Boncler, M.; Luzak, B.; Rozalski, M.; Helinska, M.; Watala, C. Structure, stability and antiplatelet activity of O-acyl derivatives of salicylic acid and lipophilic esters of acetylsalicylate. Pharmacol. Rep. 2009, 61, 476–489. [Google Scholar] [CrossRef]
- Kim, C.; Nam, S.-W.; Choi, D.-Y.; Choi, J.-H.; Park, E.-S.; Jhoo, W.-K.; Kim, H.-C. A new antithrombotic agent, aspalatone, attenuated cardiotoxicity induced by doxorubicin in the mouse; possible involvement of antioxidant mechanism. Life Sci. 1996, 60, 75–82. [Google Scholar] [CrossRef]
- Fritzson, I.; Bedingfield, P.T.P.; Sundin, A.P.; McConkey, G.; Nilsson, U.J. N-Substituted salicylamide as selective malaria parasite dihydroorotate dehydrogenase inhibitors. MedChemComm 2011, 2, 895–898. [Google Scholar] [CrossRef]
- Bassoli, A.; Borgonovo, G.; Caimi, S.; Farina, G.; Moretti, M. Oleoylsalicylate derivatives: Synthesis and antifungal activity. Open Nat. Prod. J. 2008, 1, 14–19. [Google Scholar] [CrossRef]
- Silverman, F.P.; Petracek, P.D.; Heiman, D.F.; Ju, Z.; Fledderman, C.M.; Warrior, P. Salicylate activity. 2. Potentiation of atrazine. J. Agric. Food Chem. 2005, 53, 9769–9774. [Google Scholar] [CrossRef] [PubMed]
- Nezu, Y.; Miyazaki, M.; Sugiyama, K.; Wada, N.; Kajiwara, I.; Miyazawa, T. Dimethoxypyrimidines as novel herbicides. Part 2. Synthesis and herbicidal activity of O-pyrimidinylsalicylates and analogues. Pestic. Sci. 1996, 47, 115–124. [Google Scholar] [CrossRef]
- Beber, K.; Jarosch, B.; Langen, G.; Kogel, K.-H. Expression analysis of genes induced in barley after chemical activation reveals distinct disease resistance pathways. Mol. Plant Path. 2000, 1, 277–286. [Google Scholar]
- Hause, B.; Voros, K.; Kogel, K.-H.; Besser, K.; Wasternack, C. A jasmonate-responsive lipoxygenase of barley leaves is induced by plant activators but not by pathogens. J. Plant Physiol. 1999, 154, 459–462. [Google Scholar] [CrossRef]
- The product from (a) is 2-bromophenyl 2-benzyloxybenzoate: FT-IR (ZnSe): 1749, 1470, 1204, 1034, 1018, 749, 737, 695 cm−1; 1H-NMR δ 8.21 (dd, 1H, J = 1.6, 8.1 Hz), 7.69 (d, 1H, J = 7.9 Hz), 7.54–7.61 (m, 3H), 7.27–7.42 (m, 5H), 7.20 (d, 1H, J = 7.8 Hz), 7.22–7.09 (m, 2H), 5.28 (s, 2H); 13C-NMR δ 163.0, 158.9, 148.4, 136.4, 134.4, 133.3, 132.5, 128.5, 128.4, 127.7, 127.2, 126.8, 124.1, 120.5, 118.9, 116.4, 113.7, 70.5; HRMS (ESI, positive ion mode): [M+Na]+ m/z calculated: 405.0102, found: 405.0094.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).