Abstract
A new thiosemicarbazone, 1-methyl-2-imino-N-(methanethialdiamine)-yl-4-iminoimidazolidin was synthesized and its UV-VIS, IR, and NMR spectroscopic data and CHN analysis are presented.
The chemistry of thiosemicarbazones has received considerable attention because of their variable bonding modes, promising biological implications, structural diversity, and ion-sensing ability [1,2]. They have been used as drugs and are reported to possess a wide variety of biological activities against bacteria, fungi, and certain type of tumors, and they are also a useful model for bioinorganic processes [3]. In continuation of previous studies [4,5,6,7,8,9,10,11,12,13,14], we have focused on synthesis of new heterocyclic compounds, and herein we are reporting the synthesis of 1-methyl-2-imino-N-(methanethialdiamine)-yl-4-iminoimidazolidin as new molecule (Scheme 1).
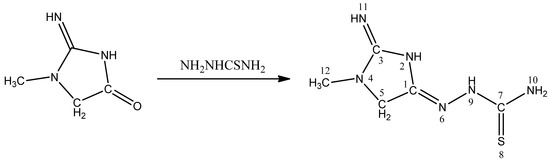
Scheme 1.
Synthesis of 1-methyl-2-imino-N-(methanethialdiamine)-yl-4-iminoimidazolidin.
Experimental
Synthesis of 2-(2-Imino-1-methylimidazolidin-4-ylidene)hydrazinecarbothioamide
A mixture of 1-methyl-4-oxo-2-iminoimidazolidin (2.26 g, 0.02 mol) and thiosemicarbazide (1.82 g, 0.02 mol) in 100 mL of ethanol was refluxed for 3 h. The solvent was evaporated on a rotary evaporator. The title compound was washed with cold ethanol, and dried under vacuum over P4O10. Yield 70%, (light brown) [7].
Melting point: 153 °C.
UV-VIS in DMF 255 and 322 nm.
FT-IR spectroscopy; 3421 cm−1 (N-H stretching vibrations, NH2); 1631 cm−1 (C=N) and 1618 cm−1 (C=N).
1H-NMR (300 MHz, DMSO-d6): 1.80 (s, 1H, NH), 2.20 (s, 3H, CH3), 2.70 (s, 2H, CH2), 8.00 (s, 1H, NH), 9.10 (s, 1H, NH) and 10.90 (s, 1H, NH).
Elemental analysis: C, 32.25 (31.91); H, 5.41 (5.11); N, 45.13 (44.74).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
This study was supported by University of Technology, Baghdad, Iraq and Universiti Kebangsaan Malaysia under the DIP-2012-02 grant.
References
- Casas, J.S.; García-Tasende, M.S.; Sordo, J. Corrigendum to Main group metal complexes of semicarbazones and thiosemicarbazones. A structural review. Coord. Chem. Rev. 2000, 209, 197–261. [Google Scholar] [CrossRef]
- Mishra, D.; Naskar, S.; Drew, M.G.B.; Chattopadhyay, S.K. Synthesis, spectroscopic and redox properties of some ruthenium(II) thiosemicarbazone complexes: Structural description of four of these complexes. Inorganica Chim. Acta 2006, 359, 585–592. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Abdulreazak, H.; Abood, H. Synthesis, Characterization, Theoretical Crystal Structure, and Antibacterial Activities of Some Transition Metal Complexes of the Thiosemicarbazone. Bioinorg. Chem. Appl. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A.; Takriff, M.S. Antimicrobial and antioxidant activities of new metal complexes derived from 3-aminocoumarin. Molecules 2011, 16, 6969–6984. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Al-Bayati, R.I.H.; Saour, K.Y.; Radi, M.F. Cytotoxicity, antioxidant and antimicrobial activities of novel 2-quinolone derivatives derived from coumarins. Res. Chem. Intermediat. 2011, 38, 559–569. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.H.; Mohamad, A.B. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules 2011, 16, 6833–6843. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, A.A.H.; Al-Amiery, A.A.; Musa, A.Y.; Mohamad, A.B. The Antioxidant Activity of New Coumarin Derivatives. Int. J. Mol. Sci. 2011, 12, 5747–5761. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, A.A.H.; Al-Amiery, A.A.; Shikara, M.; Mohamad, A. Synthesis, Structure elucidation and DFT studies of New thiadiazoles. Int. J. Phys. Sci. 2011, 6, 6692–6697. [Google Scholar]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Ibrahim, H.H.; Al-Tamimi, A.A. Antioxidant, antimicrobial, and theoretical studies of the thiosemicarbazone derivative Schiff base 2-(2-imino-1-methylimidazolidin-4-ylidene) hydrazinecarbothioamide (IMHC). Org. Med. Chem. Lett. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.A. Antifungal and Antioxidant Activities of Pyrrolidonethiosemicarbazone Complexes. Bioinorg. Chem. Appl. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Al-Amiery, A.A. Synthesis and antioxidant, antimicrobial evaluation, DFT studies of novel metal complexes derivate from Schiff base. Res. Chem. Intermediat. 2012, 38, 745–759. [Google Scholar] [CrossRef]
- Al-Amiery, A.A. Antimicrobial and Antioxidant Activities of New Metal Complexes Derived from (E)-3-((5-phenyl-1,3,4-oxadiazol-2-ylimino)methyl)naphthalen-2-ol. Med. Chem. Res. 2011. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.A. Antifungal Activities of New Coumarins. Molecules 2012, 17, 5713–5723. [Google Scholar] [CrossRef] [PubMed]
- Junaedi, S.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.A.; Takriff, M.S. Synthesis and Characterization of Novel Corrosion Inhibitor Derived from Oleic Acid: 2-Amino 5-Oleyl-1,3,4-Thiadiazol (AOT). Int. J. Electrochem. Sci. 2012, 7, 3543–3554. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).