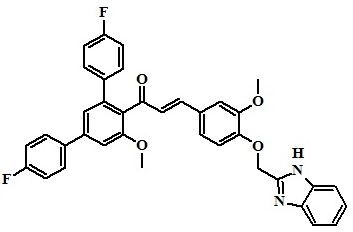
(2E)-3-[4-(1H-Benzimidazol-2-ylmethoxy)-3-methoxyphenyl]-1-(4,4''-difluoro-5'-methoxy-1,1':3',1''-terphenyl-4'-yl)prop-2-en-1-one
Abstract
:Introduction
Results and Discussion
Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Acknowledgments
References
- Gill, M.; Steglich, W. Pigments of fungi (Macromycetes). Prog. Chem. Org. Nat. Prod. 1987, 51, 1–317. [Google Scholar] [CrossRef]
- Liu, J.K. Natural terphenyls: Developments since 1877. Chem. Rev. 2006, 106, 2209–2223. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.A.; Yozhitsa, I.N.; Bugaeva, L.I.; Anisimova, V.A. Benzimidazole derivatives: Spectrum of pharmacological activity and toxicological properties (a review). Pharm. Chem. J. 1999, 33, 232–243. [Google Scholar] [CrossRef]
- Samshuddin, S.; Narayana, B.; Sarojini, B.K. Ethyl 4,4''-difluoro-5'-hydroxy-1,1':3',1''-terphenyl-4'-carboxylate. Molbank 2011, 2011, M745. [Google Scholar] [CrossRef]
- Samshuddin, S.; Narayana, B.; Shetty, D.N.; Raghavendra, R. An efficient synthesis of 2,4,6-triaryl pyridines and their biological evaluation. Der Pharma Chemica 2011, 3, 232–240. [Google Scholar]
- Samshuddin, S.; Narayana, B.; Sarojini, B.K.; Srinivasan, R.; Vinayachandra; Chandrashekar, K.R. Synthesis, characterization and biological evaluation of some pyrazoles derived from α,β-dibromo 4,4'-difluoro chalcone. Der Pharma Chemica 2012, 4, 587–592. [Google Scholar]
- Samshuddin, S.; Narayana, B.; Baktir, Z.; Akkurt, M.; Yathirajan, H.S. Synthesis, characterization and crystal structure of 1-[3,5-bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl]propan-1-one. Der Pharma Chemica 2011, 3, 487–493. [Google Scholar]
- Samshuddin, S.; Butcher, R.J.; Akkurt, M.; Narayana, B.; Yathirajan, H.S.; Sarojini, B.K. 1,3-Bis(4-fluorophenyl)-N,N-(propane-1,3-diylidene)dihydroxylamine. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, E67, o1954–o1955. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Chia, T.S.; Samshuddin, S.; Narayana, B.; Sarojini, B.K. 2-[3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl]-4,6-bis(4-fluorophenyl) pyrimidine. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, E68, o807–o808. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, J.P.; Golen, J.A.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. (6Z)-3,5-Bis(4-fluorophenyl)-6-(1-hydroxyethylidene)cyclohex-2-en-1-one. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, E68, o638–o639. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Chia, T.S.; Samshuddin, S.; Narayana, B.; Sarojini, B.K. Ethyl 4,4''-difluoro-5'-methoxy-1,1':3',1''-terphenyl-4'-carboxylate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, E68, o172. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Arshad, S.; Samshuddin, S.; Narayana, B.; Sarojini, B.K. 5-(4,4''-Difluoro-5'-hydroxy-1,1':3',1''-terphenyl-4'-yl)-3-(morpholin-4-ylmethyl)-1,3,4-oxadiazole-2(3H)-thione. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, E67, o3372. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.K.; Hemamalini, M.; Samshuddin, S.; Narayana, B.; Sarojini, B.K. 1-(4,4''-Difluoro-5'-methoxy-1,1':3',1''-terphenyl-4'-yl)ethanone. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, E68, o163. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, J.P.; Golen, J.A.; Samshuddin, S.; Narayana, B.; Yathirajan, H.S. 4-(1H-Benzimidazol-2-ylmethoxy)-3-methoxybenzaldehyde tetrahydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, E67, o2021–o2022. [Google Scholar] [CrossRef] [PubMed]

© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Samshuddin, S.; Narayana, B.; Shetty, D.N.; Srinivasan, R.; Sarojini, B.K. (2E)-3-[4-(1H-Benzimidazol-2-ylmethoxy)-3-methoxyphenyl]-1-(4,4''-difluoro-5'-methoxy-1,1':3',1''-terphenyl-4'-yl)prop-2-en-1-one. Molbank 2012, 2012, M764. https://doi.org/10.3390/M764
Samshuddin S, Narayana B, Shetty DN, Srinivasan R, Sarojini BK. (2E)-3-[4-(1H-Benzimidazol-2-ylmethoxy)-3-methoxyphenyl]-1-(4,4''-difluoro-5'-methoxy-1,1':3',1''-terphenyl-4'-yl)prop-2-en-1-one. Molbank. 2012; 2012(3):M764. https://doi.org/10.3390/M764
Chicago/Turabian StyleSamshuddin, Seranthimata, Badiadka Narayana, Divya N. Shetty, Rajagopalan Srinivasan, and Balladka Kunhanna Sarojini. 2012. "(2E)-3-[4-(1H-Benzimidazol-2-ylmethoxy)-3-methoxyphenyl]-1-(4,4''-difluoro-5'-methoxy-1,1':3',1''-terphenyl-4'-yl)prop-2-en-1-one" Molbank 2012, no. 3: M764. https://doi.org/10.3390/M764
APA StyleSamshuddin, S., Narayana, B., Shetty, D. N., Srinivasan, R., & Sarojini, B. K. (2012). (2E)-3-[4-(1H-Benzimidazol-2-ylmethoxy)-3-methoxyphenyl]-1-(4,4''-difluoro-5'-methoxy-1,1':3',1''-terphenyl-4'-yl)prop-2-en-1-one. Molbank, 2012(3), M764. https://doi.org/10.3390/M764