Abstract
An hydroxypropyl-aziridine-containing side chain is attached to an oxonaphthalene-annelated pyrrole in expectation of DNA alkylating properties. The cytotoxicity is evaluated against two cell lines, KB-31 and KB-8511, respectively.
Introduction
Chlorambucil and melphalan are chemotherapy drugs belonging to the class of nitrogen mustard alkylating agents. Both compounds are believed to exert their antitumor effects by cross-linking DNA via aziridinium cation intermediates arising from the bis(2-chloroethyl)amine moiety [1]. Besides that there are anticancer agents with the aziridine moiety, such as TEPA, thio-TEPA and triethylenemelamine with a 1,3,5-triazine nucleus. In continuation of our department’s previous studies in the field of antitumor agents [2,3,4,5,6,7,8,9,10], we are reporting in this paper the synthesis of the oxonaphthalene-annelated pyrrole 2 with an attached side chain containing an aziridine group. The rationale is that the three-membered aziridine ring is struturally analogous to the immonium-intermediate formed from the nitrogen mustards. The aziridine moietiy is not charged and the reactivity results from the strain on the three-member ring structure [11]. Recent studies with aziridine substituted quinones showed promising results against breast cancer tumor cells [12,13]. The cytotoxic activity of 2 was evaluated.
Results and Discussion
Reaction of 1 [14] with 2-(chloromethyl)oxirane with KOH in DMSO [15] afforded the N-alkylated product. The following reaction with aziridine [16] gave the target compound 2 (Scheme 1). The biological activity of 2 was tested against two cancer cell lines, KB-31 and KB-8511, respectively. KB-31 is a drug sensitive human epidermoid cell line, whereas KB-8511 is a multi-drug resistant subline, typically overexpressing P-glycoprotein. The IC50[μM] values of 2 are 9.362 (KB-31) and 6.452 (KB-8511), respectively (3 days incubation time; staining with 0.05% methylene blue; optical density measured at 665 nm; for further experimental details, see [17,18]).
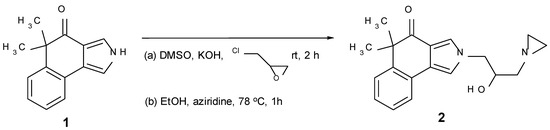
Scheme 1.
Synthesis of target compound 2.
Experimental
2-[3-(Aziridin-1-yl)-2-hydroxypropyl]-5,5-dimethyl-2,5-dihydro-4H-benzo[e]isoindol-4-one (2)
(a) To a solution of 1 [12] (0.5 g, 2.37 mmol) in 4.7 mL of dry DMSO were added at room temperature dropwise under argon 1.5 mL (18.96 mmol) of 2-(chloromethyl)oxirane. Subsequently 0.265 g (4.74 mmol) of KOH were added and stirring was continued for 2 h at room temperature. The reaction mixture was diluted with 9.5 ml of H2O, extracted with CH2Cl2, dried (Na2SO4), concentrated in vacuo and isolated after column chromatography (silica gel, ethyl acetate/light petroleum 70/30). Yield 284 mg (45%) of a colorless oil.
(b) The resulting product (284 mg) was dissolved in 4.6 ml EtOH (1% TEA) and after the addition of 0.37 mL (7.2 mmol) of aziridine under argon the reaction mixture was refluxed for 1 h. After concentration in vacuo the resulting crude product was purified by column chromatography (silica gel, ethyl acetate/ethanol 95/5) to afford 104 mg (32%) of colorless crystals of 2. m.p.: 118–119 °C (ethyl acetate/ethanol). IR (KBr): 3397, 1650, 1526, 1208, 1159 cm−1. MS (EI, 70 eV) m/z: 310 (M+, 5%), 268 (M+-42, 0.5), 101 (20), 59 (100), 58 (42), 57 (33), 56 (28), 55 (26), 45 (26). 1H-NMR (CDCl3, 200 MHz) δ = 7.56 (m, 1H, 9-H), 7.43 (m, 1H, 6-H), 7.40 (d, J = 2.2 Hz, 1H, 3-H), 7.22 (m, 2H, 7-H, 8-H), 7.10 (d, J = 2.2 Hz, 1H, 1-H), 4.14–3.99 (m, 3H, 1'-H, 2'-H), 3.8 (sbr, 1H, OH), 2.41 (dd, J = 7.5 and 12 Hz, 1H, 3'-H), 2.12 (dd, J = 3.8 and 12 Hz, 1H, 3'-H), 1.82–1.73 (m, 2H, aziridine-CH2), 1.50 (s, 6H, (CH3)2), 1.29–1.16 (m, 2H, aziridine-CH2). 13C-NMR (CDCl3, 50 MHz) δ = 198.6 (C-4), 144.1 (C-5a), 127.1(C-6), 126.8 (C-9a), 126.6 (C-7), 126.2 (C-8), 125.0 (C-9b), 124.1(C-3), 122.7 (C-9), 118.3 (C-3a), 116.1 (C-1), 70.3 (C-2'), 64.0 (C-3'), 54.3 (C-1'), 47.6 (C-5), 28.2/28.1((CH3)2), 27.6/27.2 (2× aziridine-CH2). HRMS calc. for C19H22N2O2: 310.94 Calc.: 310.1680. Found: 310.1680.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
We are indebted to Novartis AG (Vienna, Austria) for the evaluation of the cytotoxic activity.
References and Notes
- Montgomery, J.A. Agents That React with DNA. In Cancer Chemotherapeutic Agents, ACS Professional Reference Book; Foye, W.O., Ed.; American Chemical Society: Washington, DC, USA, 1995; pp. 111–121. [Google Scholar]
- Pongprom, N.; Müller, G.; Schmidt, P.; Holzer, W.; Spreitzer, H. Synthesis of anticancer compounds, III (Bioorg Med Chem Lett 17, 6091, 2007), carbinol derivatives of azanaphthoquinone annelated pyrroles. Monatsh. Chem. 2009, 140, 309–313. [Google Scholar] [CrossRef]
- Shanab, K.; Pongprom, N.; Wulz, E.; Holzer, W.; Spreitzer, H.; Schmidt, P.; Aicher, B.; Mueller, G.; Günther, E. Synthesis and biological evaluation of novel cytotoxic azanaphthoquinone annelated pyrrolo oximes. Bioorg. Med. Chem. Lett. 2007, 17, 6091–6095. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, H.; Puschmann, C. Dual function antitumor agents based on bioreduction and DNA-alkylation. Monatsh. Chem. 2007, 138, 517–522. [Google Scholar] [CrossRef]
- Haider, N.; Sotelo, E. 1,5-Dimethyl-6H-pyridazino[4,5-b]carbazole, a 3-Aza bioisoster of the antitumor alkaloid olivacine. Chem. Pharm. Bull. 2002, 50, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Kabicher, T.; Käferböck, J.; Plenk, A. Synthesis and in-vitro antitumor activity of 1-[3-(indol-1-yl)prop-1-yn-1-yl]phthalazines and related compounds. Molecules 2007, 12, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Jbara, R.; Käferböck, J.; Traar, U. Synthesis of tetra- and pentacyclic carbazole-fused imides as potential antitumor agents. ARKIVOC 2009, vi, 38–47. [Google Scholar]
- Spreitzer, H.; Puschmann, C. Regioselective alkylation of an oxonaphthalene-annelated pyrrol system. Molbank 2009, 2009, M619. [Google Scholar] [CrossRef]
- Spreitzer, H.; Puschmann, C. 2-[4-[Bis(2-chloroethyl)amino]benzyl]-5,5-dimethyl-2,5-dihydro-4H-benzo[e]isoindol-4-one (Cytotoxic Oxonaphthalene-Pyrroles, Part I). Molbank 2010, 2010, M651. [Google Scholar] [CrossRef]
- Spreitzer, H.; Holzer, W.; Puschmann, C.; Pichler, A.; Kogard, A.; Tschetschkowitsch, K.; Heinze, T.; Bauer, S.; Shabaz, N. Synthesis and NMR-investigation of annelated pyrrole derivatives. Heterocycles 1997, 45, 1989–1997. [Google Scholar] [CrossRef]
- Spreitzer, H.; Holzer, W.; Fülep, G.; Puschmann, C. N-substituted 5,5-dimethyl-2,5-dihydro-4H-isoindol-4-ones: Synthesis and NMR-investigation. Heterocycles 1996, 43, 1911–1922. [Google Scholar] [CrossRef]
- Huang, C.H.; Kuo, H.S.; Liu, J.W.; Lin, Y.L. Synthesis and Antitumor Evaluation of Novel Bis-Triaziquone Derivatives. Molecules 2009, 14, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Su, Y.T.; Chen, B.H. A study on inhibition mechanism of breast cancer cells by bis-type triziquone. Eur. J. Pharmacol. 2010, 637, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.J.; Bernstein, P.R.; Cronk, L.A.; Dosset, D.L.; Hebbel, K.C.; Maduskuie, T.P., Jr.; Shapiro, H.S.; Vacek, E.P.; Yee, Y.K.; Willard, A.K.; et al. Hydroxyacetophenone-derived antagonists of the peptidoleukotrienes. J. Med. Chem. 1989, 32, 807–826. [Google Scholar] [CrossRef] [PubMed]
- Giancaspro, G.I.; Pizzorno, M.T.; Albonico, S.M.; Bindstein, E.; Garofalo, A.; Zeichen, R. Synthesis of disubstituted tetrahydrocarbazoles with potential antidepressant activity. Farmaco 1989, 44, 483–493. [Google Scholar] [PubMed]
- Naylor, M.A.; Threadgill, M.D.; Webb, P.; Startford, I.J.; Stephens, M.A.; Fielder, G.E.; Adams, G.E. 2-Nitroimidazole dual-function bioreductive drugs: Studies on the effects of regioisomerism and side-chain structural modifications on differential cytotoxicity and radiosensitization by aziridinyl and oxiranyl derivatives. J. Med. Chem. 1992, 35, 3573–3578. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Regenass, U.; Fabbro, D.; Alteri, E.; Rösel, J.; Müller, M.; Caravatti, G.; Matter, A. A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int. J. Cancer 1989, 43, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Scarpelli, R.; Bollbuck, B.; Werschkun, B.; Pereira, M.M.; Wartmann, M.; Altmann, K.H.; Zaharevitz, D.; Guscio, R.; Giannakakou, P. Chemical synthesis and biological properties of pyridine epothilones. Chem. Biol. 2000, 7, 593–599. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).