Abstract
1-Methyl-7-(propan-2-yl)-4-oxatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione (1) as starting material and hydroxylamine were used for the preparation of the title compound 4-hydroxy-1-methyl-7-(propan-2-yl)-4-azatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione (2). This product was characterized by 1H-NMR, 13C-NMR, MS and elemental analysis.
1. Introduction
The extensively studied class of cyclic imides is known for a wide spectrum of biological activities. Less examined, but also valuable for their activities are N-hydroxy analogues. They were synthesized as potential β-adrenolytic [1], anxiolytic [2], anti-inflammatory and analgesic agents [3]. Some of the reported derivatives possess metal-chelating abilities, and a number of these compounds inhibits the growth of microorganisms [4]. Additionally, cyclic imides were tested in vitro against various bacteria including Gram-positive cocci, Gram-negtive rods and fungi species (e.g., Candida) [5,6]. The large group of 4-azatricyclo[5.2.2.02,6]undec-8-ene was tested for their affinity to 5-HT1A and 5-HT2A receptors [7]. The most popular method to produce cyclic imides and N-hydroxy analogues is the well-known Diels-Alder reaction [8], but this work describes another conventional method for the synthesis of 4-azatricyclo[5.2.2.02,6]undec-8-ene derivatives [9,10,11]. This one is reserved for N-hydroxy derivatives. The aim of our present work was to prepare 4-hydroxy-1-methyl-7-(propan-2-yl)-4-azatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione (2), starting from the corresponding anhydride 1-methyl-7-(propan-2-yl)-4-oxatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione (1) using an aqueous solution of hydroxylamine (Scheme 1).
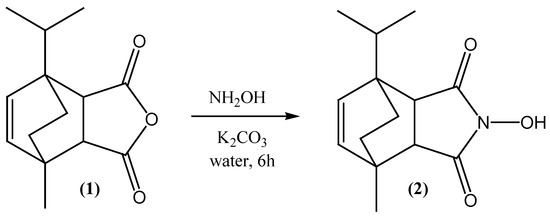
Scheme 1.
Synthesis of 4-hydroxy-1-methyl-7-(propan-2-yl)-4-azatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione.
2. Experimental
2.1. General
The melting point was determined in an open capillary and is uncorrected. The NMR spectra were recorded on a Bruker AVANCE DMX400 spectrometer, operating at 400 MHz (1H-NMR) and 100 MHz (13C-NMR). The chemical shift values are expressed in ppm relative to TMS as an internal standard. Mass spectra (ESI) measurements were carried out on a Waters ZQ Micro-mass instrument with a quadrupol mass analyzer. The spectra were recorded in positive ion mode at a declustering potential of 40–60 V. The sample was previously separated on a UPLC column (C18) using a Waters UPLC ACQUITYTM system connected with a DPA detector. Flash chromatography was performed on Merck silica gel 60 (200–400 mesh) using a chloroform/methanol (19:1 vol) mixture as eluent. Analytical TLC was carried out on silica gel F254 (Merck) plates (0.25 mm thickness).
2.2. Synthesis of 4-Hydroxy-1-methyl-7-(propan-2-yl)-4-azatricyclo[5.2.2.02,6]undec-8-ene-3,5-dione (2)
A mixture of the anhydride 1 (0.234 g, 1 mmol), K2CO3 (0.20 g, 1.4 mmol), NH2OH·HCl (0.20 g, 2.9 mmol) and H2O (5 mL) was refluxed for 5 h. The precipitate was filtered, washed with water, dried and purified by column chromatography (silica gel) to obtain a white solid.
Yield: 90%.
m.p.: 139–140 °C.
1H-NMR (400 MHz, CDCl3) δ (ppm): 5.97 (d, 1H, CH=, J = 8.4 Hz); 5.89 (d, 1H, CH=, J = 8.4 Hz); 4.18 (br s, 1H, OH); 2.93 (d, 1H, CH-C=O, J = 7.6 Hz); 2.58–2.51 (m, 2H, CH-C=O, CH=); 1.50–1.40 (m, 2H, CH2); 1.46 (s, 3H, CH3); 1.36–1.24 (m, 2H, CH2); 1.08 (d, 3H, CH3, J = 6.8 Hz); 0.98 (d, 3H, CH3, J = 6.8 Hz).
13C-NMR (100 MHz, CDCl3) δ (ppm):174.3, 172.5, 136.3, 48.9, 47.7, 45.1, 43.7, 36.9, 34.2, 29.7, 22.6, 18.4, 16.9.
ESI MS: m/z = 272.4 [M+Na] + (100%).
Elemental analysis: Calculated for C14H19NO3 (249.306): C, 67.45%, H, 7.68%, N, 5.62%. Found: C, 67.48%, H, 7.50%, N, 5.72%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Kossakowski, J.; Jarocka, M. Synthesis of new derivatives of 1-hydroxymethyl and 1-methoxymethyl-dibenzo[e.h]bicyclo[2.2.2]-octane-2,3-dicarboximide with an expected β-adrenolytic activity. Acta Polon. Pharm. 2000, 57, S60–S62. [Google Scholar]
- Kossakowski, J.; Krawiecka, M. Synthesis of N-substituted cyclic imides with an expected anxiolytic activity. XXIV. Derivatives of N-hydroxy-1-methoxybicyclo[2.2.2]oct-5-ene-2,3-dicarboximide. Acta Polon. Pharm. 2003, 60, 177–182. [Google Scholar]
- Abu-Hashem, A.A.; Gouda, M.A. Synthesis, Anti-inflammatory and Analgesic Evaluation of Certain New 3a,4,9,9a-Tetrahydro-4,9-benzenobenz[f]isoindole-1,3-diones. Arch. Pharm. Chem. Life Sci. 2011, 344, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Matsuo, K.; Nakanishi, A.; Kataoka, Y.; Takase, K.; Otsuki, S. Syntheses of Cyclic Hydroxamic Acid Derivatives, and Their Chelating Abilities and Biological Activities. Chem. Pharm. Bull. 1988, 36, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Struga, M.; Kossakowski, J.; Stefanska, J.; Zimniak, A.; Koziol, A.E. Synthesis and antibacterial activity of bis-[2-hydroxy-3-(1,7,8,9,10-pentamethyl-3,5-dioxo-4-aza-tricyclo[5.2.1.02,6]dec-8-en-4-yloxy)-propyl]-dimethyl-ammonium chloride. Eur. J. Med. Chem. 2008, 43, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Struga, M.; Krawiecka, M.; Kossakowski, J.; Stefańska, J.; Miroslaw, B.; Kozioł, A.E. Synthesis and Structural Characterisation of Derivatives of Tricyclo[5.2.1.02,6]dec-8-ene-3,5-dione with an Expected Antimicrobial Activity. J. Chin. Chem. Soc. 2008, 55, 1258–1265. [Google Scholar] [CrossRef]
- Bojarski, A.J.; Koziol, A.; Kuran, B.; Kossakowski, J.; Jagiello-Wojtowicz, E.; Chodkowska, A. Synthesis and serotonin receptor activity of the arylpiperazine alkyl/propoxy derivatives of new azatricycloundecanes. Eur. J. Med. Chem. 2009, 44, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Ogbomo, S.M.; Burnell, D.J. cis-3,5-Cyclohexadiene-1,2-diol derivatives: Facial selectivity in their Diels–Alder reactions with ethylenic, acetylenic and azo dienophiles. Org. Biomol. Chem. 2006, 4, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Fujimoto, M.; Sakai, M.; Matsumoto, A. Les Hydrazides et Hydroxamates Intramoleculaires. III. Sur la Synthese des Homologues d’Hydroxy-2 cis-Perhydroisoindolinedione-1,3. Chem. Pharm. Bull. 1968, 16, 622–625. [Google Scholar] [CrossRef]
- Stolberg, M.A.; Mosher, W.A.; Wagner-Jauregg, T. Synthesis of a Series of Vicinally Substituted Hydroxamic Acids. J. Am. Chem. Soc. 1957, 79, 2615–2617. [Google Scholar] [CrossRef]
- Bauer, L.; Miarka, S.V. Stereospecific Lossen Rearrangements. J. Org. Chem. 1959, 24, 1293–1296. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).