Abstract
A novel probe L has been synthesized by classical Schiff-base reaction between 1-benzothiophene-3-carbaldehyde and tetraethylenepentamine as diamine. The structure of compound L was confirmed by melting point, elemental analysis, ESI-MS spectrometry, IR and 13C-NMR and 1H-NMR spectroscopy.
The constant and growing interest in the development of new synthetic methods for the preparation of systems involving thiophene subunits is justified by their valuable physiological and pharmacological properties [1,2]. A large number of benzothiophene derivatives have been found to exhibit a wide variety of pharmaceutical properties such as anti-microbial [3], anti-cancer [4], and anti-HIV activity [5].
Since the last five years, our research group has been interested in the introduction of sulphur containing ligands into the skeleton of new fluorescent and colorimetric probes, and new active MALDI-TOF-MS molecules for sensing studies [6,7,8,9,10,11].
This paper describes the synthesis and characterization of a new benzothiophene di-imine probe (L), prepared by simple and classical Schiff-base reaction between 1-benzothiophene-3-carbaldehyde and tetraethylenepentamine (See scheme 1).
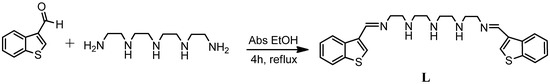
Scheme 1.
Schematic representation of compound L.
Experimental
A solution of 1-benzothiophene-3-carbaldehyde (0.361 g, 2.108 mmol) in absolute ethanol (25 mL) was added drop wise to a refluxing solution of tetraethylenepentamine (0.199 g, 1.054 mmol) in the same solvent (35 mL). The resulting solution was gently refluxed with magnetic stirring for ca. 4 h. During that period the color changed from yellow to green. The resulting solution was concentrated under vacuum to 1/3 of its original volume. Diethyl ether was added to form a green precipitate that was filtered off and dried under vacuum. The compound isolated was characterized as chemosensor L.
Melting point: 123–125 °C.
Yield: 395 mg (L) (78%).
ESI-MS: m/z (rel. int%): 478.20 (100) [L+H]+.
1H-NMR (CDCl3) (L): δ = 8.2 (s, 2H, C–H)thiophene; 8.0–7.8 (m, 8H, C–H, Ar); 7.2 (s, 2H, N=C–H); 3.8–3.2 (m, 12H, CH2); 2.6–2.2 (m, 4H, CH2); 1.8 (s, 3H, NH) ppm.
13C-NMR (CDCl3) (L): δ = 160.8; 139.9; 127.9; 126.4; 124.4; 124.3; 123.2; 122.8; 119.7; 55.3; 50.2; 49.0.
IR (cm−1) (L): 3036 (C–H, Ar), 1645 (C=N, Imine), 1598, 1493 (C=C, Ar).
Elemental analysis: Calcd for C26H31N5S2·2H2O: C, 60.79; H, 6.87; N,13.63. Found (L): C, 60.95; H, 7.20; N, 13.75.
Uv-vis (CHCl3), [L] = 1.00 ×10−5 M, λmax 287 and 302 nm. Non-emissive.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
We are grateful to Xunta de Galicia (Spain) for projects 09CSA043383PR and 10CSA383009PR (Biomedicine) and to the Scientific Association ProteoMass for financial support. C.N. thanks the Fundação para a Ciência e a Tecnologia/FEDER (Portugal/EU) programme postdoctoral contract SFRH/BPD/65367/2009. J.F.L. thanks Xunta de Galicia (Spain) for a research contract by project 09CSA043383PR in Biomedicine. J.L.C. and C.L. thank Xunta de Galicia for the Isidro Parga Pondal Research program.
References and Notes
- Sall, D.J.; Bailey, D.L.; Bastian, J.A.; Buben, J.A.; Chirgadze, N.Y.; Clemens-Smith, A.C.; Denney, M.L.; Fisher, M.J.; Giera, D.D.; Gifford-Moore, D.S.; et al. Diamino Benzo[b]thiophene Derivatives as a Novel Class of Active Site Directed Thrombin Inhibitors. 5. Potency, Efficacy, and Pharmacokinetic Properties of Modified C-3 Side Chain Derivatives. J. Med. Chem. 2000, 43, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Akay, S.; Yang, W.; Wang, J.; Lin, L.; Wang, B. Synthesis and Evaluation of Dual Wavelength Fluorescent Benzo[b]thiophene Boronic Acid Derivatives for Sugar Sensing. Chem. Biol. Drug Des. 2007, 70, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, G.; Alagarsamy, V.; Prakash, C.R.; Selvam, T.P.; Karthick, V.; Kumar, P.D. Synthesis and anti-microbial activities of some 3-(4-phenylthiazole-2-yl)/3-(3-carbethoxy-4,5,6,7-tetrahydrobenzothiophene-2yl)quinazolin-4(3H)-one. RASĀYAN J. Chem. 2009, 2, 746–752. [Google Scholar]
- Starčević, K.; Kralj, M.; Piantanida, I.; Šuman, L.; Pavelić, K.; Karminski-Zamola, G. Synthesis, photochemical synthesis, DNA binding and antitumor evaluation of novel cyano- and amidino-substituted derivatives of naphtho-furans, naphtho-thiophenes, thieno-benzofurans, benzo-dithiophenes and their acyclic precursors. Eur. J. Med. Chem. 2006, 41, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Carballo, M.; Conde, M.; Tejedo, J.; Gualberto, A.; Jimenez, J.; Monteseirín, J.; Santa María, C.; Bedoya, F.J.; Hunt, S.W., III; Pintado, E.; et al. Macrophage inducible nitric oxide synthase gene expression is blocked by a benzothiophene derivative with anti-HIV properties. Mol. Genet. Metab. 2002, 75, 360–368. [Google Scholar]
- Batista, R.M.F.; Oliveira, R.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Synthesis and Ion Sensing Properties of New Colorimetric and Fluorimetric Chemosensors Based on Bithienyl-Imidazo-Anthraquinone Chromophores. Org. Lett. 2007, 9, 3201–3204. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.M.F.; Oliveira, R.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Synthesis and Evaluation of bipendant-armed (oligo)thiophene crown ether derivatives as new chemical sensors. Tetrahedron Lett. 2008, 49, 6575–6578. [Google Scholar] [CrossRef]
- Batista, R.M.F.; Oliveira, R.; Nuñez, C.; Costa, S.P.G.; Lodeiro, C.; Raposo, M.M.M. Synthesis and Evaluation of new thienyl and bithienyl-bis-indolymethanes as colorimetric sensors for anions. J. Phys. Org. Chem. 2009, 22, 362–366. [Google Scholar] [CrossRef]
- Oliveira, E.; Baptista, R.; Costa, S.P.G.; Raposo, M.M.M.; Lodeiro, C. Exploring the Emissive Properties of New Azacrown Compounds Bearing Aryl, Furyl and Thienyl Moieties: A Special case of Chelation Enhancement of Fluorescence upon interaction with Ca(II), Cu(II) or Ni(II). Inorg. Chem. 2010, 49, 10478–10857. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Genovese, D.; Juris, R.; Zaccheroni, N.; Capelo, J.L.; Raposo, M.M.M.; Costa, S.P.G.; Prodi, L.; Lodeiro, C. Bioinspired systems for Metal-ion sensing: New emissive peptide probes based on benzo[d]oxazole derivatives and their gold and silica nanoparticles. Inorg. Chem. 2011, 50, 8834–8849. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Nunes-Miranda, J.D.; Santos, H.M. From colorimetric chemosensors to metal nanoparticles using two new tyrosine Schiff-base ligands for Cu(II) detection. Inorg. Chim. Acta 2012, 380, 22–30. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).