Abstract
Herein, we synthesized 2,3-diaminophenazine (DAP) from o-phenylenediamine (OP) using fungal laccase as a biocatalyst. The conversion ratio of OP monomer was 85% and the yield of the final purified product, DAP, was 63%. The structure of the main product, DAP, was confirmed by using several spectroscopy techniques (UV-VIS, IR, 2D-NMR, and MS).
1. Introduction
Recently, there has been increasing interest in “green” approaches for the synthesis of 2,3-diaminophenazine (DAP) because of its potential applications in fluorescence sensitive assays and in the manufacture of conductive polymers [1,2]. The chemical synthesis of DAP was first developed by oxidation of o-phenylenediamine (OP) using ferric chloride in the 1870s [3]. Later, Tarcha et al. [4] reported that enzyme (biochemical)-catalyzed oxidation of OP could also yield DAP as an alternative way. Their results showed that DAP was produced at a yield of 42% by using the horseradish peroxidase (HRP)-catalyzed oxidation. However, it is not realistic to scale up DAP biosynthesis using HRP because of the increased costs. Similar to HRP, laccase (EC 1.10.3.2), a copper-containing polyphenol oxidases produced dominantly in plants and microorganisms, also carries the ability to catalyze the oxidation of phenols and amines [5]. Recently, the potential of laccase-catalyzed polymerization of OP has been demonstrated [6] but the main products from laccase/OP reaction still needs to be further characterized. The difference between HRP and laccase-catalyzed reactions is that laccase in acetic buffer needs oxygen as an oxidant while HRP requires the involvement of hydrogen peroxide (Figure 1). Therefore, biosynthesis of DAP by laccase is expected to be a more economic and safer way than that using HRP. To this end, we studied the biosynthetic DAP from o-phenylenediamine (OP) using a fungal laccase and extensively characterized the product by several spectroscopic techniques (UV-VIS, FT- IR, 2D-NMR, and MS).
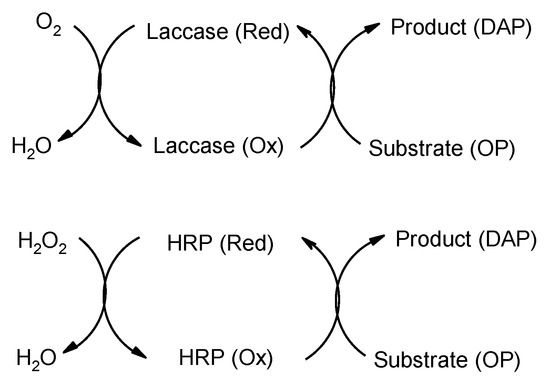
Figure 1.
Catalytic mechanism of laccase and HRP
2. Results and Discussion
A typical synthetic protocol is as follows: 5 g of o-phenylenediamine purified by distillation was dissolved in acetate buffer (0.1 mol/L, pH 4.8). The reaction was initiated by adding 1 mL of purified laccase with an activity of 6, 000 U/L, produced by a white-rot fungus Flammulina velutipes. Herein, one unit of laccase activity was defined as the amount of enzyme required to oxidize 1 μmol of ABTS (2, 2’-azinobis(3-ethylbenzthia-zoline-6-sulfonic acid)) per minute [7]. The reaction system was kept in dark under a constant temperature of 30 °C and bubbled with oxygen. After 24-h incubation, the resulted crystals were collected by filtering, washing, and drying under vacuum. The conversion ratio (85%) was calculated as the ratio of the mass of the product to the o-phenylenediamine. The product was further purified by dissolving in hot ethanol and recrystalling procedures. The final yield of product was estimated to be 63% after purification. Compared to the HRP-mediated synthesis of DAP, the laccase-oxygen system doesn’t require additional chemicals, such as, hydrogen peroxide, and provides a high conversion ratio [4].
The purified product (dissolved in methanol) was first characterized using the UV-visible spectrum (200–600 nm). As shown in Figure 2. Two characteristic absorption peaks were found at 258 nm and 426 nm. The peak at 258 nm is attributed to the benzene π → π * electronic transition, while the peak at 426 nm is ascribed to the n →π * electronic transition. Our observations agree with the UV-visible data of ferric chloride or HRP catalyzed DAP as reported in literature [8,9].
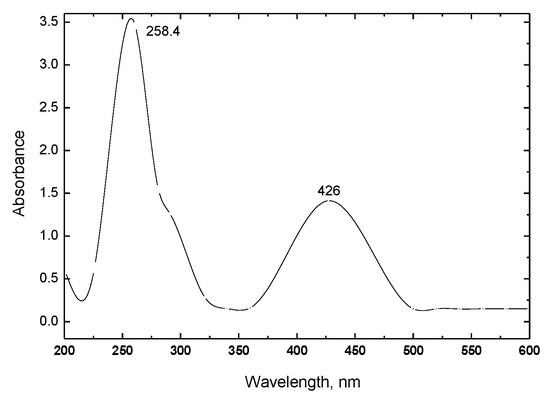
Figure 2.
UV-VIS spectrum of product in methanol.
The infrared spectrum (see Figure 3) of the purified crystals (in KBr pellets) appears similar to that of DAP reported by Jiang and Feng [9]. The characteristic bands at 3,434 cm−1, 3,307 cm−1 and 3,180 cm−1 are attributed to the N–H stretching vibrations of at least two -NH2 groups. The bands at 1,643 cm−1, 1,562 cm−1 are assigned as the deformation vibration of primary amine and C=N stretching vibration, respectively. Four bands, at 1,492 cm−1 due to a C=C stretching vibration, at 763 cm-1 due to a ring 4 adjacent hydrogen deformation vibration, and at 586 cm−1 and 487 cm−1 due to the aromatic plane and out-of-plane bending vibration, respectively, are characteristic of phenazine. The absorption at 840 cm−1 is assigned to a ring hydrogen deformation vibration isolation, 1,338 cm-1 to the C-N= stretching vibration, and 1,224 cm−1 to the aromatic ring C-NH2 stretching vibration.
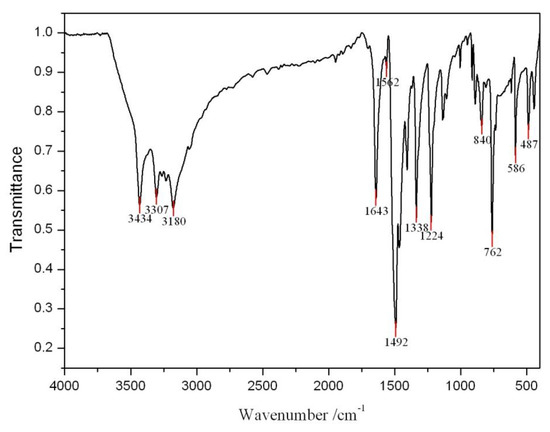
Figure 3.
FT-IR absorption spectrum of the product.
The structure of products was also characterized by two-dimensional 1H–13C Heteronuclear Single Quantum Coherence (HSQC) experiments. Referred to chemical shift data reported, the proton and carbon signals in the NMR spectrum (Figure 4) were identified, and the attribution of the various peaks shown in Figure 5a and Figure 5b below.
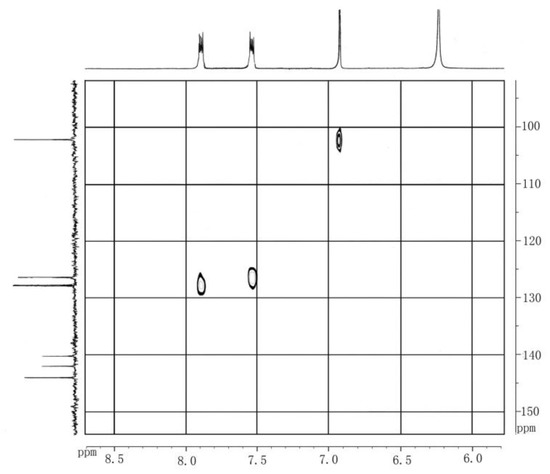
Figure 4.
HSQC spectrum of the product in methanol.
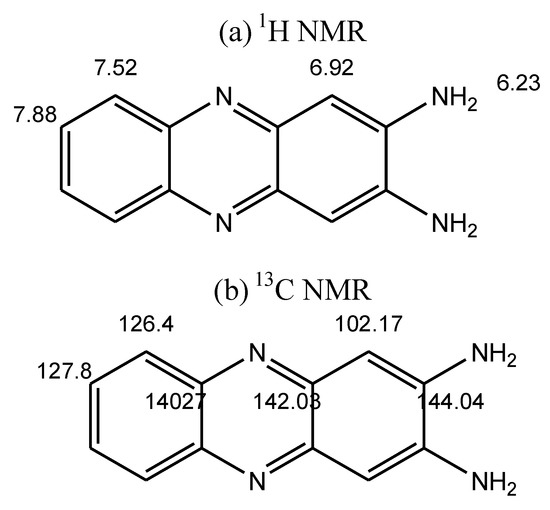
Figure 5.
Attribution of 1H–13C HSQC peaks.
The obtained mass spectrum (not shown) indicates the products exhibit an intense quasi-molecular ion (M+) of 211, suggesting that the molecular formula is C12H10N4. This is in accord with reported data which are obtained from HRP catalyzed formation. The oxidation of o-phenylenediamine via diazo reaction can thus be excluded since the expected product, 2, 2’-diaminoazobenzene [10], exhibits a molecular ion of 212. Therefore, we propose that laccase can also catalyze the monomer, o-phenylenediamine, to form free radicals. These free radicals then couple with each other and yield 2,3-Diaminophenazine.
3. Conclusions
In conclusion, laccase in the presence of oxygen can catalyze a high yield synthesis of 2,3-diamino-phenazine (DAP) from o-phenylenediamine (OP) under mild conditions. The structure of the main product, DAP, was confirmed by UV-VIS, IR, 2D-NMR, and MS spectra. The conversion ratio of o-phenylenediamine was 85% and the final yield of DAP after purification reached 63%, significantly higher than that obtained by HRP-catalyzed biosynthesis. Main text paragraph.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
This work was supported by the National Science Foundation of China (No.30771689 and 30972324) and the Major State Basic Research Development Program (No.2010BC732206). We also acknowledge an SCUT-NCSU scholar exchange program that allowed portions of this work to be possible.
References and Notes
- Piro, B.; Zhang, Q.D.; Reisberg, S.; Noel, V.; Dang, L.A.; Duc, H.T.; Pham, M.C. Direct and rapid electrochemical immunosensing system based on a conducting polymer. Talanta 2010, 82, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Mekler, V.M.; Bystryak, S.M. Application of o-phenylenediamine as a fluorogenic substrate in peroxidase-mediated enzyme-linked immunosorbent assay. Anal. Chim. Acta 1992, 264, 359–363. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Alcántara, A.R.; Carballeira, J.D.; De la Casa, R.M.; García-Burgos, C.A.; Hernáiz, M.J.; Sánchez-Montero, J.V.; Sinisterra, J.V. Candida rugosa lipase: A traditional and complex biocatalyst. Curr. Org. Chem. 2006, 10, 1053–1066. [Google Scholar] [CrossRef]
- Tarcha, P.J.; Chu, V.P.; Whittern, D. 2,3-diaminophenazine is the product from the horseradish peroxidase-catalyzed oxidation of o-phenylenediamine. Anal. Biochem. 1987, 165, 230–233. [Google Scholar] [CrossRef]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 9, 2563–2605. [Google Scholar] [CrossRef]
- Leutbecher, H.; Constantin, M.A.; Mika, S.; Conrad, J.; Beifuss, U. A new Laccase-catalyzed domino process and its application to the efficient synthesis of 2-aryl-1H-benzimidazoles. Tetrahedron Lett. 2011, 52, 604–607. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates: An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Fornera, S.; Walde, P. Spectrophotometric quantification of horseradish peroxidase with o-phenylenediamine. Anal. Biochem. 2010, 407, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Feng, C.L. The study on reaction kinetics based on a new system of the horseradish peroxidase catalyting the oxidation of o-phenylenediamine by H2O2. Spectrosc. Spectr. Anal. 2002, 22, 436–440. [Google Scholar]
- Hempen, C.; Van Leeuwen, S.M.; Luftmann, H.; Karst, U. Liquid chromatographic/mass spectrometric investigation on the reaction products in the peroxidase-catalyzed oxidation of o-phenylenediamine by hydrogen peroxide. Anal. Bioanal. Chem. 2005, 382, 234–238. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).