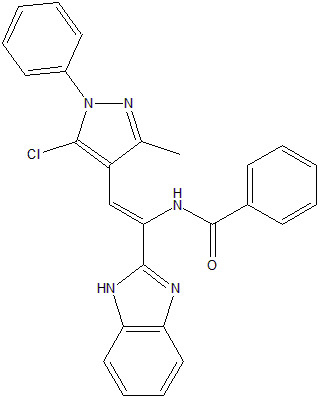
N-[1-(1H-Benzimidazol-2-yl)-2-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)vinyl]benzamide
Abstract
:1. Introduction
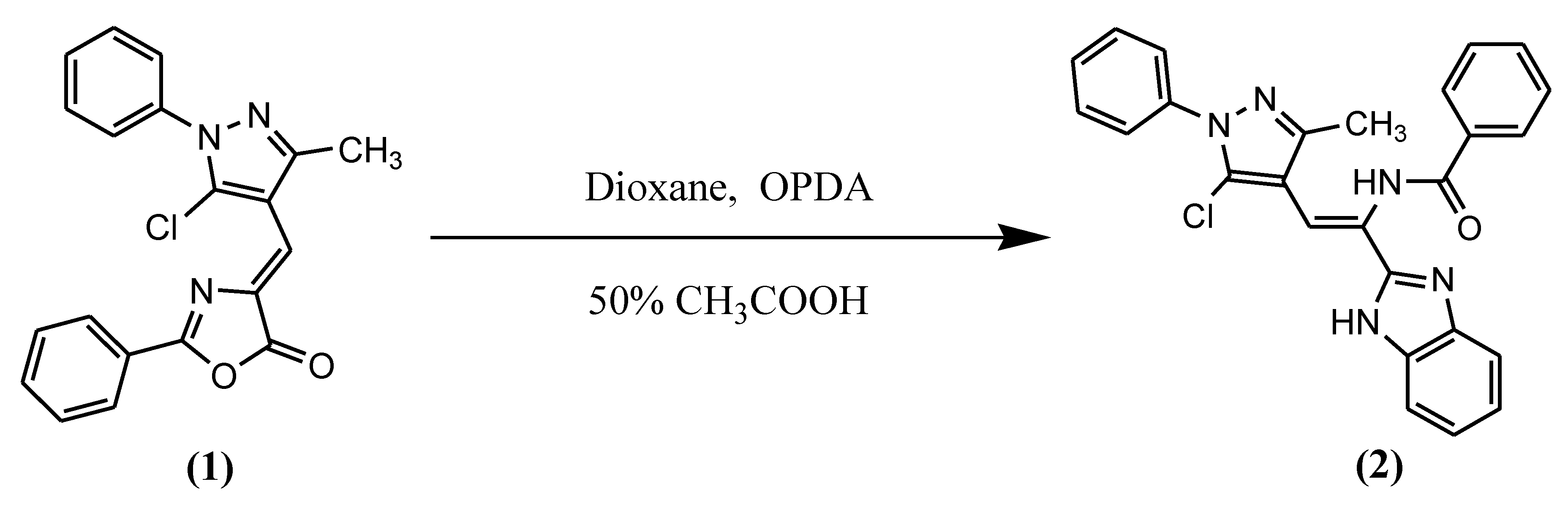
2. Results and Discussion
3. Experimental
Synthesis of N-[1-(1H-benzimidazol-2-yl)-2-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)vinyl]benzamide (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
References
- Kuzmanovic, S.O.P.; Cvetkovic, D.M. Antibacterial evaluation of some benzimidazole derivatives and their zinc(II) complexes. J. Serb. Chem. Soc. 2007, 72, 459–466. [Google Scholar] [CrossRef]
- Kus, C.; Altanlar, N. Synthesis of new benzimidazole carbamate derivatives for evaluation of antifungal activity. Turk. J. Chem. 2003, 27, 35–39. [Google Scholar]
- Gupta, H.C.; Jaiswal, V. Synthesis and antiviral activity of some new benzimidazoles. J. Ind. Council Chem. 2010, 27, 159–162. [Google Scholar]
- Vazquez, G.N.; Yepez, L.; Campos, A.H.; Tapia, A.; Luis, F.H.; Cedillo, R.; Gonzalez, J.; Fernandez, A.M.; Grueiro, M.M.; Castilloa, R. Synthesis and antiparasitic activity of albendazole and mebendazole analogues. Bioorg. Med. Chem. 2003, 11, 4615–4622. [Google Scholar] [CrossRef]
- Padilla, D.V.; Morales, S.R.; Campos, A.H.; Luis, F.H.; Mulia, L.Y.; Contreras, A.T.; Castillo, R. Synthesis and antiprotozoal activity of novel 1-methylbenzimidazole derivatives. Bioorg. Med. Chem. 2009, 17, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Sreena, K.; Ratheesh, R.; Rachana, M.; Poornima, M.; Shyni, C. Synthesis and anthelmintic activity of benzimidazole derivatives. Hygeia 2009, 1, 21–22. [Google Scholar]
- Kaushik, D.; Khan, S.A.; Chawla, G. Synthesis of (substituted benzamidostyryl) lH-benzimidazoles and their screening for anti-inflammatory activity. Med. Chem. Res. 2011. [Google Scholar] [CrossRef]
- Patil, A.; Ganguly, S.; Surana, S. Synthesis and antiulcer activity of 2-[5-substituted-1-H-benzo(d) imidazol-2-yl sulfinyl]methyl-3-substituted quinazoline-4-(3H) ones. J. Chem. Sci. 2010, 122, 443–450. [Google Scholar]
- Shah, D.I.; Sharma, M.; Bansal, Y.; Bansal, G.; Singh, M. Angiotensin II AT1 receptor antagonists: Design, synthesis and evaluation of substituted carboxamido benzimidazole derivatives. Eur. J. Med. Chem. 2008, 48, 1808–1812. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Grover, P.; Pathak, D.P. Synthesis, anticonvulsant activity and comparative QSAR study of some novel 1,2,5-trisubstituted benzimidazole derivatives. Acta Pharm. Sci. 2010, 52, 511–522. [Google Scholar]
- Akula, G.; Srinivas, B.; Vidyasagar, M.; Kandikonda, S. Synthesis of 3-(1H-benzimidazol-2-yl amino)2-phenyl-1, 3-thiazolidin -4-one as potential CNS depressant. Int. J. Pharm. Tech. Res. 2011, 3, 360–364. [Google Scholar]
- Kaushik, D.; Verma, T.; Madaan, K. 2-(Benzoylamino)-3-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)acrylic acid. Molbank 2011, 2011, M726. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kaushik, D.; Maan, N. N-[1-(1H-Benzimidazol-2-yl)-2-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)vinyl]benzamide. Molbank 2011, 2011, M731. https://doi.org/10.3390/M731
Kaushik D, Maan N. N-[1-(1H-Benzimidazol-2-yl)-2-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)vinyl]benzamide. Molbank. 2011; 2011(3):M731. https://doi.org/10.3390/M731
Chicago/Turabian StyleKaushik, Darpan, and Namita Maan. 2011. "N-[1-(1H-Benzimidazol-2-yl)-2-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)vinyl]benzamide" Molbank 2011, no. 3: M731. https://doi.org/10.3390/M731
APA StyleKaushik, D., & Maan, N. (2011). N-[1-(1H-Benzimidazol-2-yl)-2-(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)vinyl]benzamide. Molbank, 2011(3), M731. https://doi.org/10.3390/M731