Abstract
A new flavonoid glycoside (1) has been isolated from the aerial parts of Salix denticulata (Salicaceae) together with five known compounds, β-sitosterol, 2,6-dihydroxy-4-methoxy acetophenone, eugenol-1-O-β-d-glucopyranoside, 1-O-β-d-(3’-benzoyl) salicyl alcohol and luteolin-7-O-β-d-glucopyranosyl-(1-6)-glucopyranoside. The structure of 1 was elucidated as 2’,5-dihydroxy-3’-methoxyflavone-7-O-β-d-glucopyranoside by means of chemical and spectral data including 2D NMR studies.
1. Introduction
Salix denticulata which belongs to the Salicaceae family is a deciduous shrub indigenous to Central Himalayas (3000 meter) of India and is well known for its medicinal importance [1]. Previous studies on the plants of this genus led to isolation and elucidation of different compounds such as terpenoids [2], catechins [3], lignans [4], flavones [5,6] and other phenolic compounds [7]. This paper illustrates the isolation and structure revelation of a novel flavonoid glycoside (1) from the aereal parts of S. denticulata with the help of modern spectroscopic methods.
2. Results and discussion
Compound 1 was isolated as yellow crystalline solid, m.p. 210-212 °C, deduced molecular formula C22H22O11 from its FAB-MS. It gave positive Molisch test, Shinoda test and blue color with FeCl3, characteristic for flavone glycosides. The IR spectrum showed characteristic absorption bands for hydroxy (3350 cm-1) and carbonyl (1460 cm-1) functions. The 1H NMR spectrum showed doublets at δ 6.46 (J = 1.8 Hz, H-6) and δ 6.76 (J = 1.8 Hz, H-8), indicating a tetrasubstituted aromatic ring. Other doublets at δ 7.12 (J = 3.4 Hz, H-3’), 6.92 (J = 8.4 Hz, H-6’) and 7.44 (J = 3.4, 8.4 Hz, H-5’) revealed the trisubstituted aromatic ring. The position of two singlets at δ 12.9 and 9.5 indicated two hydroxy groups, in which former is chelated with a carbonyl function and assigned at position OH-5. A sharp singlet at δ 3.61 correlated to C-4’ (δ 145.81), indicating OCH3-4’. A doublet at δ 5.08 (J = 7.2 Hz) indicated anomeric signal with other signals in the range of δ 3.2-4.6 for a β linked sugar. In the 13C NMR spectrum, the downfield signal at δ 181.9 indicated a carbonyl group. The positions of substituted groups were confirmed by 1H-13C correlation in HMBC (Figure 2) and HSQC. The correlation of H-3’ (δ 7.12) to C-4’ (δ 145.81) and C-5’ (δ 116.02); H-5’ (δ 7.44) to C-3’ (δ 113.5), C-4’ (δ 145.81) and C-6’ (δ 119.21) and H-6’ (δ 6.92) to C-1’ (δ 121.42) and C-4’ (δ 145.81) reveled the substitution at C-2’ (OH) and C-4’ in ring B. The correlation between the anomeric proton (δ 5.09) and C-7 (δ 162.98) indicated position of sugar at C-7. The sugar was identified as d-glucose by hydrolysis and direct comparison (co-PC) with authentic sugar. The chemical structure of compound 1 is given in Figure 1.
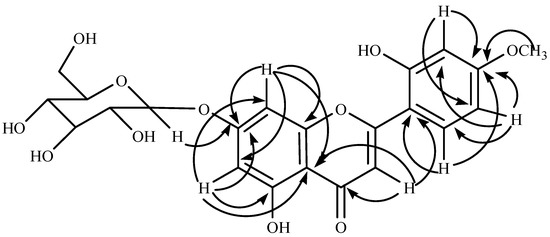
Figure 2.
Important HMBC correlations in compound 1.
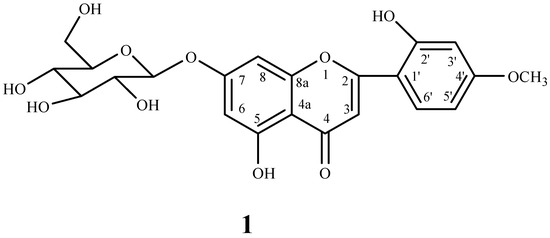
Figure 1.
Chemical structure of compound 1.
3. Experimental Section
3.1. General
Melting points were recorded on a Perfit melting point apparatus. UV spectra were measured on a Perkin-Elmer Lambda-25 spectrophotometer in methanol. IR spectra recorded on a Perkin-Elmer Spectrum RX1 FT-IR spectrometer (KBr discs). NMR spectra were obtained on Bruker Avance 300 and 500 spectrometers (300 MHz for 1H and 125 MHz for 13C, CDCl3 as solvent, TMS as internal standard). MS were recorded on Qualtro II-EIMS and Jeol SX-102 (FAB) mass spectrometer. Column chromatography was performed on silica gel (Merck 60-120 mesh, 15 × 100 cm). TLC was carried out on silica gel (Merck 10-40 μ) precoated plates, spots were visualized by spraying with 7% H2SO4.
3.2. Plant material
Aerial parts of S. denticulata were collected from Tungnath, Chamoli during the month of May and identified from Taxonomy Laboratory, Department of Botany, H.N.B. Garhwal University Srinagar. A voucher specimen (GUH-8036) of the plant has been kept in the Departmental Herbarium for future records.
3.3. Extraction and isolation
The shade dried aerial parts of S. denticulata (6 kg) were powdered and extracted exhaustively with 95% ethanol (3 times) to yield a black brown extract, which was concentrated under reduced pressure and defatted with n-hexane. The extract (380 g) was pre-adsorbed with silica gel and applied on the top of a column prepared by silica gel (500 g) in CHCl3. The elution was first started with CHCl3 and then CHCl3 with increasing amounts of MeOH (0-30%). Elution with CHCl3:MeOH = 22:3 afforded compound 1, whereas 9:1, 43:7, 41:9, 8:2 and 21:4 furnished β-sitosterol [8], 2,6-dihydroxy-4-methoxyacetophenone [9], eugenol-1-O-β-d-glucopyranoside, 1-O-β-d-(3’-benzoyl)-salicyl alcohol [10] and luteolin-7-O-β-d-glucopyranosyl-(1→6)-glucopyranoside [11,12], respectively.
3.4. 5-Hydroxy-2-(2-hydroxy-4-methoxyphenyl)-4-oxo-4H-chromen-7-yl β-d-glucopyranoside (1)
Yellow amorphous solid (60 mg); m.p. 210-212 °C (uncorr.); UV: : 253, 278 and 353 nm; IR: : 3373, 2907, 1703, 1293 cm-1; NMR data: see Table 1; FAB-MS (m/z): 462 [M]+, 300 [M-glu]+ 149 [C9H9O2]+; calcd. C 57.14, H 4.80; found C 57.86, H 4.37.

Table 1.
13C, 1H-NMR, HSQC and HMBC data of compound 1 in CDCl3.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors pay their sincere thanks to D.S.T. New Delhi, for financial assistance and R.D. Gaur, Department of Botany, Garhwal University Srinagar, for identification of the plant material.
References and Notes
- Gaur, R.D. Flora of Garhwal North West Himalaya. Trans Media: Srinagar Garhwal, India, 1999; p. 186. [Google Scholar]
- Zheng, S.; Wang, J.; Lu, J.; Shen, T.; Sun, L.; Shen, X. Two new acyclic diterpene-γ-lactones from Salix matsudan. Planta Med. 2000, 66, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.L.; Nonaka, G.I.; Nishioka, I. Acylated flavanols and procyanidins from Salix sieboldiana. Phytochemistry 1985, 24, 2089–2091. [Google Scholar] [CrossRef]
- Lee, H.; Watanabe, N.; Sasaya, T.; Ozawa, S. Extractives of short-rotation hardwood species. I. Phenolics of the wood of Salix sachalinensis Fr. Schm. Mokuzai Gakkaishi 1993, 39, 1409–1414. [Google Scholar]
- Shelyuto, V.L.; Bondarenko, V.G. Flavonoids of Salix acutifolia. Khim. Prir. Soedin. 1985, 4, 567–568. [Google Scholar]
- Kompantsev, V.A. Polyphenols of the leaves of Salix pantosericea and Salix pentandroides. Khim. Prir. Soedin. 1980, 5, 654–656. [Google Scholar]
- Shao, Y.; Lahloub, M. F.; Meier, B.; Sticher, O. Isolation of phenolic compounds from the bark of Salix pentandra. Planta Med. 1989, 55, 617–620. [Google Scholar] [CrossRef]
- Sati, O.P.; Pant, G. Steroidal constituents of Agave cantala Roxb. (Rootstaks). Pharmazie 1983, 38, 353. [Google Scholar]
- Hikino, H.; Konno, C.; Takemoto, T. Structure of pleoside from Pleopeltis thunbergiana. Yakugaku Zasshi 1969, 89, 372–374. [Google Scholar] [PubMed]
- Mizuno, M.; Kato, M.; Misu, C.; Iinuma, M.; Tanaka, T. Chaenomeloidin: A phenolic glucoside from leaves of Salix chaenomeloides. J. Nat. Prod. 1991, 54, 1447–1450. [Google Scholar] [CrossRef]
- Montoro, P.; Braca, A.; Pizza, C.; De Tommasi, N. Structure antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Chiruvella, K. K.; Mohammed, A.; Dampuri, G.; Ghanta, R. G.; Raghavan, S. C. Phytochemical and antimicrobial studies of methyl angolensate and luteolin-7-O-glucoside isolated from callus cultures of Soymida febrifuga. Int. J. Biomed. Sci. 2007, 3, 269–278. [Google Scholar] [PubMed]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).