Synthesis of 4-[10H-Phenothiazin-10-yl(1H-tetrazol-5-yl)-methyl]phenol
Abstract
:Introduction
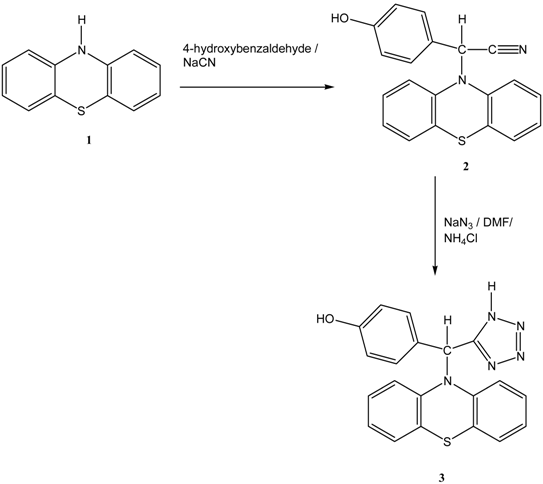
Synthesis
Preparation of (4-hydroxyphenyl)(10H-phenothiazin-10-yl)acetonitrile (2)
Preparation of 4-[10H-phenothiazin-10-yl(1H-tetrazol-5-yl)methyl]phenol (3)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References
- Sangal, S.K.; Kumar, A. J. Indian Chem. Soc. 1986, 63, 351.
- Wazir, V.; Singh, G.B.; Singh, S.; Gupta, R.; Kachroo, P.L. J. Indian Chem. Soc. 1991, 68, 305.
- Wadsworth, H.J.; Jenkins, S.M.; Orlek, B.S.; Cassidy, F.; Clark, M.S.G.; Brown, F.; Riley, G.J.; Graves, D.; Hawkins, J.; Naylor, C.B. J. Med. Chem. 1992, 35, 1280. [PubMed]
- Broughton, B.J.; Chaplen, P.; Knowles, P.; Lunt, E.; Marshall, S.M.; Pain, D.L.; Wooldridge, K.R.H. J. Med. Chem. 1975, 18, 1117. [PubMed]
- Brusova, E.G. Bull. Expt. Bio. Med. 1992, 113, 86–89.
- Ali Harb, A.E. Arch. Pharm. Res. 1991, 14, 195–198.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Venkatraman, B.R.; Kavitha, H.P. Synthesis of 4-[10H-Phenothiazin-10-yl(1H-tetrazol-5-yl)-methyl]phenol. Molbank 2009, 2009, M621. https://doi.org/10.3390/M621
Venkatraman BR, Kavitha HP. Synthesis of 4-[10H-Phenothiazin-10-yl(1H-tetrazol-5-yl)-methyl]phenol. Molbank. 2009; 2009(3):M621. https://doi.org/10.3390/M621
Chicago/Turabian StyleVenkatraman, Bathey R., and Helen P. Kavitha. 2009. "Synthesis of 4-[10H-Phenothiazin-10-yl(1H-tetrazol-5-yl)-methyl]phenol" Molbank 2009, no. 3: M621. https://doi.org/10.3390/M621
APA StyleVenkatraman, B. R., & Kavitha, H. P. (2009). Synthesis of 4-[10H-Phenothiazin-10-yl(1H-tetrazol-5-yl)-methyl]phenol. Molbank, 2009(3), M621. https://doi.org/10.3390/M621



