Abstract
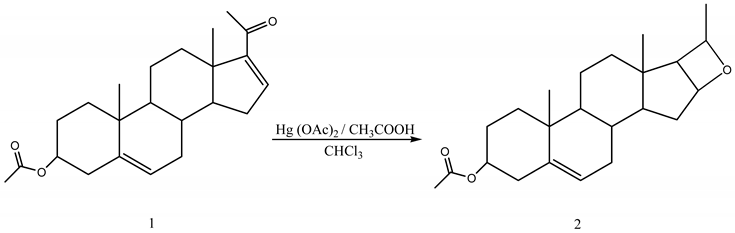
16-Dehydropregnenolone acetate (16-DPA) (1), an exocyclic α,β-conjugated ketone having a steroidal skeleton, when treated with mercuric(II) acetate in acetic acid furnished a single compound identified as (2). The structure of compound (2) has been elucidated on the basis of spectral data analysis (IR, NMR and MS).
In the course of our continuous study on the transformative reactions of steroids and triterpenoids [1,2,3,4,5], we report herein the transformation of 16-DPA into a cyclic ether formed on ring-D on the steroidal skeleton.
Oxidative transformation of steroids and triterpenoids using mercuric(II) acetate has been reported earlier by a number of research groups. Depending on the nature of the substrate, this oxidation has been reported to furnish lactones [6], cyclic ethers [6,7,8] and some other products [9,10,11,12] from steroids and triterpenoids. People have reported the formation of Δ7,9-allo-steroids from Δ7-allo-steroids [13], conversion of substituted double bond of steroidal systems into α,β-unsaturated ketones [14], mercuration and associated rearrangement of α,β-unsaturated steroidal ketones [15] by using the same reagent.
Encouraged by these findings, we undertook the present investigation towards the oxidative trans-formation of an exocyclic α,β-unsaturated ketone, taking 16-DPA as a representative steroidal skeleton. The transformation introduces a cyclic ether onto the ring-D of 16-DPA, involving the carbonyl oxygen and the double bond in its α,β-position. A single-step conversion of exocyclic α,β-conjugated ketone functionality of a steroid into a four-membered cyclic ether which may possess potent biocidal activity [16], preserves the main significance of the study. The result of this investigation is reported in this communication.

Experimental
16-DPA (0.5 g) was dissolved in chloroform (12.5 mL) and a solution of mercuric(II) acetate (9 g) in hot glacial acetic acid (70 mL) was added. The reaction mixture was maintained at 100 ºC in an oil bath for 4 hours. On cooling, the precipitated mercuric(I) acetate was filtered off and the whole filtrate was diluted with water and extracted with chloroform. The chloroform layer was washed well with water and then dried over anhydrous sodium sulphate. Removal of chloroform gave an orange solid which was dissolved in pyridine and H2S was passed for 2 hours. The black reaction mixture was filtered and pyridine was removed by very dilute HCl and then extracted with ether. The brownish black residue (0.38 g) so obtained was then chromatographed over silica gel. The product was obtained using ethyl acetate and petroleum ether mixture (1:6) as eluent and it was recrystallised from absolute alcohol to get white crystals of m.p. 132-134 ºC, Yield: 0.25 g.
IR (KBr, cm-1): υ 1726, 1696, 1240, 1030 and 668.
1H-NMR (300 MHz, CDCl3): δ 5.37 (s, 1H, CH), 4.61 (s, 1H, CH), 3.85-4.20 (m, 2H, CH), 2.95 (dd, J = 3.0 Hz and 6.0 Hz,1H, CH), 2.18 (d, J = 6.0 Hz, 3H, CH3), 2.03 (s, 3H, CH3), 1.02 (s, 3H, CH3), 0.66 (s, 3H, CH3).
13C-NMR (300 MHz, CDCl3): δ 170.5 (>CO), 139.6 (=C5), 121.9 (=C6H), 73.7 (-O-CH), 71.1 (-O-CH), 70.6 (-C3H), 55.6, 49.6, 47.0, 45.6, 38.5, 37.9, 36.8, 36.5, 33.3, 31.8, 31.5, 31.3, 27.6, 21.4, 20.8, 19.2, 14.2.
MS: m/z = 358 (M+), 343, 315, 298, 297 (100%).
Elemental Analysis: Calculated for C23H34O3 (358.52): C, 77.05%; H, 9.56%; O, 13.39%. Found: C, 77.08%; H, 9.61%; O, 13.45%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
The authors thank to CDRI, Lucknow, India, for the mass spectral data and elemental analysis.
References and Notes
- Pradhan, B.P.; Ghosh, P.; Chakraborty, S. Oxidation of triterpenoids: Part X–Oxidation of lupenyl acetate and methyl acetylbetulenate with selenium dioxide and hydrogen peroxide in t-butanol. Ind. J. Chem. 1991, 30, 549–553. [Google Scholar]
- Pradhan, B.P.; Dutta, S.; Ghosh, R.K.; Ghosh, P. Studies on oxidation of triterpenoids: Part XI–Oxygenation of friedelin in presence of potassium t-butoxide in t-butanol. Ind. J. Chem. 1991, 30, 7–12. [Google Scholar]
- Pradhan, B.P.; Ghosh, P. Studies on reactions of 2- bromo 3- keto triterpenoids: Part IV–debromination and dehydrobromination of 2α-bromo and 2,2-dibromoderivatives of lupanone and methyl dihydrobetulonate. Ind. J. Chem. 1993, 32, 1068–1069. [Google Scholar]
- Pradhan, B.P.; Ghosh, P. Studies on the action of N–bromo succinimide on 3-oximinolupanes in CHCl3–DMSO. Ind. J. Chem. 1993, 32, 491–493. [Google Scholar]
- Pradhan, B.P.; Ghosh, P. Studies on reactions of 2-bromo-3-keto triterpenoids: Part IV–debromination and dehydrobromination of 2α-bromo and 2,2-dibromoderivatives of lupanone and methyl dihydrobetulonate. Ind. J. Chem. 1994, 33, 73–75. [Google Scholar]
- Dutta, G. Chemical Investigation of Naturally Occurring Carbocyclic Ring Compounds. Ph.D Thesis, University of North Bengal, Darjeeling, India, 1989. [Google Scholar]
- Vystrcil, A.; Blecha, Z. A revised structure for the product of oxidation of betulin with mercuric acetate. Chem. Ind. 1969, 418–419. [Google Scholar]
- Vystrcil, A.; Blecha, Z. Dehydrogenation of 20(29)-lupane derivatives with mercuric acetate. Coll. Czech. Chem. Commun. 1970, 35, 3309–3319. [Google Scholar] [CrossRef]
- Ortar, G.; Torrini, I. Metal salt oxidations of steroid olefins: Reaction of 17-methylene-5α-androstan-3β-yl acetate with lead(IV), thallium(III) and mercury(II) acetates in methanol. Tetrahedron 1977, 33, 859–863. [Google Scholar] [CrossRef]
- Romo, J.; Rosenkranz, G.; Djerassi, C. Steroids. XXIII. Δ7,9(11)-allopregnadiene-3β,20β-diol and related compounds. J. Am. Chem. Soc. 1951, 73, 5489–5490. [Google Scholar]
- Turner, A.B. Chapter 12. Terpenoids and steroids. Annu. Rep. Prog. Chem., Sect. B: Org. Chem. 1969, 66, 389–422. [Google Scholar] [CrossRef]
- Heilbron, I.M.; Kennedy, T.; Spring, F.S.; Swain, G. Studies in the sterol group. Part XXXVII. The structure of lumisterol and its stereoisomers. J. Chem. Soc. 1938, 869–876. [Google Scholar] [CrossRef]
- Ruyle, W.V.; Jacob, T.A.; Chemerda, J.M.; Chamberlin, E.M.; Rosenburg, D.W.; Sita, G.E.; Erickson, R.L.; Aliminosa, L.M.; Tishler, M. The Preparation of Δ7,9(11)-allo-steroids by the action of mercuric acetate on Δ7-allo-steroids. J. Am. Chem. Soc. 1953, 75, 2604–2609. [Google Scholar] [CrossRef]
- Blossey, E.C.; Kucinski, P. Unusual mercury(II) acetate oxidation of steroidal alkenes. J. Chem. Soc., Chem. Commun. 1973, 56–57. [Google Scholar] [CrossRef]
- Smith, R.G.; Ensley, H.E.; Smith, H.E. Mercuration of α,β-unsaturated steroidal ketones and other unsaturated systems. J. Org. Chem. 1972, 37, 4430–4435. [Google Scholar] [CrossRef] [PubMed]
- Angelastro, M.R.; Marquart, A.L.; Weintraub, P.M.; Gates, C.A.; Laughlin, M.E.; Blohm, T.R.; Peet, N.P. Time-dependent inactivation of steroid C17(20) lyase by 17β-cyclopropyl ether-substituted steroids. Bioorg. Med. Chem. Lett. 1996, 6, 97–100. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
