Synthesis of 3-Methyl-1-morpholin-4-ylmethyl-2,6-diphenylpiperidin-4-one
Abstract
:Introduction
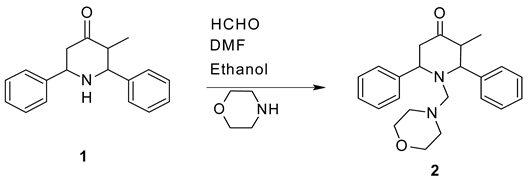
Synthesis
Preparation of 3-methyl-1-morpholin-4-ylmethyl-2, 6-diphenylpiperidin-4-one 2
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgement
References
- El-Subbagh, H.I.; Abu-Zaid, S.M.; Mahran, M.A.; Badria, F.A.; Alofaid, A.M. J. Med. Chem. 2000, 43, 2915. [CrossRef]
- Watson, A.A.; Fleet, G.W.J.; Asano, N.; Molyneux, R.J.; Nugh, R.J. Phytochemistry 2001, 56, 265. [PubMed]
- Ganellin, C.R.; Spickett, R.G. J. Med. Chem. 1965, 8, 619. [CrossRef]
- Hagenbach, R.E.; Gysin, H. Experimentia 1952, 8, 184.
- Ileana, B.; Dobre, V.; Nicluescu-Duvaz, I. J. Prakt. Chem. 1985, 327, 667.
- Mokio, I.G.; Soldatenkov, A.T.; Federov, V.O.; Agreev, E.A.; Sergeeva, N.D.; Lin, S.; Stashenku, E.E.; Prostakov, N.S.; Andreeva, E.L. Khim. Farm. Zh. 1989, 23, 421.
- Noller, C.R.; Baliah, V. J. Chem. Soc. 1948, 70, 3853. [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kumar, S.S.; Kavitha, H.P.; Venkatraman, B.R. Synthesis of 3-Methyl-1-morpholin-4-ylmethyl-2,6-diphenylpiperidin-4-one. Molbank 2009, 2009, M617. https://doi.org/10.3390/M617
Kumar SS, Kavitha HP, Venkatraman BR. Synthesis of 3-Methyl-1-morpholin-4-ylmethyl-2,6-diphenylpiperidin-4-one. Molbank. 2009; 2009(3):M617. https://doi.org/10.3390/M617
Chicago/Turabian StyleKumar, Samiappan Sathish, Helen P. Kavitha, and Bathey R. Venkatraman. 2009. "Synthesis of 3-Methyl-1-morpholin-4-ylmethyl-2,6-diphenylpiperidin-4-one" Molbank 2009, no. 3: M617. https://doi.org/10.3390/M617
APA StyleKumar, S. S., Kavitha, H. P., & Venkatraman, B. R. (2009). Synthesis of 3-Methyl-1-morpholin-4-ylmethyl-2,6-diphenylpiperidin-4-one. Molbank, 2009(3), M617. https://doi.org/10.3390/M617



