Abstract
Reaction of 5-chloro-1,3-dimethyl-1H-pyrazole with I2/HIO3 in refluxing acetic acid gives the title compound in good yield. Detailed spectroscopic data (1H NMR, 13C NMR, 15N NMR, IR, MS) are presented.
(Hetero)aryl halides are valuable starting materials for different transition-metal-catalyzed cross coupling reactions. In the recent past, these reactions have emerged as extraordinaryly important methods for C–C and also C–X (X = O, N, S) bond formation in organic chemistry [1,2].
In the Sonogashira coupling, terminal acetylenes react with, for instance, (hetero)aryl halides or triflates to afford the corresponding (hetero)aryl alkynes [3,4]. Comparing the reactivity of the possible aryl reactants, the general order of reactivity is aryl iodides > aryl triflates ≥ aryl bromides >> aryl chlorides [4]. Accordingly, the best results in many reactions can be obtained with aryl iodides. This is also confirmed for pyrazolyl halides, in which a clear preference for iodides over bromides and chlorides is evident [5].
In the course of a synthetic program dedicated to the functionalization of halogenopyrazoles [6,7,8,9], we were interested in 4-iodopyrazole 2, which was – amongst others – considered as a possible precursor in Sonogashira-type couplings. The synthesis of compound 2 was achieved by reaction of commercially available 5-chloro-1,3-dimethyl-1H-pyrazole (1) with I2/HIO3 in refluxing acetic acid (Scheme 1). Thus, the desired iodopyrazole 2 was obtained in 75% yield after flash chromatography.
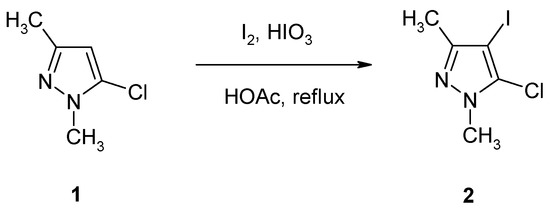
Scheme 1.
Experimental
The melting point was determined on a Reichert–Kofler hot-stage microscope and is uncorrected. Mass spectrum: Shimadzu QP 1000 instrument (EI, 70 eV). IR spectrum: Perkin-Elmer FTIR Spectrum 1000 instrument (KBr-disc). The elemental analysis was performed at the Microanalytical Laboratory, University of Vienna. 1H and 13C NMR spectra were recorded on a Varian UnityPlus 300 spectrometer at 28 °C (299.95 MHz for 1H, 75.43 MHz for 13C). The centre of the solvent signal was used as an internal standard which was related to TMS with δ = 7.26 ppm (1H in CDCl3) and δ = 77.0 ppm (13C in CDCl3). The digital resolutions were 0.2 Hz/data point in the 1H and 0.4 Hz/data point in the 1H-coupled 13C-NMR spectra (gated decoupling). The 15N NMR spectrum (50.68 MHz, refocused and decoupled INEPT) was obtained on a Bruker Avance 500 instrument with a ‘directly’ detecting broadband observe probe (BBFO) and was referenced against external nitromethane.
5-Chloro-4-iodo-1,3-dimethyl-1H-pyrazole (2)
To a solution of 5-chloro-1,3-dimethyl-1H-pyrazole (1) (2.500 g, 19.15 mmol) in glacial acetic acid (10 mL) was added HIO3 (674 mg, 3.8 mmol) and the mixture was stirred for 10 minutes. Then I2 (3.884 g, 15.3 mmol) was added and the mixture was heated to reflux for 4 h. After cooling, the mixture was treated with 2N NaOH until the dark color disappeared, then some drops of Na2S2O3 solution were added to obtain a colorless solution. The mixture was exhaustively extracted with dichloromethane, the combined organic layers were washed with water, dried over anhydrous Na2SO4 and evaporated under reduced pressure. The residue was purified by column chromatography (silica gel, eluent: light petroleum–ethyl acetate, 10:1) to afford 3.683 g (75%) of 2 as colorless crystals, mp 64–65 °C.
IR (KBr) ν (cm-1): 2923, 1497, 1350, 1271, 1107, 1053, 1022, 638.
MS (EI, 70 eV): (m/z, %) 256/258 (M+, 19/7), 160 (14), 128 (24), 96 (18), 64 (100).
1H NMR (CDCl3): δ (ppm) 2.22 (s, 3H, 3-Me), 3.84 (s, 3H, 1-Me).
13C NMR (CDCl3): δ (ppm) 14.4 (3-Me, 1J = 128.3 Hz), 37.1 (1-Me, 1J = 141.1 Hz), 60.8 (C-4, 3J(C4,3-Me) = 4.3 Hz), 131.3 (C-5, 3J(C5,1-Me) = 2.4 Hz), 150.4 (C-3, 2J(C3,3-Me) = 6.9 Hz).
15N NMR (CDCl3): δ (ppm) −186.1 (N-1), −77.5 (N-2).
Anal. Calcd for C5H6ClIN2: C, 23.42%; H, 2.36%; N, 10.92%. Found: C, 23.76%; H, 2.34%; N, 10.81%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
Gytė Vilkauskaitė thanks the Erasmus student exchange program for providing a scholarship.
References and Notes
- Negishi, E.; de Meijere, A. Handbook of Organopalladium Chemistry for Organic Synthesis; John Wiley & Sons: New York, NY, USA, 2002; Vol. 1 and Vol. 2. [Google Scholar]
- de Meijere, A.; Diederich, F. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: Weinheim, Germany, 2004; Vol. 1 and Vol. 2. [Google Scholar]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. The Sonogashira reaction: A booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Vasilevsky, S. F.; Tretyakov, E. V.; Elguero, J. Synthesis and properties of acetylenic derivatives of pyrazoles. Adv. Heterocycl. Chem. 2002, 82, 1–99. [Google Scholar]
- Heinisch, G.; Holzer, W.; Huber, T. Ein effizienter Zugang zu Aryl- oder Benzyl-4-pyrazolylketonen und –carbinolen. Arch. Pharm. (Weinheim) 1987, 320, 1267–1272. [Google Scholar] [CrossRef]
- Heinisch, G.; Holzer, W.; Obala, C. Beiträge zur Chemie von Pyrazolylalkinen. Monatsh. Chem. 1988, 119, 253–262. [Google Scholar] [CrossRef]
- Hahn, M.; Heinisch, G.; Holzer, W.; Schwarz, H. Synthesis of novel heteroaryl 4-pyrazolyl ketones. J. Heterocycl. Chem. 1991, 28, 1189–1192. [Google Scholar] [CrossRef]
- Arbačiauskienė, E.; Vilkauskaitė, G.; Eller, G. A.; Holzer, W.; Šačkus, A. Pd-catalyzed cross-coupling reactions of halogenated 1-phenylpyrazol-3-ols and related triflates. Tetrahedron 2009, 65, 7817–7824. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).