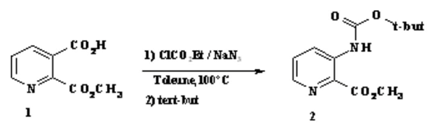
The discussion and purpose for the synthesis of the 2, 3-pyridinecarboxylicacid-2-methylester 1 has been reported elsewhere [1]. To a solution of half-ester (1.0 g, 5.52 mmol) in dry tetrahydrofuran (15 ml) cooled at –10°C was added drop-wise triethylamine (1.52 ml, 11.04 mmol) then ethyl chloroformate (0.79 ml, 8.28 mmol). The mixture was stirred for 30 minutes at –10°C. Analysis by tlc showed complete conversion to a very non-polar product. A solution of sodium azide (0.61 g, 9.38 mmol) in water (4 ml) was then added drop wise continued stirring for 1h at –10°C. The resulting mixture was filtered, evaporated and the aqueous phase was extracted with ethyl acetate (3 x 15 ml). The combined organic layer were washed with brine, dried (MgSO4), filtered and concentrated in vacuo. The acyle azide was slowly brought to reflux in toluene for 2h to give by Curtius rearrangement cleanly isocyanate. The subsequent reaction of isocyanate with 2-Methyl-2-propanol at 100°C for 3 hours. After evaporation in vacuo, the crude product was purified by chromatography on silica gel (9/1 : EtOAc/petroleum ether) to give 3-tert-Butoxycarbonylamino-pyridine-2-carboxylic acid methyl ester 2 (1.03 g, 80%).
Rf: 0.36 (9:1, EtOAc/petroleum ether)
Melting point: 94°C (pale white crystals, from hexane)
IR (KBr,υ, cm-1): 3298, 3000 (NH), 1721, 1691 (CO).
1H-NMR (300 MHz, DMSO-d6, δ, ppm): 1.47 (s, 9H, CH3), 3.85 (s, 3H, CH3), 7.55 ( dd, 1H, J = 8.5, 4.3 Hz, H-5), 8.32 (dd, 1H, J = 4.3, 1.3 Hz, H-4), 8.41 (dd, 1H, J = 8.5, 1.3, H-6), 9.90 (s, 1H, NH).
13C- NMR (75 MHz, DMSO-d6, δ, ppm): 27.82 (3CH3), 52.44 (CH3O), 80.62 (C(CH3)3), 127.54, 127.71, 134.25, 137.28, 142.25, 152.17, 167.00 (CO).
MS m/z: 253 [M+1], 197.5, 165.0, 153.0, 139.0, 121.5.
Elemental analysis: Calculated C12H16 N2O4: C, 57.13; H, 6.39; N, 11.10 Found: C, 57.21; H, 6.45; N, 11.07.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
We wish to thank the ministry ESRSFC of the Moroccan Government (PROTARS-P2T2/07) for financial support.
References
- Mamouni, R.; Aadil, M.; Akssira, M.; Lasri, J.; Sepulveda-Arques, J. Tetrahedron Lett. 2003, 44, 2745–2747.
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.