Stereoselective Synthesis of a New cis Monocyclic β-lactam Bearing a Sugar Moiety at Its N1 Position and Its Physical Characterization
Abstract
:Introduction
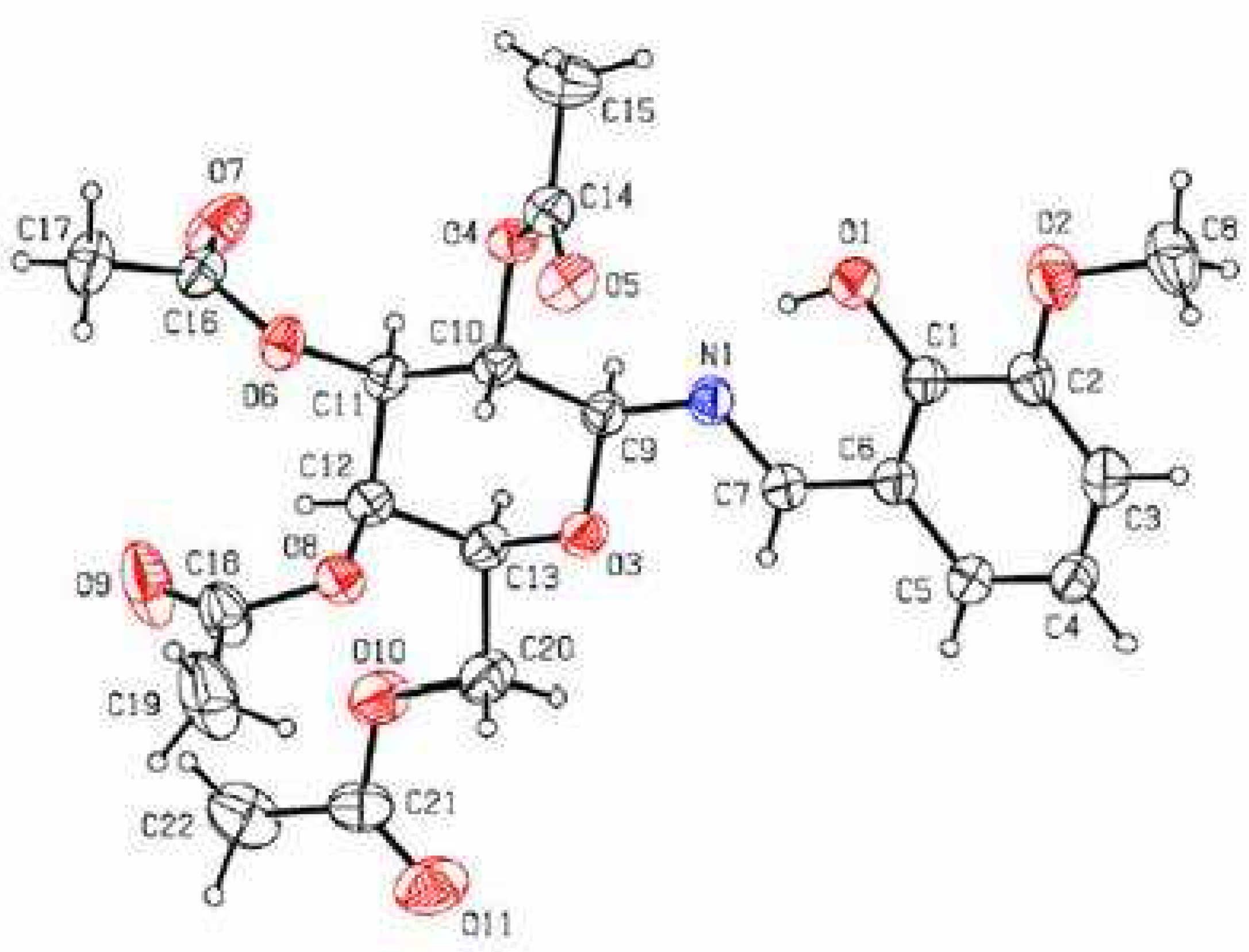
Results and Discussion

Experimental
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgment
References
- Kunz, H.; Pfrengle, W. Angew. Chem. Int. Ed. Engl. 1989, 28, 1067. [CrossRef]
- Kunz, H.; Pfrengle, W. J. Am. Chem. Soc. 1988, 110, 651. [CrossRef]
- Kunz, H.; Sager, W. Angew. Chem. Int. Ed. Engl. 1987, 26, 557. [CrossRef]
- Babiano, R.; Fuentes Mota, J. Carbohydr. Res. 1986, 154, 280.
- Cusack, N.J.; Hildick, B.J.; Robinson, D.H.; Rugg, P.W.; Shaw, G. J. Chem. Soc. 1973, 1720–1731.
- Cusack, N.J.; Robinson, D.H.; Rugg, P.W.; Shaw, G.; Lofthouse, R. J. Chem. Soc. 1974, 73–81.
- Kunz, H. Modern Amination Methods; Ricci, A., Ed.; WILEY-VCH: Weinheim, 2000; p. 103. [Google Scholar]
- Jarrahpour, A.A.; Shekarriz, M.; Taslimi, A. Molecules 2004, 9, 29–38. [PubMed]Jarrahpour, A.A.; Alvand, P. Iranian Journal of Science and Technology Transaction A 2007. (accepted).
- Georg, G.I.; Mashava, P.M.; Akgun, E.; Milstead, M.W. Tetrahedron Lett. 1991, 32, 3151.
- Akkurt, M.; Yıldırım Ozturk, S.; Jarrahpour, A.A.; Khalili, D.; Buyukgungor, O. Acta Cryst. 2006, E62.
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19.
- Frisch, M.J.; et al. GAUSSIAN03, Revision A.1; Frisch, M.J., et al., Eds.; Gaussian, Inc.: Pittsburgh PA, 2003. [Google Scholar]
- Foresman, J.B. Æ Frisch, Exploring Chemistry with Electronic Structure Methods, 2nd edition; Gaussian, INC: Pittsburgh PA, 1996. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook, NIST Standard Reference Database Number 69, July 2001; National Institute of Standards and Technology: Gaithersburg, MD; Volume 20899.
- Jalbout, A.F.; Solimannejad, M.; Labonowski, J.K. Chem. Phys. Letts. 2003, 379, 503.
- Jalbout, A.F.; Jiang; Quasri, A.; Jeghnou, H.; Rhandour, A. Vib. Spect. 2006, (in press).
- Jalbout, A.F.; Nazara, F.; Turker, L. J. Mol. Struct. (THEOCHEM) 2004, 627, 1, (Invited Review). [CrossRef]

| T (K) | Cp (J/mol.K) | S (J/mol.K) | H (kJ/mol) |
|---|---|---|---|
| 100.00 | 319.39 | 684.05 | 20.67 |
| 200.00 | 486.26 | 957.20 | 61.03 |
| 298.15 | 654.17 | 1182.29 | 116.91 |
| 300.00 | 657.42 | 1186.35 | 118.12 |
| 400.00 | 829.87 | 1399.33 | 192.57 |
| 500.00 | 984.08 | 1601.52 | 283.47 |
| 600.00 | 1113.54 | 1792.76 | 388.55 |
| 700.00 | 1220.54 | 1972.71 | 505.42 |
| 800.00 | 1309.30 | 2141.66 | 632.05 |
| 900.00 | 1383.52 | 2300.29 | 766.80 |
| 1000.00 | 1446.07 | 2449.39 | 908.37 |
| Fitted Thermodynamic Equation (T/1000=t) | |
| Cp | 6.75142*t +2502.912*t2 -1184.28159*t3 +117.34776*t-2 |
| S | -255.24191*ln(t) +3247.10622*t -1361.86454*t2/2 -334.36163 *t3/3 -4.23578/(2*t2) -10.40244 |
| ∆H | 274.78754*t +1460.20852*t2/2+97.78182*t3/3 -368.91094*t4/4 +2.49268/t -38.3513 |
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Jarrahpour, A.; Jalbout, A.F.; Alvand, P. Stereoselective Synthesis of a New cis Monocyclic β-lactam Bearing a Sugar Moiety at Its N1 Position and Its Physical Characterization. Molbank 2007, 2007, M544. https://doi.org/10.3390/M544
Jarrahpour A, Jalbout AF, Alvand P. Stereoselective Synthesis of a New cis Monocyclic β-lactam Bearing a Sugar Moiety at Its N1 Position and Its Physical Characterization. Molbank. 2007; 2007(3):M544. https://doi.org/10.3390/M544
Chicago/Turabian StyleJarrahpour, Aliasghar, Abraham F. Jalbout, and Parvaneh Alvand. 2007. "Stereoselective Synthesis of a New cis Monocyclic β-lactam Bearing a Sugar Moiety at Its N1 Position and Its Physical Characterization" Molbank 2007, no. 3: M544. https://doi.org/10.3390/M544
APA StyleJarrahpour, A., Jalbout, A. F., & Alvand, P. (2007). Stereoselective Synthesis of a New cis Monocyclic β-lactam Bearing a Sugar Moiety at Its N1 Position and Its Physical Characterization. Molbank, 2007(3), M544. https://doi.org/10.3390/M544




