Physiological and Molecular Response Mechanisms of Betaphycus gelatinus to Low- and High-Temperature Stress
Abstract
1. Introduction
2. Results
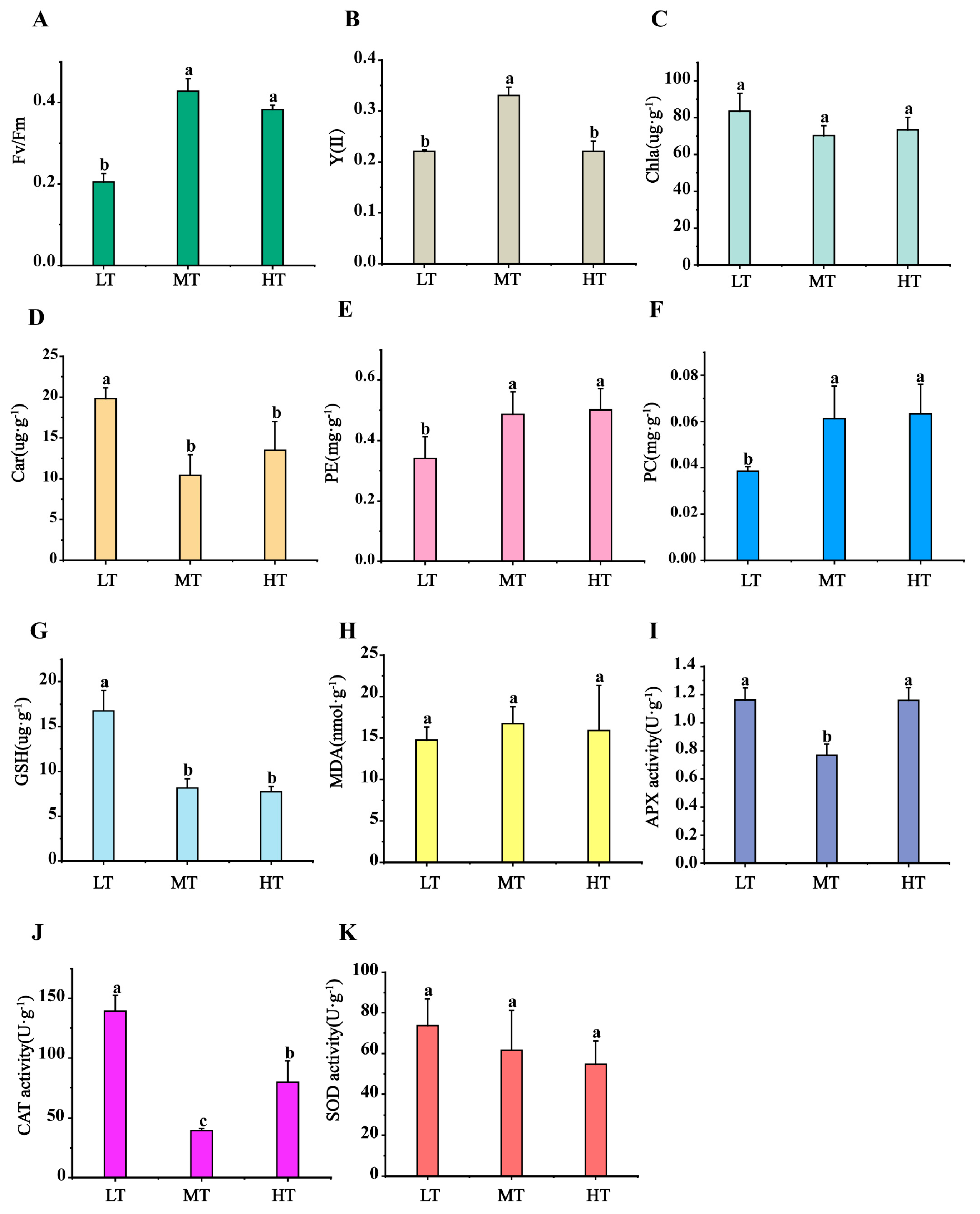
2.1. Physiological Change
2.2. Transcriptome and Metabolome Response Under Temperature Stress
2.3. Effects of Temperature Stress on Photosynthesis
2.4. Effects of Temperature Stress on Carbohydrate and Energy Metabolism
2.5. Effects of Temperature Stress on Amino Acid Biosynthesis
2.6. Effects of Temperature Stress on Porphyrin and Vitamin B6 Metabolism
3. Discussion
3.1. Inhibition of Photosynthesis
3.2. Remodelling of Carbohydrate and Energy Metabolism
3.3. Antioxidant Effects of Amino Acid Metabolism
3.4. Inhibition of Porphyrin and Vitamin B6 Metabolism
4. Materials and Methods
4.1. Cultivation Conditions and Experimental Design
4.2. Physical Analysis
4.3. Transcriptome Analysis and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.4. Metabolomic Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global seaweed farming and processing in the past 20 years. Food Prod. Process. Nutr. 2022, 4, 23, Correction in Food Prod. Process. Nutr. 2022, 4, 28. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Jacob, A.; Nader, C.; Oliveira, C.D.L.; Matos, Â.P.; Araújo, E.S.; Shabnam, N.; Ashok, B.; Gálvez, A.O. An overview on microalgae as renewable resources for meeting sustainable development goals. J. Environ. Manage 2022, 320, 115897. [Google Scholar] [CrossRef]
- Dumilag, R.V.; Liao, L.M.; Lluisma, A.O. Phylogeny of Betaphycus (Gigartinales, Rhodophyta) as inferred from COI sequences and morphological observations on B. philippinensis. J. Appl. Phycol. 2014, 26, 587–595. [Google Scholar] [CrossRef]
- Jamaluddin; Yahya, M.; Rauf, R.F.; Rivai, A.A. Drying kinetics and quality characteristics of Eucheuma cottonii seaweed in various drying methods. J. Food Process. Preserv. 2022, 46, e16258. [Google Scholar] [CrossRef]
- Lim, Y.K.; Tan, I.S.; Foo, H.C.Y.; Tan, Y.H.; Lam, M.K.; Wong, M.K. Exergetic and exergoeconomic analyses of Eucheuma cottoni residue biorefinery for co-production of polylactic acid and electricity. Energy 2024, 300, 131598. [Google Scholar] [CrossRef]
- Wang, X.; He, L.; Ma, Y.; Huan, L.; Wang, Y.; Xia, B.; Wang, G. Economically important red algae resources along the Chinese coast: History, status, and prospects for their utilization. Algal Res. 2020, 46, 101817. [Google Scholar] [CrossRef]
- Trung, V.T.; Huynh, T.V.; Duc, T.M.; Nghia, L.T.; Hung, P.D. Research on the effects of temperature, salinity and nutrition to the growth of the Betaphycus gelatinus (Esper) Doty. J. Biol. 2021, 43, 119–126. [Google Scholar] [CrossRef]
- Deser, C.; Alexander, M.A.; Xie, S.P.; Phillips, A.S. Sea surface temperature variability: Patterns and mechanisms. Ann. Rev. Mar. Sci. 2010, 2, 115–143. [Google Scholar] [CrossRef]
- Jing, X.; Zhen, Y.; Mi, T.; Yu, Z.; Wang, Y.; Wang, X. Transcriptome response of diatom Skeletonema marinoi to lower temperature. Mar. Biol. 2024, 171, 115. [Google Scholar] [CrossRef]
- Vasechkina, E.F.; Naumenko, I.P. Variability of photosynthetic parameters of macroalgae and seagrasses based on laboratory experiments. Ecol. Model. 2023, 486, 110512. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative damage and antioxidative system in algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Sun, P.; Mao, Y.; Li, G.; Cao, M.; Kong, F.; Wang, L.; Bi, G. Comparative transcriptome profiling of Pyropia yezoensis (Ueda) M.S. Hwang & H.G. Choi in response to temperature stresses. BMC Genom. 2015, 16, 463. [Google Scholar] [CrossRef]
- Shin, H.; Hong, S.J.; Yoo, C.; Han, M.A.; Lee, H.; Choi, H.K.; Cho, S.; Lee, C.G.; Cho, B.K. Genome-wide transcriptome analysis revealed organelle specific responses to temperature variations in algae. Sci. Rep. 2016, 6, 37770. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chang, R.; Sun, Y.; Li, B. iTRAQ-Based Quantitative Proteomic Analysis of Spirulina platensis in Response to Low Temperature Stress. PLoS ONE 2016, 11, e016687, Correction in PLoS ONE 2018, 13, e0196442. [Google Scholar] [CrossRef]
- Su, J.; Ye, M.; Lou, Y.; Yang, Z.; Sun, T.; Zhang, R.; Xu, J.; Zhou, C.; Yan, X. Low-molecular-mass organic acid and lipid responses of Isochrysis galbana Parke to high temperature stress during the entire growth stage. Algal Res. 2017, 26, 93–103. [Google Scholar] [CrossRef]
- Lideman; Gregory, N.N.; Noro, T.; Terada, R. In Vitro Growth and Photosynthesis of Three Edible Seaweeds, Betaphycus gelatinus, Eucheuma serra and Meristotheca papulosa (Solieriaceae, Rhodophyta). Aquacult. Sci. 2011, 59, 563–571. [Google Scholar] [CrossRef]
- Yee, T.V.; Rodrigues, K.F.; Wong Vui Ling, C.M.; Thau Lym, W.Y. Comparison of gene expression patterns of Kappaphycus alvarezii (Rhodophyta, Solieriaceae) under different light wavelengths and CO2 enrichment. bioRxiv 2017, 188250. [Google Scholar] [CrossRef]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and challenges for industrial production of seaweed bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef]
- Tian, H.; Deng, Y.; Liao, K.; Xu, S.; Chen, J.; He, L. Physiological, transcriptomic, and metabolomic analyses reveal the adaptation mechanism of Betaphycus gelatinus under different salinity conditions. Algal Res. 2025, 86, 103894. [Google Scholar] [CrossRef]
- Bhagooli, R.; Mattan-Moorgawa, S.; Kaullysing, D.; Louis, Y.D.; Gopeechund, A.; Ramah, S.; Soondur, M.; Pilly, S.S.; Beesoo, R.; Wijayanti, D.P.; et al. Chlorophyll fluorescence—A tool to assess photosynthetic performance and stress photophysiology in symbiotic marine invertebrates and seaplants. Mar. Pollut. Bull. 2021, 165, 112059. [Google Scholar] [CrossRef]
- Banks, J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practitioners. Tree Physiol. 2017, 37, 1128–1136. [Google Scholar] [CrossRef]
- He, W.; Yoo, G.; Ryu, Y. Evaluation of effective quantum yields of photosystem II for CO2 leakage monitoring in carbon capture and storage sites. PeerJ 2021, 9, e10652. [Google Scholar] [CrossRef]
- Endo, H.; Okumura, Y.; Sato, Y.; Agatsuma, Y. Interactive effects of nutrient availability, temperature, and irradiance on photosynthetic pigments and color of the brown alga Undaria pinnatifida. J. Appl. Phycol. 2016, 29, 1683–1693. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Fan, M.; Sun, X.; Xu, N.; Liao, Z.; Li, Y.; Wang, J.; Fan, Y.; Cui, D.; Li, P.; Miao, Z. Integration of deep transcriptome and proteome analyses of salicylic acid regulation high temperature stress in Ulva prolifera. Sci. Rep. 2017, 7, 11052. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, L.; Pang, T.; Liu, J. Comparative transcriptome profiling of Kappaphycus alvarezii (Rhodophyta, Gigartinales) in response to two extreme temperature treatments: An RNA-seq-based resource for photosynthesis research. Eur. J. Phycol. 2019, 54, 162–174. [Google Scholar] [CrossRef]
- Dai, W.; Wang, X.; Zhuang, M.; Sun, J.; Shen, Y.; Xia, Z.; Wu, T.; Jiang, R.; Li, A.; Bi, F.; et al. Responses of photosynthesis-related genes in Sargassum horneri to high temperature stress. Mar. Pollut. Bull. 2024, 199, 115944. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Heinrich, S.; Valentin, K.; Frickenhaus, S.; John, U.; Wiencke, C. Transcriptomic Analysis of Acclimation to Temperature and Light Stress in Saccharina latissima (Phaeophyceae). PLoS ONE 2012, 7, e44342. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, J.; Wang, S.; Chen, X.; Liao, J.; Guo, Y.; Xin, R.; Huang, B.; Xie, E. Transcriptome analysis reveals the molecular mechanisms of adaptation to high temperatures in Gracilaria bailinae. Front. Plant Sci. 2023, 14, 1125324. [Google Scholar] [CrossRef]
- Oliver, T.; Kim, T.D.; Trinugroho, J.P.; Cordon-Preciado, V.; Wijayatilake, N.; Bhatia, A.; Rutherford, A.W.; Cardona, T. The Evolution and Evolvability of Photosystem II. Annu. Rev. Plant Biol. 2023, 74, 225–257. [Google Scholar] [CrossRef]
- Fu, F.; Zhang, X.; Zhang, X.; Li, Q.; Sun, L. Comprehensive analysis and identification of heat-responsive genes in Agarophyton vermiculophyllum by RNA-sequencing. Bot. Mar. 2020, 63, 479–490. [Google Scholar] [CrossRef]
- Pintscher, S.; Pietras, R.; Mielecki, B.; Szwalec, M.; Wojcik-Augustyn, A.; Indyka, P.; Rawski, M.; Koziej, L.; Jaciuk, M.; Wazny, G.; et al. Molecular basis of plastoquinone reduction in plant cytochrome b(6)f. Nat. Plants 2024, 10, 1814–1825. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Ostrowska, A.; Gadzinowska, J.; Grzesiak, M.T.; Dziurka, K.; Dubas, E. Rieske iron-sulfur protein of cytochrome-b6f is involved in plant recovery after drought stress. Environ. Exp. Bot. 2018, 156, 228–239. [Google Scholar] [CrossRef]
- Lee, H.-J.; Park, E.-J.; Choi, J.-i. Isolation, Morphological Characteristics and Proteomic Profile Analysis of Thermo-tolerant Pyropia yezoensis Mutant in Response to High-temperature Stress. Ocean Sci. J. 2018, 54, 65–78. [Google Scholar] [CrossRef]
- Barten, R.; Kleisman, M.; D’Ermo, G.; Nijveen, H.; Wijffels, R.H.; Barbosa, M.J. Short-term physiologic response of the green microalga Picochlorum sp. (BPE23) to supra-optimal temperature. Sci. Rep. 2022, 12, 3290. [Google Scholar] [CrossRef]
- Zhu, S.; Gu, D.; Lu, C.; Zhang, C.; Chen, J.; Yang, R.; Luo, Q.; Wang, T.; Zhang, P.; Chen, H. Cold stress tolerance of the intertidal red alga Neoporphyra haitanensis. BMC Plant Biol. 2022, 22, 1–17. [Google Scholar] [CrossRef]
- Liang, M.H.; Jiang, J.G.; Wang, L.; Zhu, J. Transcriptomic insights into the heat stress response of Dunaliella bardawil. Enzyme Microb. Technol. 2020, 132, 109436. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Wu, S.; Gu, W.; Jia, S.; Wang, L.; Wang, L.; Liu, X.; Zhou, L.; Huang, A.; Wang, G. Proteomic and biochemical responses to different concentrations of CO2 suggest the existence of multiple carbon metabolism strategies in Phaeodactylum tricornutum. Biotechnol. Biofuels 2021, 14, 235. [Google Scholar] [CrossRef]
- Heinrich, S. Effects of Multiple Abiotic Stresses on Gene Expression in Saccharina latissima (Phaeophyceae). Ph.D. Thesis, Universität Bremen, Bremen, Germany, 2012. [Google Scholar]
- Zhang, N.; Mattoon, E.M.; McHargue, W.; Venn, B.; Zimmer, D.; Pecani, K.; Jeong, J.; Anderson, C.M.; Chen, C.; Berry, J.C.; et al. Systems-wide analysis revealed shared and unique responses to moderate and acute high temperatures in the green alga Chlamydomonas reinhardtii. Commun Biol 2022, 5, 460. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Liu, X.; Zhong, M.; Chen, W.; Wang, F.; Du, H. Response of Gracilaria lemaneiformis to nitrogen deprivation. Algal Res. 2018, 34, 82–96. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Li, Z.; Tu, M.; Qin, F.; Shui, G.; Xu, D.; Zang, X. Identification of Indicator Genes for Agar Accumulation in Gracilariopsis lemaneiformis (Rhodophyta). Int. J. Mol. Sci. 2024, 25, 4606. [Google Scholar] [CrossRef]
- Bertels, L.-K.; Fernández Murillo, L.; Heinisch, J.J. The Pentose Phosphate Pathway in Yeasts–More Than a Poor Cousin of Glycolysis. Biomolecules 2021, 11, 725. [Google Scholar] [CrossRef]
- Nagappan, S.; Devendran, S.; Tsai, P.-C.; Jayaraman, H.; Alagarsamy, V.; Pugazhendhi, A.; Ponnusamy, V.K. Metabolomics integrated with transcriptomics and proteomics: Evaluation of systems reaction to nitrogen deficiency stress in microalgae. Process Biochem. 2020, 91, 1–14. [Google Scholar] [CrossRef]
- Hu, C.; Cui, D.; Sun, X.; Shi, J.; Xu, N. Primary metabolism is associated with the astaxanthin biosynthesis in the green algae Haematococcus pluvialis under light stress. Algal Res. 2020, 46, 101768. [Google Scholar] [CrossRef]
- He, Y.; Hu, C.; Wang, Y.; Cui, D.; Sun, X.; Li, Y.; Xu, N. The metabolic survival strategy of marine macroalga Ulva prolifera under temperature stress. J. Appl. Phycol. 2018, 30, 3611–3621. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Moenne, F.; Rodríguez-Rojas, F.; Pardo, D.; Lavergne, C.; Moenne, A.; Brown, M.T.; Huovinen, P.; Gómez, I.; Navarro, N.; et al. Antarctic intertidal macroalgae under predicted increased temperatures mediated by global climate change: Would they cope? Sci. Total Environ. 2020, 740, 140379. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.J.; Oh, Y.S.; Kim, M.Y.; Park, H.-S.; Yum, S. Transcriptional responses in Ecklonia cava to short-term exposure to hyperthermal stress. Toxicol. Environ. Health Sci. 2016, 8, 181–188. [Google Scholar] [CrossRef]
- Machado Monteiro, C.M.; Li, H.; Bischof, K.; Bartsch, I.; Valentin, K.U.; Corre, E.; Collén, J.; Harms, L.; Glöckner, G.; Heinrich, S. Is geographical variation driving the transcriptomic responses to multiple stressors in the kelp Saccharina latissima? BMC Plant Biol. 2019, 19, 513. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Wang, Y.; Xu, D.; Zhang, J.; Ye, N. Exploring Core Response Mechanisms to Multiple Environmental Stressors Via A Genome-Wide Study in the Brown Alga Saccharina japonica (Laminariales, Phaeophyceae). J. Phycol. 2021, 57, 345–354. [Google Scholar] [CrossRef]
- Thompson, J.F. 10—Arginine Synthesis, Proline Synthesis, and Related Processes. In Amino Acids and Derivatives; Miflin, B.J., Ed.; Academic Press: Cambridge, MA, USA, 1980; pp. 375–402. [Google Scholar]
- Cunin, R.; Glansdorff, N.; Piérard, A.; Stalon, V. Biosynthesis and metabolism of arginine in bacteria. Microbiol. Rev. 1986, 50, 314–352. [Google Scholar] [CrossRef]
- Glansdorff, N.; Charlier, D. Biosynthesis of Arginine and Polyamines. Ecosal Plus 2004, 1, 408–433. [Google Scholar] [CrossRef]
- Kiss, E.; Talbot, J.; Adams, N.B.P.; Opekar, S.; Moos, M.; Pilny, J.; Kvasov, T.; Schneider, E.; Konik, P.; Simek, P.; et al. Chlorophyll biosynthesis under the control of arginine metabolism. Cell Rep. 2023, 42, 113265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, S.; Hu, C.; Chen, X.; Sun, X.; Xu, N. Physiological and Transcriptome Analysis of Exogenous L-Arginine in the Alleviation of High-Temperature Stress in Gracilariopsis lemaneiformis. Front. Mar. Sci. 2021, 8, 784586. [Google Scholar] [CrossRef]
- Yang, W.; Liu, D.; Gao, P.; Wu, Q.; Li, Z.; Li, S.; Zhu, L. Oxidative stress and metabolic process responses of Chlorella pyrenoidosa to nanoplastic exposure: Insights from integrated analysis of transcriptomics and metabolomics. Environ. Pollut. 2024, 357, 124466. [Google Scholar] [CrossRef]
- Heinrich, S.; Valentin, K.; Frickenhaus, S.; Wiencke, C. Temperature and light interactively modulate gene expression in Saccharina latissima (Phaeophyceae). J. Phycol. 2015, 51, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, Y.; Mo, J.; Sun, H.; Li, Q. Sulfamethoxazole-Altered Transcriptomein Green Alga Raphidocelis subcapitata Suggests Inhibition of Translation and DNA Damage Repair. Front. Microbiol. 2021, 12, 541451. [Google Scholar] [CrossRef]
- Guo, J.; Ma, Z.; Peng, J.; Mo, J.; Li, Q.; Guo, J.; Yang, F. Transcriptomic analysis of Raphidocelis subcapitata exposed to erythromycin: The role of DNA replication in hormesis and growth inhibition. J. Hazard. Mater. 2021, 402, 123512. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B6 and Its Role in Cell Metabolism and Physiology. Cells 2018, 7, 84. [Google Scholar] [CrossRef]
- Fan, S.; Li, W.; Chen, Z.; Wang, Z.; Cheng, X.; Zhang, S.; Dai, M.; Yang, J.; Chen, L.; Zhao, G. Pyridoxine dehydrogenase SePdx regulates photosynthesis via an association with the phycobilisome in a cyanobacterium. Plant J. 2025, 121, e70055. [Google Scholar] [CrossRef]
- Tambasco-Studart, M.; Titiz, O.; Raschle, T.; Forster, G.; Amrhein, N.; Fitzpatrick, T. Vitamin B6 biosynthesis in higher plants. Proc. Natl. Acad. Sci. USA 2005, 102, 13687–13692. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S.J.; Xu, N.; Shan, T.F.; Sun, S.; Hu, X.; Yang, J.Q. Ulva diversity in the Yellow Sea during the large-scale green algal blooms in 2008–2009. Phycol. Res. 2010, 58, 270–279. [Google Scholar] [CrossRef]
- Beer, S.; Eshel, A. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Mar. Freshwater Res. 1985, 36, 785–792. [Google Scholar] [CrossRef]
- Ji, Z.; Zou, D.; Gong, J.; Liu, C.; Ye, C.; Chen, Y. The different responses of growth and photosynthesis to NH4+ enrichments between Gracilariopsis lemaneiformis and its epiphytic alga Ulva lactuca grown at elevated atmospheric CO2. Mar. Pollut. Bull. 2019, 144, 173–180. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef]
- Smith-Unna, R.; Boursnell, C.; Patro, R.; Hibberd, J.M.; Kelly, S. TransRate: Reference-free quality assessment of de novo transcriptome assemblies. Genome Res. 2016, 26, 1134–1144. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Flick, P.; Sato, K.; Ramírez, F.; Klopfenstein, D.V.; Mungall, C.J.; Yunes, J.M.; Pedersen, B.S. GOATOOLS: Tools for Gene Ontology; Zenodo: Geneva, Switzerland, 2015. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Deng, Y.; Xu, S.; Liao, K.; He, L. Physiological and Molecular Response Mechanisms of Betaphycus gelatinus to Low- and High-Temperature Stress. Int. J. Mol. Sci. 2026, 27, 593. https://doi.org/10.3390/ijms27020593
Deng Y, Xu S, Liao K, He L. Physiological and Molecular Response Mechanisms of Betaphycus gelatinus to Low- and High-Temperature Stress. International Journal of Molecular Sciences. 2026; 27(2):593. https://doi.org/10.3390/ijms27020593
Chicago/Turabian StyleDeng, Yongqiu, Siqi Xu, Kangtai Liao, and Linwen He. 2026. "Physiological and Molecular Response Mechanisms of Betaphycus gelatinus to Low- and High-Temperature Stress" International Journal of Molecular Sciences 27, no. 2: 593. https://doi.org/10.3390/ijms27020593
APA StyleDeng, Y., Xu, S., Liao, K., & He, L. (2026). Physiological and Molecular Response Mechanisms of Betaphycus gelatinus to Low- and High-Temperature Stress. International Journal of Molecular Sciences, 27(2), 593. https://doi.org/10.3390/ijms27020593





