Abstract
Repeated exposure to low-level blast overpressure (BOP) during controlled detonations is an emerging occupational health concern for military breachers and Special Operations Forces personnel, given accumulating evidence that chronic exposure may produce subtle, subclinical neurotrauma. This study derived a latent neuroinjury construct integrating three complementary domains of brain health—post-concussive symptoms, working-memory performance, and circulating biomarkers—to determine whether breachers exhibit coherent patterns of neurobiological alteration. Symptom severity was assessed using the Rivermead Post-Concussion Questionnaire (RPQ), and working memory was assessed with the N-Back task and a panel of thirteen neuroproteomic biomarkers was measured reflecting astroglial activation, neuronal and axonal injury, oxidative stress, inflammatory signaling, and neurotrophic regulation. Experienced Canadian Armed Forces breachers with extensive occupational BOP exposure were compared with unexposed controls. Bayesian latent-variable modeling provided probabilistic evidence for a chronic, subclinical neurobiological signal, with the strongest contributions arising from self-reported symptoms and smaller but consistent contributions from the biomarker domain. Working-memory performance did not load substantively on the latent factor. Several RPQ items and circulating biomarkers showed robust loadings, and the latent neuroinjury factor was elevated in breachers relative to controls (97% posterior probability). The pattern is broadly consistent with subclinical neurobiological stress in the absence of measurable cognitive impairment, suggesting early or compensated physiological alterations rather than overt dysfunction. This multidomain, biomarker-informed framework provides a mechanistically grounded and scalable approach for identifying subtle neurobiological strain in military personnel routinely exposed to repetitive low-level blast. It may offer value for risk stratification, operational health surveillance, and the longitudinal monitoring of neurobiological change in high-risk occupations.
Keywords:
axonal injury; blast overpressure; brain health monitoring; traumatic brain injury; military breachers; occupational exposure; subconcussive neurotrauma; blood biomarkers; GFAP; neurofilament light chain; tau protein; Bayesian latent-variable modeling; working memory; Rivermead Post-Concussion Symptoms Questionnaire 1. Introduction
Explosive breaching, a core tactic for gaining rapid entry and tactical advantage, exposes military personnel to repeated low-level blast overpressure (BOP) generated by user-directed munitions during controlled detonations [1,2]. Within the Canadian Armed Forces (CAF), tactical breachers represent one of the most chronically exposed groups, often performing multiple breaches per day across successive training cycles and operational deployments [3]. Although single events typically fall below diagnostic thresholds for traumatic brain injury (TBI) or concussion, emerging evidence indicates that repeated subconcussive blasts can produce subtle, cumulative alterations in central nervous system (CNS) structure and function [4,5,6,7,8,9,10]. Over time, this additive exposure burden has been linked to measurable disturbances in cognition, balance, sleep regulation, and hearing [11,12,13,14,15,16], as well as elevated rates of depression, post-traumatic stress disorder (PTSD), and other neurobehavioral symptoms [17,18,19,20,21].
BOP exposure begins with the rapid transmission of a shock front generated by explosive detonation, producing an instantaneous pressure rise followed by a nonlinear decay [22,23]. Propagating at supersonic speed, this primary blast wave imparts complex baromechanical loads to the body and skull, driving shear, tensile, and rotational forces through cerebrospinal fluid (CSF) and cranial tissues that induce diffuse axonal injury (DAI), blood–brain barrier (BBB) disruption, and microvascular strain [24,25,26,27,28]. Once delivered to the head, blast-induced loading may involve direct transmission, skull flexure, rapid head acceleration, and CSF cavitation—mechanisms consistently implicated in both human and experimental models of blast neurotrauma [29,30]. Although this mechanism remains controversial, some evidence suggests a potential thoracic-mediated contribution to blast neurotrauma, whereby pressure-wave transmission through the systemic circulation may indirectly stress cerebral and perivascular structures, producing transient cerebrovascular pressure surges that elevate intracranial pressure, compromise BBB integrity, and impair glymphatic clearance of neurotoxic proteins such as amyloid (A)-β and tau [31,32,33,34,35,36,37,38]. These primary mechanical insults are thought to initiate secondary cascades involving neuroinflammation, oxidative stress, astroglial activation, endothelial dysfunction, and disrupted neurovascular coupling [33,39,40,41,42,43,44,45], ultimately destabilizing synaptic signaling and metabolic homeostasis [46,47,48,49]. With repeated exposures, these perturbations accumulate into diffuse network dysfunction rather than focal lesions, manifesting clinically as cognitive fatigue, slowed processing speed, irritability, and affective dysregulation commonly reported among experienced breachers and other high-exposure personnel [13,18,19,50,51,52].
Blast-related neurotrauma is frequently accompanied by the release of brain-enriched proteins into the peripheral circulation, reflecting mechanical stress, astroglial activation, axonal strain, and disruption of neurovascular homeostasis [11,53,54,55,56,57,58]. Quantifiable using ultrasensitive immunoassays, these circulating biomarkers provide a minimally invasive means of detecting subtle neuronal, glial, and vascular perturbations even when conventional structural neuroimaging appears normal [5,53,54,55,56]. Collectively, they index complementary injury domains, including neuronal soma and dendritic injury (UCH-L1, NSE, NRGN, VILIP-1), axonal degeneration (NfL, pNF-H), astroglial activation and BBB disruption (GFAP, S100β), oxidative and mitochondrial stress (PRDX-6, CK-BB), inflammatory signaling (MCP-1), and neurotrophic regulation (BDNF) [57,58,59]. Within this expanding biomarker landscape, A-β40/42 peptides and phosphorylated (p)-tau isoforms have emerged as particularly sensitive indicators of cumulative CNS stress and early degenerative change, offering a plausible mechanistic link between repetitive subconcussive blast exposure and longer-term neurodegenerative risk [60,61,62,63,64]. Together, these neurobiological measures support a mechanistically grounded and scalable framework for probabilistic risk stratification and longitudinal monitoring of blast-related neurobiological strain, with potential relevance for evidence-informed return-to-duty decisions and individualized exposure management in high-risk military populations [65,66,67].
Despite major advances, most studies remain constrained by narrow molecular biomarker panels and a focus on acute, single-event exposures [67,68,69,70]. The chronic evolution of biomarker expression under career-long, low-level exposure remains poorly characterized [17,53]. Conventional imaging and behavioral tools lack the sensitivity to capture diffuse microscopic or network-level dysfunction [5,71]. In this context, circulating biomarkers offer a biologically grounded complement to neuroimaging and neurocognitive assessment, potentially capturing subclinical injury processes [69,72,73,74]. However, systematic investigations linking biomarkers with cognitive and symptomatic domains remain limited, particularly in studies of long-term exposure among high-risk occupational groups.
In this study, we extend prior research in CAF breachers and range staff showing persistent post-concussive symptoms, slowed cognitive–motor performance, and poorer self-reported health compared with unexposed controls [12,14,74]. These findings suggest that low-level BOP exposure may produce a latent, cumulative form of neurological stress spanning behavioral, cognitive, and molecular domains [45,75,76]. To further characterize these effects, we integrated three complementary dimensions of brain health—self-reported symptoms, working-memory performance, and biomarkers of astroglial activation, axonal injury, oxidative stress, inflammation, and neurotrophic regulation. Using a Bayesian latent-variable framework, we tested whether breachers with chronic BOP exposure exhibited higher latent neuroinjury scores than unexposed controls and identified the symptom and neurobiological features contributing most to this construct [77]. This probabilistic approach aims to estimate subclinical CNS perturbations that cannot be directly measured by capturing shared variance across behavioral, cognitive, and biomarker domains—offering a mechanistically integrated alternative to conventional analyses [78]. Within this model, biomarkers index neuronal and glial stress, symptoms reflect functional impact, and N-Back performance represents frontoparietal network efficiency; regions vulnerable to blast-related microstructural disruption [79,80]. This multidomain Bayesian framework improves our understanding of blast-related neurobiological change, highlights potential indicators of subclinical injury, and establishes a scalable foundation for precision monitoring of occupational brain health in military and other high-risk groups repetitively exposed to low-level blast.
2. Results
2.1. Participant Characteristics
Participant demographics and service characteristics are summarized in Table 1. Both blast-exposed breachers and unexposed controls were predominantly male (89% each), with a median age of 32 years (IQR: 26–37). Breachers were primarily senior-ranking personnel (61%), evenly split between Regular Force and Primary Reserve, and reported a median of 6.5 years (IQR: 4–10) of breaching experience. Nearly two-thirds (63%) had prior war-zone deployments. In contrast, controls were more frequently Primary Reserve members (58%), mostly junior NCMs (68%), and had no prior deployments.

Table 1.
Participant demographics and service characteristics.
N-Back working-memory scores were comparable between groups across all task levels. Group-level summaries for biomarker distributions (Low/Medium/High) and RPQ symptom severity categories (Low/Moderate/Severe), as well as associated cut-points, are provided in Supplementary Tables S1 and S2.
2.2. Item Contributions to the Latent Neuroinjury Score
The posterior histograms of the per-item loadings on the latent neuroinjury variable are shown in Figure 1. Among the three domains, symptom measures from the RPQ were the strongest contributors to the latent neuroinjury score (mean loading = 0.8 SD units; 90% Credible Interval [CrI]: 0.3–1.3). Biomarker variables contributed moderately (mean = 0.3; 90% CrI: –0.1–0.7), while working-memory performance (not shown on the plot) contributed minimally (mean = 0.1; 90% CrI: 0–0.2). Within the RPQ domain, ‘Frustration’, ‘Forgetful’, ‘Poor Concentration’, and ‘Taking Longer to Think’ showed the strongest associations, while top biomarker contributors included BDNF, GFAP, PRDX-6, Tau, and VILIP-1.
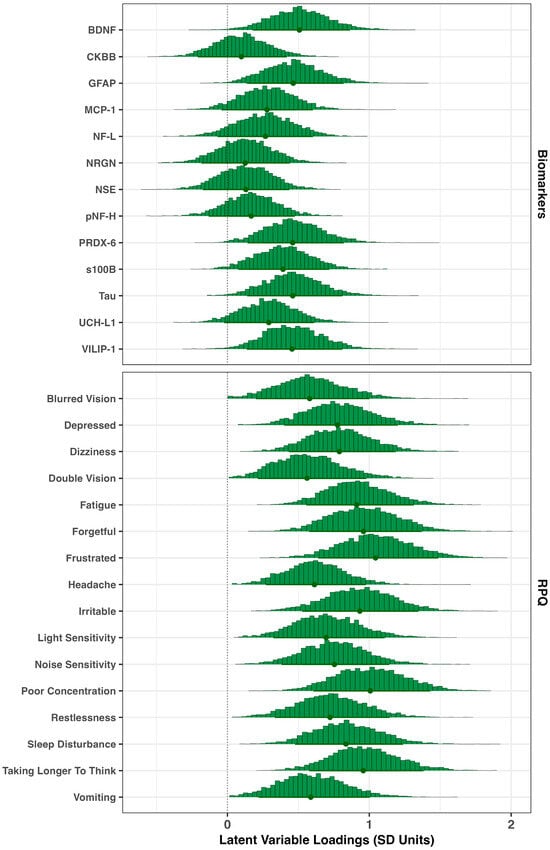
Figure 1.
Item loadings on latent neuroinjury. Posterior histograms for item loadings linking biomarkers and RPQ symptoms to the latent neuroinjury factor. Higher magnitudes indicate stronger associations; positive values denote greater symptom severity (or poorer task performance) or biomarker concentration with higher latent neuroinjury. Dots represent the posterior means for each loading, and the thicker line across the bottom represents the 90% credible interval.
2.3. Latent Neurological Injury Scores in Breachers and Controls
Posterior densities estimates for the group-level latent neuroinjury scores in Breachers and Controls can be seen in Figure 2. The posterior probability quantifies how likely it is, given the data and model assumptions, that one group’s score exceeds another. Breachers showed a higher latent neuroinjury score than controls, with a 97% posterior probability that the group difference was greater than zero (pprob > 0). The mean latent score for Breachers was 0.2 SD units (90% CrI: −0.3 to 0.7), compared with −0.4 SD units in controls (90% CrI: −0.6 to −0.2). Although the absolute difference was modest, the high posterior probability supports a consistent shift toward greater neurobiological stress in the blast-exposed cohort.
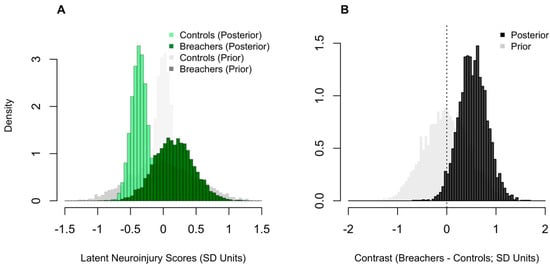
Figure 2.
Comparison of latent neuroinjury scores between Breachers and Controls. Posterior histograms of latent neuroinjury scores (A); standard-deviation, SD units) for Controls and Breachers, with corresponding prior densities overlaid in lighter grey colouring. Posterior histogram of the group contrast (B); Breachers–Controls, SD units), with the prior contrast shown in light grey. The vertical line marks zero (no difference). Higher values indicate greater latent neuroinjury.
2.4. Practical Implications of Elevated Neuroinjury Scores
To illustrate how elevated latent neuroinjury manifests across domains, we generated model-based predictions for biomarker concentrations, memory performance, and RPQ symptom severity. These estimates quantify the expected expression of neuroinjury, captured by the latent model, across biological and symptomatic measures, contrasting Breachers and Controls. Unlike the raw group-level summaries presented in Table 1 and the supplementary biomarker (Table S1) and RPQ (Table S2) data, the model-based predictions isolate neuroinjury-specific variance by accounting for shared covariance across domain items. In doing so, they distinguish variance attributable specifically to the underlying latent process from that arising from uncontrolled sources of individual or measurement variability.
For instance, consistent with higher latent neuroinjury scores in Breachers, the model predicted a greater estimated probability of ‘High’ BDNF concentrations in Breachers (>328.5 pg/mL): 40% (90% CrI = 26–56%) versus Controls (mean estimate: 35%, 90% CrI: 22–48%), representing an average difference of 6% (90%, 0–15%; pprob = 88%). Conversely, low BDNF concentrations (<181.3 pg/mL) were less likely in Breachers (26%, 15–39%) than in Controls (32%, 20–44%), a 5% difference (90% CrI: −2–13%; pprob = 89%). There was no meaningful difference between groups in the probability of medium BDNF concentrations (181.3–328.5 pg/mL). These results indicate that group differences in BDNF are stable but slight, and concentrated at the higher end of the concentration range, rather than reflecting a uniform shift across all levels. Similar distributional patterns were observed for GFAP, VILIP-1, Tau, and PRDX-6 (Figure 3).
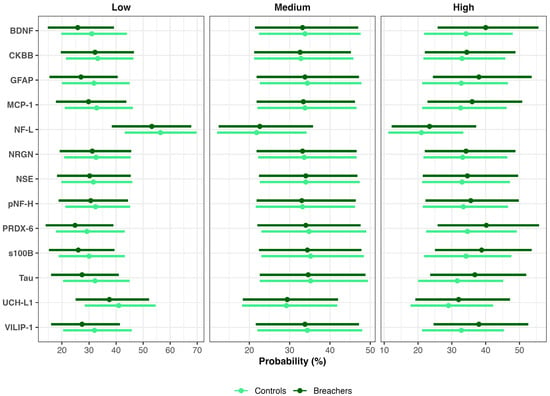
Figure 3.
Posterior probabilities of biomarker concentration categories in Breachers and Controls. Bayesian posterior mean probabilities (points) and 90% credible intervals (bars) for low, medium, and high biomarker concentrations in Breachers (dark green) and Controls (light green).
Parallel analyses of RPQ items demonstrated that Breachers were more likely to endorse frustration across all severity levels, reflecting a rightward shift in symptom distribution. The probability of reporting no frustration (“None”) was lower in Breachers (52%, 36–68%) than in Controls (63%, 48–77%), a 10% difference (90% CrI: 0–22%; pprob = 95%). Mild frustration (scores 1–2) was slightly more frequent among Breachers (29%, 16–44%) compared with Controls (24%, 13–38%), a 4% difference (90% CrI: 2–11%; pprob = 88%), while severe frustration (scores 3–4) occurred in 19% (8–32%) of Breachers versus 13% (5–23%) of controls (Δ = 6%; 90% CrI: 0–14%; pprob = 93%). Collectively, these findings indicate that Breachers were less likely to be symptom-free and more likely to report either mild or severe frustration, consistent with higher latent neuroinjury scores. Similar rightward-shifted distributions were observed for other RPQ items, including Poor Concentration, Taking Longer to Think, Irritability, Noise Sensitivity, Forgetfulness, and Depression (Figure 4).
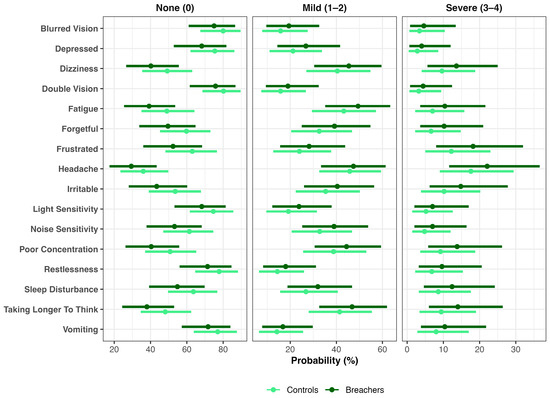
Figure 4.
Distributional shifts in post-concussive symptoms by group. Bayesian ordinal models estimated the probability of endorsing None (0), Mild (1–2), or Severe (3–4) symptom levels for each Rivermead Post-Concussion Questionnaire (RPQ) item in Breachers and Controls. Points represent posterior means and horizontal bars denote 90% credible intervals.
Despite group-level differences in symptom reporting and biomarker profiles, no differences were observed in N-Back working-memory performance between Breachers and Controls. This suggests that subjective symptom burden and underlying neurobiological alterations may emerge independently of overt cognitive impairment detectable by standard behavioral testing (Figure 5).
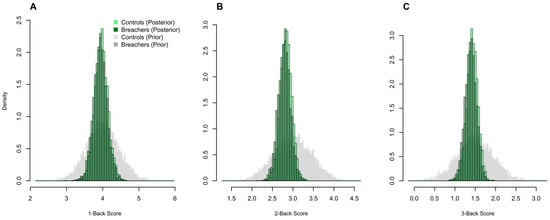
Figure 5.
Working-memory performance does not differ between Breachers and Controls. Posterior histograms of N-Back sensitivity (d′) for 1-Back (A), 2-Back (B), and 3-Back (C) conditions show substantial overlap between Breachers and Controls, with no meaningful group differences in accuracy or sensitivity. Corresponding prior histograms overlaid in grey.
3. Discussion
3.1. Overview and Principal Findings
This study provides convergent probabilistic evidence that chronic occupational exposure to low-level BOP among experienced breachers is associated with a latent pattern of subclinical neurobiological stress. Using a Bayesian latent-variable framework, we integrated biomarker, symptom, and cognitive domains into a unified latent neuroinjury construct. Breachers exhibited higher latent scores than military controls with a high posterior probability (97%). This elevation was driven primarily by post-concussive symptom burden—most notably difficulties with concentration, irritability, forgetfulness, and slowed thinking—with more modest contributions from circulating biomarkers indexing astroglial activation (GFAP), oxidative stress (PRDX-6), synaptic and neurotrophic signaling (VILIP-1, BDNF), and tau-related cytoskeletal strain. In contrast, working-memory performance contributed minimally to the latent construct, consistent with preserved task-level accuracy despite underlying symptomatic and biological differences. Posterior predictive contrasts further revealed rightward shifts in symptom distributions and subtle, non-uniform biomarker alterations in breachers—patterns that may reflect cumulative astroglial–axonal stress and compensatory neural adaptation, while acknowledging that alternative explanations cannot be excluded. Although exploratory, this work represents one of the most comprehensive multimodal assessments of circulating neurobiomarkers in a highly exposed operational cohort to date and highlights the utility of probabilistic modeling for characterizing subtle neurobiological variation in complex military populations exposed to repetitive blast.
3.2. Alignment with Prior Human Blast Biomarker Studies
The present findings align with a growing body of human research showing that both acute and chronic BOP exposure elicit quantifiable perturbations in astroglial, axonal, vascular, and neurotrophic proteins [17,53,67]. Although effect sizes were modest and inter-individual variability was evident, the biomarker pattern observed in this breacher cohort is broadly consistent with established pathophysiological processes implicated in blast-related neurotrauma. Experimental and human studies suggest that primary blast waves impart shear, tensile, and compressive forces to neural and vascular tissues, altering endothelial tight junctions and compromising BBB stability, with downstream consequences for astroglial activation, oxidative balance, and peripheral biomarker release [28,81,82]. Controlled live-fire, breaching, and heavy-weapons investigations consistently demonstrate rapid post-blast elevations in canonical CNS-derived biomarkers—including GFAP, UCH-L1, total and phosphorylated tau, and NfL—within hours to days after detonation [56,65,68,83].
Early field investigations established the translational sensitivity of these markers. Tate et al. first reported acute elevations in GFAP, UCH-L1, and αII-spectrin breakdown products in New Zealand breachers, with increases correlating with slowed reaction time and transient symptoms [11]. Carr et al. replicated acute UCH-L1 elevations in U.S. Army breachers over a two-week course—evidence of reproducible neuronal–glial stress in the absence of diagnosed concussion [72]. In a 5-year follow-up of the New Zealand cohort, Kamimori et al. observed no chronic change in GFAP or UCH-L1 among breachers repeatedly exposed to <4 psi, suggesting a physiological threshold separating safe from cumulatively injurious exposure [84]. Using ultrasensitive immunoassays, Boutté et al. found acute increases in tau, NfL, and Aβ40/42 with reduced GFAP within one-hour post-blast, correlating with reaction-time slowing [85]. Edwards et al. similarly reported biphasic tau and NfL responses after moderate blast (>5 psi), consistent with transient cytoskeletal disruption followed by delayed repair [73].
Comparable kinetics appear across operational contexts. Thangavelu et al. observed increased Aβ42 and reduced GFAP over three days of 0.50-caliber sniper training, linking impulse exposure to amyloidogenic processing and altered glial responsivity [86]. Tschiffely et al. reported acute GFAP reductions in experienced breachers that scaled inversely with cumulative exposure, suggesting adaptive or exhausted astroglial release dynamics under repeated low-level BOP [87]. Eonta et al. documented acute post-blast rises in GFAP and UCH-L1 after moderate BOP [50]. The magnitude of GFAP (~1.6-fold) and UCH-L1 (~3-fold) elevations in our chronically exposed breachers parallels these acute effects, implying that transient molecular responses may consolidate into altered steady-state levels with repeated exposure.
Beyond classical injury markers, convergent inflammatory and vascular signatures have been repeatedly observed. Gill et al. showed that moderate blast (~8 psi) down-regulated amyloid precursor protein and transiently elevated IL-6 and TNF-α [88,89]. Wang et al. identified down-regulation of Aβ40/42 alongside up-regulation of vascular and immune genes (THBS1, MMP9, CLU, LRP1) in U.S. breachers [67]. These signatures parallel BBB stress, altered endothelial signaling, and impaired clearance pathways reported in experimental models, including increases in S100B, MBP, NF-H, oxidative vascular injury, and microglial priming [90,91]. Elevated PRDX-6 and MCP-1 in our cohort are consistent with these processes, supporting ongoing oxidative and endothelial stress under repeated low-level BOP.
With increasing cumulative exposure, transient perturbations may progress toward chronic dysregulation. Career breachers, artillery operators, and SOF personnel demonstrate sustained elevations in GFAP, NfL, tau, and S100β proportional to exposure duration and symptom burden [5,92,93]. Agoston et al. reported prolonged elevations of GFAP, UCH-L1, claudin-5, occludin, MMP-9, IL-6, and brain-reactive autoantibodies following heavy-weapons training, suggesting persistent glio-vascular remodeling [94]. Rhind et al. documented chronic increases in anti-GFAP autoantibodies and endothelial injury markers, reinforcing BBB compromise and neuroimmune activation [45,74]. Edwards et al. further demonstrated that neuronal extracellular vesicle–associated tau and NfL tracked neurobehavioral symptom burden [92]. Longitudinal ADVANCE-TBI data show GFAP elevations persisting up to eight years post-injury [95]. Consistent with these reports, our cohort displayed parallel astroglial, axonal, and oxidative signatures within a single operationally exposed population.
Larger and multimodal studies converge on tau-related risk. In 106 active-duty personnel, Boutté et al. reported elevations in tau, UCH-L1, and Aβ40/42 proportional to cumulative impulse exposure [69]. Dickstein et al. linked blast exposure to tauopathy and axonal injury in veterans, demonstrating increased [18F]flortaucipir binding, elevated NfL, and perivascular tau deposition with astroglial scarring [96]. Vorn et al. observed acute increases in p-tau181, T-tau, and NfL during live-fire training [97]. Gilmore et al. associated cumulative blast exposure with elevated plasma p-tau181, neuroinflammatory markers, and increased cortical tau binding [98]. The mean plasma tau concentration in our breachers approximates ranges reported acutely post-blast, suggesting maintenance of low-grade cytoskeletal stress with repeated exposure.
The PRDX-6 elevation observed here extends prior evidence of glial and vascular activation to include mitochondrial redox stress, consistent with human breacher data and experimental models [29,50]. Although human data on VILIP-1 and BDNF in blast-exposed military populations remain limited, animal models and collision-sport studies demonstrate parallel dysregulation of calcium signaling and neurotrophic pathways [29,99]. Di Battista and colleagues, together with Buonora et al., reported elevations in PRDX-6, BDNF, and MCP-1 following mild TBI and concussion, linking oxidative stress and altered neurotrophic signaling to neuroinflammatory activation and delayed recovery [58,100,101]. Similar vascular–oxidative–neurotrophic patterns appear in breacher and SOF cohorts with sustained elevations of GFAP, NfL, tau, and endothelial activation markers [89]. These networks parallel microglial activation, cortical thinning, and astroglial scarring reported in MRI, PET, and postmortem studies [5,96,102,103]. In our cohort, elevated BDNF may reflect compensatory neurotrophic adaptation, while increased VILIP-1 suggests calcium-dependent synaptic stress—patterns paralleling working-memory inefficiencies and supporting a shared oxidative–neurotrophic axis across blunt and blast neurotrauma [65,104,105].
Together, convergent molecular, imaging, and neuropathological evidence outlines a reproducible vascular–glial–axonal continuum under chronic low-level blast exposure. The integrated biomarker signature observed here, GFAP, UCH-L1, tau, NfL, S100β, PRDX-6, VILIP-1, and BDNF, captures biological processes repeatedly documented in acute paradigms but expressed in a more persistent form. Modeled within a Bayesian latent-variable framework, these biomarkers clustered with symptom probability distributions and exceeded single-exposure magnitudes, consistent with cumulative neurobiological burden. Collectively, these findings support a cautious model in which repetitive low-level blast exposure may contribute to sustained vascular, glial, and metabolic stress, while underscoring the need for longitudinal, multimodal confirmation before causal inferences can be drawn.
3.3. Methodological and Analytical Advances
We implemented a principled Bayesian workflow designed to model the narratively generative data process through which occupational BOP exposure may give rise to neuroinjury across multiple domains. A strength of this approach (outlined in the ‘Workflow’ section of the methods) is its transparency and replicability: all scientific and statistical assumptions were made explicit, allowing other researchers to scrutinize, extend, or challenge the model directly; all our inferential claims are fully conditional on the assumptions we explicitly specified.
Our framework builds on the philosophical foundation articulated by I.J Good and others [106], who argued that probability represents a rational degree of belief rather than an objective property of the world. Accordingly, we emphasize that scientific rigor arises not from claims of objectivity, but from making our subjective modeling assumptions explicit and reproducible. Methodologically, we followed a principled Bayesian workflow espoused most prominently by Betancourt [107] and McElreath [108], including prior simulations, prior predictive checks, fake-data simulations, and visual posterior predictive validation—all of which are publicly available in our GitHub repository (see Data Availability Statement).
We view this iterative workflow as essential to professionalizing statistical practice in research. While it does not preclude errors in scientific reasoning and statistical inference, it promotes transparency, facilitates replication and iterative improvement in future studies, and reduces the risk of drawing nonsensical or misleading conclusions that have historically impeded scientific progress.
3.4. Reconciling “Normal” Cognition with Subclinical Neural Stress
Despite the robust symptom and biomarker signals in the present cohort, breachers did not differ from controls on conventional indices of working-memory accuracy or speed. This dissociation between molecular pathology and apparently intact behavior underscores a central challenge in studying subconcussive blast exposure: most neuropsychological tools were designed to detect overt impairment, not the diffuse, small-effect, network-level inefficiencies that accrue under chronic low-level BOP [12,13,109,110,111,112,113]. Aggregate scores on tasks such as the N-back can remain within normative bounds even as distributed cortical and subcortical systems operate at increased metabolic cost and reduced efficiency [114,115]. “Normal” performance may therefore be sustained by compensatory recruitment of additional neural resources—masking emerging dysfunction until physiological reserves are exceeded [116,117].
Multiple lines of evidence support this compensation-without-decompensation model in blast-exposed populations [17,118,119]. Consistent with this view, Di Battista et al. [120] recently applied Bayesian factor-analytic modeling to a larger cohort of CAF breachers, snipers, and military controls and found that, although individual task scores on four-choice reaction time, delayed matching-to-sample, N-back, and Stroop largely fell within normative ranges, snipers exhibited lower latent neurocognitive status than both breachers and controls, with effects driven primarily by higher-load working-memory demands. Complementing this, Martindale et al. [21] reported in a large LIMBIC-CENC cohort that low-level blast exposure did not independently predict decrements on standard cognitive tests but interacted with PTSD and deployment-related mild TBI to selectively worsen memory and processing speed, indicating that subtle blast-related vulnerability may emerge most clearly when network load is compounded by comorbid stressors. Early operational studies using computerized screening batteries (e.g., ANAM) likewise reported minimal group-mean decrements despite elevated subjective complaints [121,122,123,124]. Subsequent neuroimaging shows that such behavioral “normality” can coexist with reduced white-matter integrity, cortical thinning, and altered network topology within executive and attention systems [5,102,113,125,126,127]. Task-based fMRI reveals fronto-parietal hyperactivation and broader spatial recruitment during working-memory paradigms—signatures of compensatory up-regulation to maintain output [13,128,129,130].
Kashou et al.’s systematic review similarly emphasizes that resting-state fMRI findings in TBI are mixed and often subtle, with connectivity alterations emerging most clearly when analyses or tasks stress large-scale networks, further highlighting why early dysfunction may not appear on standard cognitive indices. For example, Pagulayan et al. [111] showed that Veterans with blast-related mTBI display widespread resting-state hyperconnectivity in frontal working-memory networks despite normal behavioral performance—a pattern interpreted as compensatory recruitment to maintain working-memory function. Consistent with this view, recent imaging reveals persistent resting-state hyperconnectivity in chronically exposed personnel and transient hypoconnectivity after acute blast—a dynamic pattern interpreted as adaptive reconfiguration under load [131,132,133]. Supporting this, Lam et al. [130] demonstrated a similar biphasic network pattern: in a large CAF breacher–sniper cohort, acute low-level blast exposure was associated with reduced global efficiency and focal hypoconnectivity in the pre-supplementary motor area, whereas chronic, career-long exposure showed increased global efficiency and nodal hyperconnectivity in fusiform regions. This acute-to-chronic shift—initial network suppression followed by later hyperconnective reorganization—suggests a potential compensatory adaptation to repeated low-level blast.
Cognitive–motor integration (CMI) paradigms sharpen this picture by taxing concurrent executive control, visuospatial transformation, and motor execution—functions supported by fronto-parietal–cerebellar circuits that are sensitive to diffuse axonal injury and network disconnection [75,134]. In CAF breachers and range staff, CMI testing revealed slower sensorimotor processing and greater intra-individual variability even when standard working-memory indices were unaffected [12,21,75]. These CMI-specific delays have been linked to disrupted coupling between associative and motor cortices and map onto subtle decrements in balance, visuomotor coordination, and dual-tasking—capabilities central to operational readiness [135,136,137]. The selectivity of these effects helps explain why coarse, single-domain tasks (accuracy, simple reaction time) may fail to register change, while integrated, network-demanding tasks do [138]. Together with the latent cognitive changes reported by Di Battista et al. [120] and the context-dependent blast effects observed by Martindale et al. [21], these findings support the view that diffuse network inefficiency often manifests behaviorally only under tasks that tax distributed circuits or when cognitive load is amplified by comorbid conditions—aligning with the present observation that early neural stress is more readily captured by integrated tasks and molecular signatures than by accuracy-based metrics alone.
Mechanistically, preserved performance alongside biomolecular disturbance aligns with established blast pathophysiology [139]. Repetitive low-level blast imposes baromechanical strain on the neurovascular unit, transiently increases BBB permeability, and perturbs glymphatic clearance, initiating chronic neuroinflammatory and oxidative cascades [24,33,47,96,102,140]. In our cohort, elevations in GFAP and PRDX-6 indicate astrocytic activation and redox imbalance; higher tau and VILIP-1 implicate cytoskeletal instability and dysregulated calcium signaling; and modulation of BDNF suggests altered neurotrophic tone and plasticity. These mechanisms plausibly degrade neural efficiency without reducing overt accuracy—particularly in highly trained operators capable of recruiting redundant pathways and cognitive strategies [113,141].
Crucially, compensation is neither cost-free nor indefinite [142]. Network over-recruitment elevates metabolic demand, and chronic reliance on compensatory circuits may accelerate neuroenergetic fatigue—precipitating subtle symptoms (mental tiredness, distractibility, slowed thinking) before standardized tests deteriorate [115]. Electrophysiological studies also show slowed oscillatory synchronization and altered beta-band coherence during cognitive effort, indicating an energetic premium to preserve performance [10,102,143,144]. Longitudinal work in repetitive head-impact and blast cohorts suggests these compensatory states plateau or erode with continued exposure, progressing to processing-speed decline, attentional lapses, and cognitive–motor slowing [21,112,145]. The latent neuroinjury factor identified here likely indexes this subclinical stage—capturing shared variance between biomarkers of astroglial/axonal stress and subjective cognitive-affective symptoms while overt behavior remains stable.
These insights carry methodological and clinical implications. First, reliance on single-domain neuropsychological tests risks false reassurance in high-exposure operators. Multidomain probes that stress integration—CMI tasks, dual-task paradigms, sustained attention under load, and decision-making in complex environments—are more likely to reveal the hidden inefficiency of diffuse network stress [12,21,75,146,147]. Second, coupling such tasks with physiological readouts—task-based fMRI, resting-state connectivity, quantitative EEG/MEG, and oculomotor metrics—can detect compensatory recruitment and altered timing before accuracy declines [5,102,112,130]. Third, integrating blood-based biomarkers adds mechanistic specificity: elevations in GFAP, PRDX-6, VILIP-1, BDNF, and tau isoforms provide convergent evidence of astroglial reactivity, oxidative stress, synaptic dysregulation, and cytoskeletal strain consistent with this compensatory framework [113,147,148,149,150].
Finally, the compensation model offers a path for operational monitoring and intervention. Periodic multidomain assessment—combining biomarker profiling with CMI and network-level neurophysiology—could identify personnel whose performance is maintained by costly compensation, guiding exposure management (e.g., training adjustments, recovery intervals), targeted rehabilitation (aerobic conditioning, vestibular/oculomotor therapy, cognitive pacing), and individualized follow-up. In research contexts, Bayesian latent-state models integrating serial biomarkers with digital cognitive and physiological data can quantify transitions from compensated to decompensated states and estimate dose–response relationships at the individual level [151,152,153].
3.5. Integrating Symptom, Neurobiological, and Mechanistic Domains
The latent neuroinjury construct defined in this study unifies biomarker perturbations and symptomatology into a probabilistic expression of subclinical neurobiological stress. Among molecular contributors, BDNF and VILIP-1—key mediators of synaptic plasticity and calcium regulation—showed the strongest loadings, reflecting sensitivity to excitatory imbalance and neuroplastic strain after blast exposure [69,90]. GFAP and PRDX-6, indices of astroglial activation and oxidative stress, likewise featured prominently, underscoring the recurring role of vascular–glial and redox dysregulation in blast pathophysiology [50,96,154]. Together these indicators suggest a coordinated neurobiological response to cumulative baromechanical loading, in which impaired proteostasis, mitochondrial dysfunction, and sustained glial activation contribute to chronic CNS stress.
Biomarker deviations were not uniformly distributed but concentrated in the upper tails, suggesting heterogeneous susceptibility among individuals. Similar “tail-heavy” elevations in p-tau181, NfL, and amyloid-β peptides have been reported in blast-exposed and contact-sport cohorts [73,85,97]. Such skew likely reflects variation in cumulative blast dose, BBB resilience, metabolic capacity, and immune priming [37,38]. Transcriptomic and proteomic studies support this view, showing repeated low-level blast modifies gene networks governing endothelial integrity, Toll-like receptor signaling, oxidative defense, and amyloid-β transport [67,155]. Within this framework, heterogeneity may reflect different positions along a shared cascade of vascular, glial, and proteostatic stress—where some individuals compensate effectively while others shift toward more persistent neuroinflammatory activation.
The symptom dimensions most predictive of the latent factor, difficulties with concentration, irritability, forgetfulness, and slowed thinking, mirror cognitive-affective and fatigue-somatic clusters typical of mTBI/concussion [156]. These experiences may arise from disrupted communication within fronto-limbic and thalamo-cortical networks, regions particularly vulnerable to diffuse axonal injury and glial dysfunction. Functional neuroimaging in blast-exposed personnel demonstrates altered prefrontal, cingulate, and parietal connectivity correlated with symptoms [9,21,130], implicating reduced network efficiency as a plausible bridge between molecular disturbance and subjective experience. Thus, co-expression of biomarker and symptom variance within a single latent dimension reflects a biologically coherent pattern: astroglial and oxidative stress align with network inefficiency manifesting as cognitive fatigue and affective dysregulation, even when task performance remains intact.
Previous CAF breacher studies further support this symptom–biomarker coupling. Astroglial and inflammatory markers (GFAP, MPO, MMP-9) covaried with subjective complaints—particularly cognitive fatigue and irritability—but not with standard neuropsychological scores [45]. Subsequent cohorts found CNS-directed autoantibodies correlated with irritability and slowed thinking, consistent with chronic neuroimmune activation [74]. These associations indicate that subjective symptoms capture a biologically meaningful dimension of CNS strain in populations where compensatory mechanisms may mask overt deficits. Integrating symptom-, biomarker-, and network-level evidence within a unified probabilistic framework therefore offers a cautious but mechanistically grounded approach to detecting early, potentially reversible stages of blast-related neurobiological dysfunction—linking cellular stress to subjective experience before measurable cognitive decline is apparent.
3.6. Implications for Clinical Translation and Operational Readiness
Mechanistically, the latent neuroinjury signature reflects a coordinated disturbance of vascular, glial, and metabolic homeostasis that may accrue silently under repetitive blast exposure. The neurobiomarker constellation observed here—elevated GFAP, PRDX-6, tau, VILIP-1, and altered BDNF—broadly aligns with the vascular–glial–tauopathy framework described above and corresponds to histopathological evidence of astroglial scarring, microvascular fragmentation, and perivascular tau deposition in blast-exposed military members and Veterans [2,19,157].
Clinically, this subclinical neurobiological pattern may contribute to the persistent cognitive fatigue, irritability, and affective dysregulation reported in breachers, artillery operators, and SOF personnel. Recognition of this potential prodromal phase highlights an opportunity for earlier characterization and targeted support before overt impairment emerges. Integrating validated biomarker panels—such as GFAP, NfL, PRDX-6, VILIP-1, and emerging markers such as Aβ40/42, p-tau181/217, and brain derived-tau—may enhance sensitivity to underlying neural stress beyond neurocognitive testing or symptom reports alone in high-exposure populations [103,158]. Importantly, such approaches are most feasible within specialized or high-risk units, pilot programs, or targeted surveillance initiatives, rather than force-wide implementation.
Operationally, these findings highlight the value of multimodal, precision-health frameworks that pair blood-based biomarkers with advanced neuroimaging, digital cognitive tools, and wearable blast dosimetry [67,155]. Such integrated systems may help refine recovery intervals, inform exposure limits, and support objective return-to-duty decisions while enabling targeted interventions to sustain performance. Alignment with NATO and Five-Eyes initiatives focused on biomarker harmonization and exposure standardization would further strengthen the translational utility of this approach [65,159].
Ultimately, embedding biomarker-based assessments within routine occupational health monitoring may help bridge mechanistic neuroscience and operational medicine, enabling early characterization of neurobiological strain and supporting evidence-informed strategies to mitigate long-term neurological risk and sustain operational readiness [53,66].
3.7. Limitations and Future Directions
Several limitations should be considered when interpreting these findings. First, the cross-sectional design and modest, occupation-specific sample preclude causal inference and limit generalizability beyond similarly exposed military populations and is susceptible to erroneous causal assumptions. Although blast exposure was well characterized at the occupational level for this cohort, individual-level blast overpressure was not directly quantified. The absence of wearable blast telemetry—reflecting practical and logistical constraints during training—limits precise dose–response modeling and constrains mechanistic interpretation. Future acute exposures and longitudinal studies integrating validated personal dosimetry with repeated biomarker and performance assessments will be essential to resolve exposure thresholds, recovery trajectories, and inter-individual susceptibility.
Second, while the present analysis focused on working memory as an index of performance resilience, this domain alone may underestimate subtle or context-dependent impairments. Prior work suggests that executive control, sustained attention, cognitive–motor integration, and decision-making under load may be more sensitive to diffuse network inefficiency associated with chronic low-level blast exposure. Expanding neurobehavioral assessment batteries, particularly under conditions of increased cognitive or physiological load, will be important for capturing compensatory limits that may not be evident in single-domain or low-demand tasks.
Third, self-reported symptoms contributed more strongly to the latent neuroinjury construct than either cognitive performance or individual biomarkers. This pattern underscores the relevance of subjective symptom burden in subclinical, high-functioning populations, while also warranting caution in interpreting latent constructs that integrate heterogeneous data streams. Multimodal frameworks that combine circulating biomarkers with neuroimaging, digital performance analytics, and physiological measures may better resolve how neural efficiency, compensatory mechanisms, and symptom expression interact across different stages of exposure and recovery. For example, incorporation of continuous physiological monitoring, such as wearable-derived exertion, recovery, and autonomic metrics, may improve sensitivity to compensatory strain and enable more precise optimization of exposure–recovery balance in operational settings.
Finally, although the biomarker-informed framework presented here shows promise for characterizing neurobiological strain in high-exposure personnel, its scalability and operational feasibility will depend on careful prioritization of markers, cost–benefit considerations, and integration with existing health surveillance systems. Future efforts should emphasize parsimonious, harmonized monitoring strategies aligned with allied initiatives, enabling translation from research cohorts to broader operational force health protection.
4. Materials and Methods
4.1. Participants
This prospective, cross-sectional cohort study enrolled Canadian Forces School of Military Engineering (CFSME) breacher instructors and range staff (n = 18; hereafter referred to as Breachers) with extensive occupational exposure to repetitive low-level blast overpressure accrued across their military careers. These personnel routinely conduct and supervise controlled explosive breaching activities as part of training and instructional duties.
The control group comprised CAF personnel (n = 19) with comparable age and sex distributions and no occupational history of blast exposure. Controls were selected to be similar to Breachers with respect to military experience, work schedules, and exposure to general operational, physical, and psychological stressors, differing primarily in the absence of routine blast exposure, as reported previously [12].
No participants in either group reported a clinically diagnosed history of moderate or severe TBI, major neurological disorder, psychotic illness, learning disability, or current substance abuse. Lifetime history of mild TBI/concussion was assessed by self-report in both groups. A subset of participants in each group reported remote, self-limited concussions earlier in their careers, as previously described [12,45]; the prevalence and distribution of head injury history were comparable between Breachers and Controls and were not associated with current symptom burden. Participants were excluded if they were taking medications known to affect cognitive or neurological function, or if they were acutely symptomatic due to infection, illness, or seasonal allergies at the time of testing.
The study was approved by the Defence Research and Development Canada (DRDC) Human Research Ethics Committee (HREC Protocol No. 2016-006) and conducted in accordance with the ethical principles of the Declaration of Helsinki. All participants received a detailed explanation of study procedures and potential risks and provided written informed consent prior to participation. Participant demographics and selected military characteristics are summarized in Table 1.
4.2. Experimental Design and Procedures
Data collection for CFSME Breachers and Controls was conducted during a single visit at one of two locations: Canadian Forces Base Gagetown (CFB Gagetown, NB) for Breachers and Defence Research and Development Canada–Toronto Research Centre (DRDC-TRC, ON) for controls. The identical protocol was followed at both sites. Following informed consent, participants provided demographic information and a comprehensive health and medical history, including details on operations (e.g., number of war-zone deployments, exposure to blasts, and proximity to explosions) and any history of concussions or brain injuries. Participants also completed occupational questionnaires about breaching and explosive experience, including intensities and frequencies of exposure.
Although individual-level blast overpressure telemetry was not collected in the present cohort, exposure characterization was informed by occupational role and prior empirical assessments conducted within the same training environment. Breacher instructors and range staff at the CFSME routinely supervise and participate in repeated controlled detonations during breaching training exercises. Importantly, DRDC-led field measurements using blast gauges (BlackBox Biometrics Inc., Rochester NY, USA), with sensors positioned on the helmet, non-firing shoulder, and chest, were previously conducted in a comparable cohort of CFSME instructors and range staff performing similar activities on the same breaching range. These assessments demonstrated that more than 30% of recorded blast wave events exceeded the commonly cited 4 psi (27.6 kPa) threshold during routine training operations [14,160]. Although these measurements were not acquired contemporaneously with the present study and do not permit direct estimation of individual cumulative blast dose, they provide relevant contextual evidence that CFSME breacher instructors are repeatedly exposed to BOP within a range previously associated with adverse alterations in brain health and performance in both human and experimental studies [161,162]. Accordingly, the occupational role–based exposure classification used here reflects a real-world training environment characterized by documented, repetitive exposure to biologically meaningful blast intensities.
Consistent with this exposure context, participants in the current breacher group typically contributed to 8–20 breaching training courses per year, each comprising 1–2 days on the breaching range with more than six controlled detonations per day. Breachers commonly delivered these courses over periods of one to three years, resulting in substantial cumulative exposure of variable frequency and magnitude [12]. To minimize potential confounding from acute physiological effects, participants were instructed to abstain from blast exposure for at least 48 h and to avoid strenuous physical activity for 24 h prior to testing. All data were de-identified and securely stored in a protected database to ensure participant confidentiality and data integrity. None of the participants reported a history of acquired brain injury, major neurological or psychiatric disorder, learning disability, or active substance abuse.
4.2.1. Rivermead Post-Concussion Symptoms Questionnaire
We administered the Rivermead Post Concussion Symptoms Questionnaire to measure post-concussive symptoms [163,164]. The RPQ includes 16 items, each of which represents a symptom associated with concussion. For each symptom, participants were asked to indicate whether they had experienced it as a function of injury to the head using a 5-point scale (0 = not experienced at all, 4 = a severe problem).
4.2.2. N-Back Test of Working Memory
This task assesses working-memory updating and maintenance across three load levels (1–3 back) [165]. Performance sensitivity was quantified using d′ derived from signal detection theory [166], where higher values denote greater discriminability and d′ ≈ 0 reflects chance-level performance. Because d′ is undefined when hit rates are 100% or false-alarm rates are 0% (i.e., when probability terms cannot be computed), standard correction procedures were applied. Participants with perfect hit rates or zero false alarms were reassigned adjusted values of 0.99 and 0.01, respectively, yielding a maximum possible d′ of 4.65. These corrections ensure stable estimation of sensitivity across participants and load conditions.
4.3. Blood Sample Collection, Processing and Storage
Peripheral blood samples were collected from participants in a fasting state by a trained medical technologist using standard phlebotomy procedures. Venous blood was drawn into 10 mL K2EDTA tubes (BD Vacutainer®, Franklin Lakes, NJ, USA), promptly centrifuged at 1600× g for 15 min at 4 °C, and separated into plasma aliquots for storage at −80 °C until further analysis. To maintain consistency, all samples were processed uniformly at the same time of day.
4.4. Plasma Neurological Biomarker Analyses
A panel of 13 neurological injury biomarkers was selected based on their brain specificity and potential to reflect distinct injury mechanisms: (1) neuronal cell body damage [neuron-specific enolase (NSE), neurogranin (NRGN), visinin-like protein (VILIP)-1, ubiquitin carboxy-terminal hydrolase (UCH-L1)]; (2) diffuse axonal injury [neurofilament light chain (NF-L), phosphorylated neurofilament heavy chain (pNF-H)]; (3) astroglial damage/gliosis [S100 calcium-binding protein beta (S100B), glial fibrillary acidic protein (GFAP)]; (4) cerebral ischemia/oxidative stress [creatine kinase brain isoenzyme (CK-BB), peroxiredoxin-6 (PRDX-6)]; (5) neuroinflammation [monocyte chemoattractant protein (MCP-1)]; (6) neural repair/plasticity [brain-derived neurotrophic factor (BDNF)]; (7) chronic neurodegeneration [total tau (T-tau]). Biomarkers were quantified using a combination of ultra-sensitive multiplexed neurological immunoassay panels from MesoScale Diagnostics, LLC (MSD®, Gaithersburg, MD, USA) MULTI-ARRAY™ Human Electrochemiluminescence assay and SINGLE MOLECULE ARRAY (SiMoA™) Human Neurology 4-Plex assay (Quanterix®, Lexington, MA, USA), as previously reported [101,167,168,169,170,171]. Biomarker values were considered usable if they were within the detection limits specified by the manufacturer and displayed a coefficient of variation (CV) of <20% between duplicate samples. All biomarkers were analyzed neat and in duplicate, with the mean value of the duplicates used as the final value. To minimize assay variation, all specimens were analyzed on the same day, in random order, by the same technician who was blinded to the participant’s status. Limits of detection (LLOD) and dynamic ranges are available on the MSD website (https://www.mesoscale.com/en (accessed 20 November 2025)).
4.5. Data Analysis
4.5.1. Workflow
The data analysis followed principled Bayesian workflow practices outlined by McElreath [108] and Betancourt [107]. The workflow began with a graphical model (Figure 6) that made explicit all scientific assumptions derived from domain expertise, providing a transparent foundation for subsequent statistical decisions. From this foundation, we constructed a prior-only statistical model that encoded our beliefs about plausible parameter values and outcome ranges. Prior predictive simulations were then used to calibrate the model, allowing us to detect pathologies, refine priors, and validate that the generative assumptions could reproduce biologically and clinically credible patterns. We then fit the model to simulated data to evaluate parameter recovery and check for redundancies and degeneracies. After iterating between these stages as needed, we fit the model to observed data and evaluated its adequacy with posterior retrodictive checks.
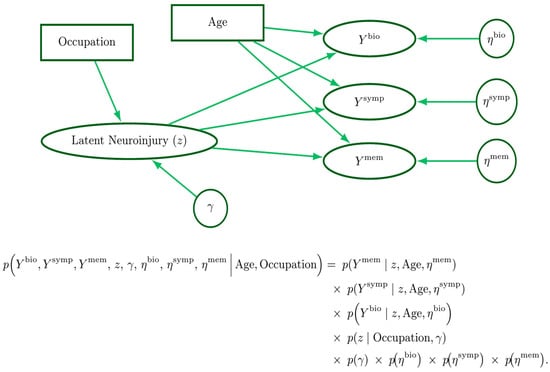
Figure 6.
A narratively generative variate–covariate model. Rectangles represent fixed covariates, ellipses denote variates (outcomes), and circles represent parameters. The latent neuroinjury () node is drawn as an ellipse for convenience, as it is both a parameter and variate. Beneath the graphical model is the corresponding joint probability factorization.
4.5.2. Scientific Modeling Assumptions
The primary aim of this study was two-fold: (1) to characterize a latent neuroinjury process that integrates molecular markers of brain injury, chronic brain injury symptoms, and measures of working memory, and (2) to quantitatively compare this process between military members in occupations with presumed higher repetitive exposure to low-level blast (breachers) and their non-exposed colleagues. Our scientific model followed the assumption that ‘neuroinjury’, could be quantified as a single metric informed by blood levels of biomarkers commonly associated with neuroinjury, self-reported post-concussion/brain injury symptoms, and the N-Back test of working memory. We hypothesized that breachers, due to their history of blast exposure, would display elevated levels of this latent neuroinjury factor compared to non-breacher counterparts. In, addition, we considered that age could influence each of the neuroinjury domains (biomarkers, symptoms, memory), independently of occupational exposure.
4.5.3. Statistical Modeling Assumptions
Our statistical model was constructed to reflect the above scientific assumptions. In addition, we assumed that the biomarker and symptom domains could both be modeled as ordinal outcomes arising from underlying continuous distributions. Specifically, biomarkers were divided into roughly equal tertiles corresponding to low, medium, and high categories. This approach served two purposes. First, it addressed the presence of values below the limit of assay detection. Second, it acknowledged our assumption that small numeric differences in biomarker levels are unlikely to have profound implications, whereas broader categorical distinctions may better reflect biologically and clinically relevant variation. This is an especially relevant assumption in small sample sizes like the current study, where ordinal categorization produces a sensible trade off of precision for stability, limiting the influence of biological noise and extreme values.
Symptom responses on the RPQ were collapsed from five categories to three: Low (0/1), Medium (1/2), Severe (3/4). This decision was made to mitigate sparse categories, stabilize threshold estimation in a small sample, and improve interpretability.
Finally, we modeled performance on the 1-, 2-, and 3-back tasks jointly using a multivariate normal likelihood to account for within-subject correlations across tests. This was motivated by the fact that N-back performance was summarized using signal-detection sensitivity (d′), defined as the difference between z-transformed hit rates and false alarm rates, which yields continuous scores.
A visual diagram of the combined scientific and statistical modeling assumptions in variate-covariate from [172], as well as the full joint conditional model can be seen in Figure 6.
4.5.4. Data
The dataset for this study consisted of a 37 row by 39 column matrix including seven participant demographic and characteristic variables (Group, Sex, Age, Military Status, Rank, years Breaching, Warzone Deployment), three N-Back variables (-1, -2, -3 Back d′), 13 Biomarkers, and 16 RPQ questions.
4.5.5. Estimands (Quantities of Interest)
Our primary estimands were (1) the individual-level latent neuroinjury score defined jointly by biomarkers, symptoms, and memory performance; and (2) the group-level contrast in these scores between Breachers and Controls. A secondary estimand was the individual loadings or contributions of each item within each domain (biomarkers, symptoms, memory) to the latent neuroinjury score.
4.5.6. Estimators (Statistical Models Used)
A linear regression was used to model individual-level latent neuroinjury scores (Equation (1)), with group membership as the sole covariate. Here, is the latent neuroinjury score for individual , modeled with unit variance for scale identification. is the expected latent score, determined by an intercept and the group indicator for breachers, for non breachers. represents the group effect, interpreted in standard deviation units of the latent scale. Weakly informative priors were placed on the and parameters:
Biomarkers (Equation (2)) and RPQ symptoms (Equation (3)) were treated as ordered categorical outcomes and modeled using ordinal logistic regression, with age and the latent neuroinjury scores as covariates. This specification followed an item-response theory framework: each biomarker or symptom was an “item” whose probability of occupying a higher category depends on the individual’s latent neuroinjury score and age, with item-specific coefficients indicating how strongly each item reflects variation in the latent neuroinjury process. Individuals were indexed by . Biomarker “items” were indexed by and RPQ symptom items by . Age for individual is and is the reference age (e.g., the sample mean) used for mean-centering. The parameter represents the linear model inside the cumulative link function for each biomarker or RPQ item, with representing the cutpoints. Parameters and represent the latent neuroinjury and age covariates, respectively.
N-Back performance for tasks was modeled jointly with a multivariate normal likelihood to account for within-subject correlation. Where are task-specific intercepts, are loadings on the latent neuroinjury score , and are mean centered age effects. The covariance across tasks was parameterized as , with task-specific standard deviations and correlation matrix assigned an LKJ prior.
4.5.7. Estimates (Posterior Inferences)
Posterior samples were generated via Hamiltonian Monte Carlo (HMC) as implemented in STAN [173]. These samples approximate the posterior distribution, and reported results are summaries of these draws. Typically, estimates are presented as posterior means with 90% credible intervals (CrIs). The summaries correspond to parameters or derived quantities constructed from the posterior parameter draws. For the latent neuroinjury score, group expectations were summarized directly from the derived of the linear model in Equation (1), and the group contrast was obtained by subtracting expectations between groups at each draw. Latent neuroinjury item loadings were summarized as the mean and 90% CrIs of the latent neuroinjury parameters for each item. For biomarkers and RPQ items, expected group differences were obtained by marginalizing the posterior predictive distribution of category probabilities, conditional on group differences in the latent neuroinjury score. At each posterior draw, predicted probabilities of falling in each category were computed, and group contrasts were summarized by their posterior means, 90% CrIs, and posterior probability that the contrast was greater than zero. For N-Back tasks, posterior draws of the regression parameters were used to generate predicted group-specific expectations (μ) for each task from the multivariate normal model. Group contrasts were then calculated and summarized analogously with posterior means, 90% CrIs, and the posterior probability that the contrast was greater than zero.
4.5.8. Software
The software Stan (Version 0.8.0) [173] provided the HMC engine which was interfaced through CmdStanR (run on R (Version 4.5.1) [174], using the RStudio integrated development environment (Version 2025.9.0.387) [174]. Model checks were aided by the model checking and plotting utilities created by Michael Betancourt [175]. To aid in the summarizing and visualizing of posterior samples the R package ‘rethinking’ was used [176]. Tables were made using the ‘gt’ [177] and ‘gtsummary’ [178] packages, with aid from the ‘dplyr’ [179] ‘tidyr’ [180] and ‘stringr’ [181] packages. The neuroinjury loadings plot was created using ‘ggplot2’ [182] with the aid of the ‘cowplot’, [183] and ‘forcats’ [184] packages (all accessed 20 November 2025). The ‘TinyTex’ [185] ‘magick’ [186] and ‘pdftools’ [187] packages were used to create the variate-covariate diagram seen in Figure 6 (all accessed 20 November 2025).
4.5.9. Reproducibility
Code and augmented data used for all statistical modeling in this manuscript can be found in a public GitHub repository (https://github.com/dibatti5/dibattista_et_al_2025_latent_neuroinjury_in_military_breachers, accessed on 22 November 2025).
5. Conclusions
This study provides probabilistic evidence that military breachers with extensive occupational exposure to low-level blast overpressure may exhibit a latent neurobiological profile characterized by modest but coordinated elevations in symptom burden and select circulating neuroinjury biomarkers, occurring in the context of preserved working-memory performance. The multidomain pattern observed—integrating post-concussive symptoms with biomarkers related to astroglial activity, oxidative stress, and cytoskeletal integrity—is broadly consistent with prior human and experimental research describing vascular–glial contributions to cumulative blast-related neural strain, while remaining subclinical and heterogeneous in expression. Importantly, cognitive performance measures did not contribute substantively to the latent construct, reinforcing a dissociation between molecular and symptomatic signals and overt task impairment.
By applying a Bayesian latent-variable framework, this work demonstrates a principled and transparent approach for integrating symptom, cognitive, and biomarker data into a unified probabilistic construct, enabling characterization of subtle neurobiological strain that may not be captured by single-domain analyses alone. While preliminary and not intended for diagnostic application, this framework provides a scalable foundation for future longitudinal investigations incorporating objective exposure metrics and repeated assessments. Taken together, these findings suggest that probabilistic integration of molecular indicators and symptom profiles may have translational relevance for occupational health surveillance, individualized risk stratification, and longitudinal monitoring in military personnel routinely exposed to repetitive low-level blast, while underscoring the need for continued validation in larger, dose-informed cohorts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms27020592/s1.
Author Contributions
Conceptualization, S.G.R., O.V., A.P.D.B., C.T., A.N., M.Y.S., T.L. and J.V.; methodology, S.G.R., O.V., A.P.D.B., C.T., A.N., M.Y.S., T.L. and J.V.; software, S.G.R., A.P.D.B., O.V. and M.Y.S.; validation, S.G.R., O.V., A.P.D.B. and M.Y.S.; formal analysis, A.P.D.B., S.G.R., O.V., M.Y.S. and C.T.; investigation, S.G.R., O.V., A.P.D.B., M.Y.S., C.T., A.N., T.L. and J.V.; resources, O.V., S.G.R., A.N., A.P.D.B. and C.T.; data curation, A.P.D.B., S.G.R., O.V., M.Y.S., C.T., T.L. and J.V.; writing—original draft preparation, S.G.R., A.P.D.B., O.V. and M.Y.S.; writing—review and editing, S.G.R., A.P.D.B., O.V., M.Y.S., C.T., A.N., T.L. and J.V.; visualization, S.G.R., A.P.D.B., C.T., O.V., M.Y.S., T.L. and J.V.; supervision, S.G.R., O.V., C.T., A.N., M.Y.S. and A.P.D.B.; project administration, S.G.R., O.V., M.Y.S., C.T., A.N. and A.P.D.B.; funding acquisition, S.G.R., O.V., M.Y.S., C.T., A.P.D.B. and A.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by funding from the Canada’s Department of National Defence (DND) and Defence Research & Development Canada (DRDC) and approved by the CAF Surgeon General’s Health Research Program. In accordance with the DND policy, this paper was reviewed and approved for submission without modification by the DRDC Publications Office.
Institutional Review Board Statement
This research was conducted in accordance with the Declaration of Helsinki, and was approved by the DRDC Human Research Ethics Committee (HREC Protocol No. 2016-006, 26 February 2016).
Informed Consent Statement
Informed consent was obtained from all participants involved in the current research study.
Data Availability Statement
Aggregate, group-level data supporting the findings of this study are included within the article and its Supplementary Materials. In addition, a GitHub repository containing the analysis code and non-identifiable supporting materials is available. Individual-level datasets generated and analyzed during the study are not publicly available due to privacy, ethical, and governance restrictions associated with research involving Department of National Defence (DND) and Canadian Armed Forces (CAF) personnel. Specifically, variables such as study site, dates of data collection, date of birth, and participant gender could permit re-identification. De-identified datasets with these variables removed may be made available by the corresponding author upon reasonable request, subject to institutional ethics approval and applicable data protection policies.
Acknowledgments
The authors would like to sincerely thank all of the CFSME and CAF members who volunteered their time to participate in this study, along with Kristen King, Doug Saunders, Witty Tse, and Shyanne Hang for their technical support. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Aβ | Amyloid-β |
| Aβ40/42 | Amyloid-β 40 and 42 peptides |
| AD | Alzheimer’s disease |
| ANAM | Automated Neuropsychological Assessment Metrics |
| APP | Amyloid precursor protein |
| ATP | Adenosine triphosphate |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BD-tau | Brain-derived tau |
| BOP | Blast overpressure |
| CAF | Canadian Armed Forces |
| CI | Credible interval |
| CMI | Cognitive–motor integration |
| CNS | Central nervous system |
| CREB | cAMP response element-binding protein |
| CSF | Cerebrospinal fluid |
| CTE | Chronic traumatic encephalopathy |
| DTI | Diffusion tensor imaging |
| EEG | Electroencephalography |
| EV | Extracellular vesicle |
| fMRI | Functional magnetic resonance imaging |
| GFAP | Glial fibrillary acidic protein |
| IL-6 | Interleukin-6 |
| IRR | Injury risk ratio (if present) |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinase |
| MBP | Myelin basic protein |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MEG | Magnetoencephalography |
| MMP | Matrix metalloproteinase |
| mBDNF | Mature BDNF |
| mTBI | Mild traumatic brain injury |
| NfL | Neurofilament light chain |
| pNF-H | Phosphorylated neurofilament heavy chain |
| NGRN | Neurogranin |
| NSE | Neuron-specific enolase |
| PET | Positron emission tomography |
| PCL | PTSD Checklist |
| PI3K | Phosphoinositide 3-kinase |
| PLCγ | Phospholipase C-gamma |
| PRDX-6 | Peroxiredoxin-6 |
| PTSD | Post-traumatic stress disorder |
| RBANS | Repeatable Battery for the Assessment of Neuropsychological Status |
| RPQ | Rivermead Post-Concussion Questionnaire |
| S100b | S100 calcium-binding protein beta |
| SOF | Special Operations Forces |
| T-tau | Total tau |
| TBI | Traumatic brain injury |
| TNF-α | Tumor necrosis factor-alpha |
| TrkB | Tropomyosin receptor kinase B |
| UCH-L1 | Ubiquitin carboxy-terminal hydrolase L1 |
| VCAM-1/ICAM-1 | Vascular/Intercellular cell adhesion molecules |
| VILIP-1 | Visinin-like protein 1 |
References
- Belding, J.N.; Kolaja, C.A.; Rull, R.P.; Trone, D.W. Single and repeated high-level blast, low-level blast, and new-onset self-reported health conditions in the U.S. Millennium Cohort Study: An exploratory investigation. Front. Neurol. 2023, 14, 1110717. [Google Scholar] [CrossRef] [PubMed]
- Martindale, S.L.; Belding, J.N.; Crawford, C.D.; Rowland, J.A. Validation of Military Occupational Specialty as a Proxy for Blast Exposure Using the Salisbury Blast Interview. J. Neurotrauma 2023, 40, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Kamimori, G.H.; Reilly, L.A.; LaValle, C.R.; Da Silva, U.B.O. Occupational overpressure exposure of breachers and military personnel. Shock Waves 2017, 27, 837–847. [Google Scholar] [CrossRef]
- Carr, W.; Dell, K.C.; Yanagi, M.A.; Hassan, D.M.; LoPresti, M.L. Perspectives on repeated low-level blast and the measurement of neurotrauma in humans as an occupational exposure risk. Shock Waves 2017, 27, 829–836. [Google Scholar] [CrossRef]
- Stone, J.R.; Avants, B.B.; Tustison, N.J.; Wassermann, E.M.; Gill, J.; Polejaeva, E.; Dell, K.C.; Carr, W.; Yarnell, A.M.; LoPresti, M.L.; et al. Functional and Structural Neuroimaging Correlates of Repetitive Low-Level Blast Exposure in Career Breachers. J. Neurotrauma 2020, 37, 2468–2481. [Google Scholar] [CrossRef]
- Martindale, S.L.; Ord, A.S.; Rowland, J.A. Influence of blast exposure on cognitive functioning in combat veterans. Neuropsychology 2020, 34, 735–743. [Google Scholar] [CrossRef]
- de Souza, N.L.; Lindsey, H.M.; Dorman, K.; Dennis, E.L.; Kennedy, E.; Menefee, D.S.; Parrott, J.S.; Jia, Y.; Pugh, M.J.V.; Walker, W.C.; et al. Neuropsychological Profiles of Deployment-Related Mild Traumatic Brain Injury: A LIMBIC-CENC Study. Neurology 2024, 102, e209417. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.R.; Martindale, S.L.; Rowland, J.A.; Walton, S.; Talmy, T.; Walker, W.C. Blast-related mild TBI: LIMBIC-CENC focused review with implications commentary. NeuroRehabilitation 2024, 55, 329–345. [Google Scholar] [CrossRef]
- Martindale, S.L.; Kolaja, C.A.; Belding, J.N.; Liu, L.; Rull, R.P.; Trone, D.W.; Rowland, J.A. Blast exposure and long-term diagnoses among veterans: A millennium cohort study investigation of high-level blast and low-level blast. Front. Neurol. 2025, 16, 1599351. [Google Scholar] [CrossRef]
- Solar, K.G.; Ventresca, M.; Zamyadi, R.; Zhang, J.; Jetly, R.; Vartanian, O.; Rhind, S.G.; Dunkley, B.T. Repetitive subconcussion results in disrupted neural activity independent of concussion history. Brain Commun. 2024, 6, fcae348. [Google Scholar] [CrossRef]
- Tate, C.M.; Wang, K.K.; Eonta, S.; Zhang, Y.; Carr, W.; Tortella, F.C.; Hayes, R.L.; Kamimori, G.H. Serum brain biomarker level, neurocognitive performance, and self-reported symptom changes in soldiers repeatedly exposed to low-level blast: A breacher pilot study. J. Neurotrauma 2013, 30, 1620–1630. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, O.; Tenn, C.; Rhind, S.G.; Nakashima, A.; Di Battista, A.P.; Sergio, L.E.; Gorbet, D.J.; Fraser, D.D.; Colantonio, A.; King, K.; et al. Blast in Context: The Neuropsychological and Neurocognitive Effects of Long-Term Occupational Exposure to Repeated Low-Level Explosives on Canadian Armed Forces’ Breaching Instructors and Range Staff. Front. Neurol. 2020, 11, 588531. [Google Scholar] [CrossRef]
- Vartanian, O.; Coady, L.; Blackler, K.; Fraser, B.; Cheung, B. Neuropsychological, Neurocognitive, Vestibular, and Neuroimaging Correlates of Exposure to Repetitive Low-Level Blast Waves: Evidence from Four Nonoverlapping Samples of Canadian Breachers. Mil. Med. 2021, 186, e393–e400. [Google Scholar] [CrossRef]
- Nakashima, A.; Vartanian, O.; Rhind, S.G.; King, K.; Tenn, C.; Jetly, C.R. Repeated Occupational Exposure to Low-level Blast in the Canadian Armed Forces: Effects on Hearing, Balance, and Ataxia. Mil. Med. 2022, 187, e201–e208. [Google Scholar] [CrossRef]
- Brown, K. Relationship Between Cognition and Blast Exposure Symptom Severity in Military Veterans and Servicemembers. Arch. Phys. Med. Rehabil. 2025, 106, e106. [Google Scholar] [CrossRef]
- Shea, K.; Vartanian, O.; Rhind, S.G.; Tenn, C.; Nakashima, A. Impact of Low-Level Blast Exposure from Military Training and Career Cumulation on Hearing Outcomes. Mil. Med. 2025, 190, e1999–e2006. [Google Scholar] [CrossRef]
- Belanger, H.G.; Bowling, F.Y.; Yao, E.F. Low-Level Blast Exposure in Humans a Systematic Review of Acute and Chronic Effects. J. Spec. Oper. Med. 2020, 20, 87–93. [Google Scholar] [CrossRef]
- Vartanian, O.; Rhind, S.G.; Nakashima, A.; Tenn, C.; Lam, T.K.; Shiu, M.; Caddy, N.; King, K.; Natale, A.; Jetly, R. Blast effects on post-concussive and mental health outcomes: Data from Canadian Armed Forces breachers and snipers. J. Mil. Veter. Fam. Health 2022, 8, 82–96. [Google Scholar] [CrossRef]
- Belding, J.N.; Bonkowski, J.; Englert, R. Traumatic brain injury and occupational risk of low-level blast exposure on adverse career outcomes: An examination of administrative and medical separations from Service (2005–2015). Front. Neurol. 2024, 15, 1389757. [Google Scholar] [CrossRef] [PubMed]
- Epshtein, E.; Shraga, S.; Radomislensky, I.; Martindale, S.L.; Bushinsky, S.; Benov, A.; Almog, O.; Tsur, A.M.; Talmy, T.; Bahouth, H.; et al. Blast injury and chronic psychiatric disability in military personnel: Exploring the association beyond posttraumatic stress disorder. J. Psychiatr. Res. 2025, 184, 515–521. [Google Scholar] [CrossRef]
- Martindale, S.L.; Bailie, J.M.; Miles, S.R.; Walker, W.C.; Babakhanyan, I.; Davenport, N.D.; Magnante, A.T.; Hinds, S.R.; Craig, K.M.; Rowland, J.A. Cumulative and Contextual Effects of Low-Level Blast Exposure on Cognitive Function in Military Personnel: Interactions with PTSD and Mild TBI. J. Head Trauma Rehabil. 2025. [Google Scholar] [CrossRef]
- Rutter, B.; Song, H.; DePalma, R.G.; Hubler, G.; Cui, J.; Gu, Z.; E Johnson, C. Shock Wave Physics as Related to Primary Non-Impact Blast-Induced Traumatic Brain Injury. Mil. Med. 2021, 186, 601–609. [Google Scholar] [CrossRef]
- Liang, R.; Wang, H.; Gao, J.; Wang, J.; Zhang, W.; Qian, A. Assessment model of blast injury: A narrative review. iScience 2025, 28, 112830. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, M.; Younger, D.; Ravula, A.R.; Alay, E.; Rao, K.V.R.; Chandra, N. Synergistic Role of Oxidative Stress and Blood-Brain Barrier Permeability as Injury Mechanisms in the Acute Pathophysiology of Blast-induced Neurotrauma. Sci. Rep. 2019, 9, 7717. [Google Scholar] [CrossRef] [PubMed]
- Donat, C.K.; Lopez, M.Y.; Sastre, M.; Baxan, N.; Goldfinger, M.; Seeamber, R.; Müller, F.; Davies, P.; Hellyer, P.; Siegkas, P.; et al. From biomechanics to pathology: Predicting axonal injury from patterns of strain after traumatic brain injury. Brain 2021, 144, 70–91. [Google Scholar] [CrossRef]
- Uzunalli, G.; Herr, S.; Dieterly, A.M.; Shi, R.; Lyle, L.T. Structural disruption of the blood–brain barrier in repetitive primary blast injury. Fluids Barriers CNS 2021, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Nguyen, T.-T.; Wu, T.; Ghajari, M. Non-Lethal Blasts Can Generate Cavitation in Cerebrospinal Fluid While Severe Helmeted Impacts Cannot: A Novel Mechanism for Blast Brain Injury. Front. Bioeng. Biotechnol. 2022, 10, 808113. [Google Scholar] [CrossRef]
- Zhu, X.; Chu, X.; Wang, H.; Liao, Z.; Xiang, H.; Zhao, W.; Yang, L.; Wu, P.; Liu, X.; Chen, D.; et al. Investigating neuropathological changes and underlying neurobiological mechanisms in the early stages of primary blast-induced traumatic brain injury: Insights from a rat model. Exp. Neurol. 2024, 375, 114731. [Google Scholar] [CrossRef]
- Siedhoff, H.R.; Chen, S.; Song, H.; Cui, J.; Cernak, I.; Cifu, D.X.; DePalma, R.G.; Gu, Z. Perspectives on Primary Blast Injury of the Brain: Translational Insights into Non-inertial Low-Intensity Blast Injury. Front. Neurol. 2021, 12, 818169. [Google Scholar] [CrossRef]
- Sachdeva, T.; Ganpule, S.G. Twenty Years of Blast-Induced Neurotrauma: Current State of Knowledge. Neurotrauma Rep. 2024, 5, 243–253. [Google Scholar] [CrossRef]
- Courtney, A.; Courtney, M. A thoracic mechanism of mild traumatic brain injury due to blast pressure waves. Med. Hypotheses 2009, 72, 76–83. [Google Scholar] [CrossRef]
- Rubio, J.E.; Skotak, M.; Alay, E.; Sundaramurthy, A.; Subramaniam, D.R.; Kote, V.B.; Yeoh, S.; Monson, K.; Chandra, N.; Unnikrishnan, G.; et al. Does Blast Exposure to the Torso Cause a Blood Surge to the Brain? Front. Bioeng. Biotechnol. 2020, 8, 573647. [Google Scholar] [CrossRef]
- Sosa, M.A.G.; De Gasperi, R.; Pryor, D.; Garcia, G.S.P.; Perez, G.M.; Abutarboush, R.; Kawoos, U.; Hogg, S.; Ache, B.; Janssen, W.G.; et al. Low-level blast exposure induces chronic vascular remodeling, perivascular astrocytic degeneration and vascular-associated neuroinflammation. Acta Neuropathol. Commun. 2021, 9, 167. [Google Scholar] [CrossRef]
- Kawoos, U.; Abutarboush, R.; Gu, M.; Chen, Y.; Statz, J.K.; Goodrich, S.Y.; Ahlers, S.T. Blast-induced temporal alterations in blood–brain barrier properties in a rodent model. Sci. Rep. 2021, 11, 5906. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.E.; Unnikrishnan, G.; Sajja, V.S.S.S.; Van Albert, S.; Rossetti, F.; Skotak, M.; Alay, E.; Sundaramurthy, A.; Subramaniam, D.R.; Long, J.B.; et al. Investigation of the direct and indirect mechanisms of primary blast insult to the brain. Sci. Rep. 2021, 11, 16040. [Google Scholar] [CrossRef]
- Elster, N.; Boutillier, J.; Magnan, P.; Naz, P.; Willinger, R.; Deck, C. A critical review of experimental analyses performed on animals, post-mortem human subjects, and substitutes to explore primary blast-induced Traumatic Brain Injuries. Front. Mech. Eng. 2023, 9, 1185231. [Google Scholar] [CrossRef]
- Abutarboush, R.; Reed, E.; Chen, Y.; Gu, M.; Watson, C.; Kawoos, U.; Statz, J.K.; Tschiffely, A.E.; Ciarlone, S.; Perez-Garcia, G.; et al. Exposure to Low-Intensity Blast Increases Clearance of Brain Amyloid Beta. J. Neurotrauma 2024, 41, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Sevao, M.; A Keil, S.; Gino, E.; Wang, M.X.; Lee, J.; A Haveliwala, M.; Klein, E.; Agarwal, S.; Pedersen, T.; et al. Macroscopic changes in aquaporin-4 underlie blast traumatic brain injury-related impairment in glymphatic function. Brain 2024, 147, 2214–2229. [Google Scholar] [CrossRef]
- Readnower, R.D.; Chavko, M.; Adeeb, S.; Conroy, M.D.; Pauly, J.R.; McCarron, R.M.; Sullivan, P.G. Increase in blood–brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res. 2010, 88, 3530–3539. [Google Scholar] [CrossRef]
- Huber, B.; Meabon, J.; Hoffer, Z.; Zhang, J.; Hoekstra, J.; Pagulayan, K.; McMillan, P.; Mayer, C.; Banks, W.; Kraemer, B.; et al. Blast exposure causes dynamic microglial/macrophage responses and microdomains of brain microvessel dysfunction. Neuroscience 2016, 319, 206–220. [Google Scholar] [CrossRef]
- Shively, S.B.; Horkayne-Szakaly, I.; Jones, R.V.; Kelly, J.P.; Armstrong, R.C.; Perl, D.P. Characterisation of interface astroglial scarring in the human brain after blast exposure: A post-mortem case series. Lancet Neurol. 2016, 15, 944–953. [Google Scholar] [CrossRef]
- Hernandez, A.; Tan, C.; Plattner, F.; Logsdon, A.F.; Pozo, K.; Yousuf, M.A.; Singh, T.; Turner, R.C.; Lucke-Wold, B.P.; Huber, J.D.; et al. Exposure to mild blast forces induces neuropathological effects, neurophysiological deficits and biochemical changes. Mol. Brain 2018, 11, 64, Correction in Mol. Brain 2021, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Iacono, D.; Murphy, E.K.; Stimpson, C.D.; Leonessa, F.; Perl, D.P. Double Blast Wave Primary Effect on Synaptic, Glymphatic, Myelin, Neuronal and Neurovascular Markers. Brain Sci. 2023, 13, 286. [Google Scholar] [CrossRef]
- Nishii, K.; Satoh, Y.; Higashi, T.; Matsui, T.; Ishizuka, T.; Kashitani, M.; Saitoh, D.; Kobayashi, Y. Evans blue and FITC-dextran double labeling reveals precise sequence of vascular leakage and glial responses after exposure to mild-level blast-associated shock waves. J. Neurotrauma 2023, 40, 1228–1242. [Google Scholar] [CrossRef] [PubMed]
- Rhind, S.G.; Shiu, M.Y.; Tenn, C.; Nakashima, A.; Jetly, R.; Sajja, V.S.S.S.; Long, J.B.; Vartanian, O. Repetitive Low-Level Blast Exposure Alters Circulating Myeloperoxidase, Matrix Metalloproteinases, and Neurovascular Endothelial Molecules in Experienced Military Breachers. Int. J. Mol. Sci. 2025, 26, 1808. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.A.; Kim, J.H.; Situ, R.; Taylor, W.; Westmoreland, T.; Du, F.; Parks, S.; Ling, G.; Hwang, J.Y.; Rapuano, A.; et al. Neuronal and glial changes in the brain resulting from explosive blast in an experimental model. Acta Neuropathol. Commun. 2016, 4, 124. [Google Scholar] [CrossRef]
- Elder, G.A.; Sosa, M.A.G.; De Gasperi, R.; Garcia, G.P.; Perez, G.M.; Abutarboush, R.; Kawoos, U.; Zhu, C.W.; Janssen, W.G.M.; Stone, J.R.; et al. The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma. Int. J. Mol. Sci. 2024, 25, 1150. [Google Scholar] [CrossRef]
- Kilgore, M.O.; Hubbard, W.B. Effects of Low-Level Blast on Neurovascular Health and Cerebral Blood Flow: Current Findings and Future Opportunities in Neuroimaging. Int. J. Mol. Sci. 2024, 25, 642. [Google Scholar] [CrossRef]
- Norris, C.; Murphy, S.F.; VandeVord, P.J. Acute astrocytic and neuronal regulation of glutamatergic protein expression following blast. Neurosci. Lett. 2025, 848, 138108. [Google Scholar] [CrossRef]
- E Eonta, S.; Kamimori, G.H.; Wang, K.K.W.; Carr, W.; LaValle, C.R.; Egnoto, M.J.; Tate, C.M. Case Study of a Breacher: Investigation of Neurotrauma Biomarker Levels, Self-reported Symptoms, and Functional MRI Analysis Before and After Exposure to Measured Low-Level Blast. Mil. Med. 2020, 185, e513–e517. [Google Scholar] [CrossRef]
- Frueh, B.C.; Madan, A.; Fowler, J.C.; Stomberg, S.; Bradshaw, M.; Kelly, K.; Weinstein, B.; Luttrell, M.; Danner, S.G.; Beidel, D.C. “Operator syndrome”: A unique constellation of medical and behavioral health-care needs of military special operation forces. Int. J. Psychiatry Med. 2020, 55, 281–295. [Google Scholar] [CrossRef]
- Englert, R.M.; Belding, J.N.; Thomsen, C.J. Self-Reported Symptoms in U.S. Marines Following Blast- and Impact-Related Concussion. Mil. Med. 2023, 188, e2118–e2125. [Google Scholar] [CrossRef]
- Beard, K.; Gauff, A.K.; Pennington, A.M.; Marion, D.W.; Smith, J.; Sloley, S. Biofluid, Imaging, Physiological, and Functional Biomarkers of Mild Traumatic Brain Injury and Subconcussive Head Impacts. J. Neurotrauma 2024, 42, 1601–1620. [Google Scholar] [CrossRef]
- Mathew, D.; Purohit, P.; Gadwal, A.; Anil, A.; Sharma, R.K.; Meshram, V.P.; Setia, P. Integrated Assessment of GFAP and UCH-L1 for their utility in severity assessment and outcome prediction in Traumatic Brain Injury. Int. J. Leg. Med. 2024, 138, 2559–2568. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Zetterberg, H.; Buki, A.; Dengler, B.A.; Diaz-Arrastia, R.; Korley, F.K.; Lazarus, R.; Meier, T.B.; Mondello, S.; Moritz, K.; et al. Blood-Based Biomarkers for Improved Characterization of Traumatic Brain Injury: Recommendations from the 2024 National Institute for Neurological Disorders and Stroke Traumatic Brain Injury Classification and Nomenclature Initiative Blood-Based Biomarkers Working Group. J. Neurotrauma 2025, 42, 1065–1085. [Google Scholar] [CrossRef] [PubMed]
- Lisi, I.; Moro, F.; Mazzone, E.; Marklund, N.; Pischiutta, F.; Kobeissy, F.; Mao, X.; Corrigan, F.; Helmy, A.; Nasrallah, F.; et al. Exploiting blood-based biomarkers to align preclinical models with human traumatic brain injury. Brain 2025, 148, 1062–1080. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.P.; Rhind, S.G.; Baker, A.J. Application of blood-based biomarkers in human mild traumatic brain injury. Front. Neurol. 2013, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.P.; Buonora, J.E.; Rhind, S.G.; Hutchison, M.G.; Baker, A.J.; Rizoli, S.B.; Diaz-Arrastia, R.; Mueller, G.P. Blood Biomarkers in Moderate-To-Severe Traumatic Brain Injury: Potential Utility of a Multi-Marker Approach in Characterizing Outcome. Front. Neurol. 2015, 6, 110. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Rhind, S.G.; Baker, A.J.; Jetly, R.; Debad, J.D.; Richards, D.; Hutchison, M.G. An investigation of neuroinjury biomarkers after sport-related concussion: From the subacute phase to clinical recovery. Brain Inj. 2018, 32, 575–582. [Google Scholar] [CrossRef]
- Rubenstein, R.; Chang, B.; Yue, J.K.; Chiu, A.; Winkler, E.A.; Puccio, A.M.; Diaz-Arrastia, R.; Yuh, E.L.; Mukherjee, P.; Valadka, A.B.; et al. Comparing Plasma Phospho Tau, Total Tau, and Phospho Tau–Total Tau Ratio as Acute and Chronic Traumatic Brain Injury Biomarkers. JAMA Neurol. 2017, 74, 1063–1072. [Google Scholar] [CrossRef]
- Huibregtse, M.E.; Bazarian, J.J.; Shultz, S.R.; Kawata, K. The biological significance and clinical utility of emerging blood biomarkers for traumatic brain injury. Neurosci. Biobehav. Rev. 2021, 130, 433–447. [Google Scholar] [CrossRef]
- Miner, A.E.; Groh, J.R.; Tripodis, Y.; Adler, C.H.; Balcer, L.J.; Bernick, C.; Zetterberg, H.; Blennow, K.; Peskind, E.; Ashton, N.J.; et al. Examination of plasma biomarkers of amyloid, tau, neurodegeneration, and neuroinflammation in former elite American football players. Alzheimer’s Dement. 2024, 20, 7529–7546. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kc, N.; Paneque, A.; Cole, P.D. Tau, Glial Fibrillary Acidic Protein, and Neurofilament Light Chain as Brain Protein Biomarkers in Cerebrospinal Fluid and Blood for Diagnosis of Neurobiological Diseases. Int. J. Mol. Sci. 2024, 25, 6295. [Google Scholar] [CrossRef]
- Luo, R.; Yu, J.-T. New biomarkers for early-stage tau pathology in Alzheimer’s disease. Nat. Aging 2025, 5, 734–735. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.J.; Keyser, D.O.; Rowe, S.S.; Hernandez, R.S.; Dovel, M.; Romero, H.; Lee, D.; Menezes, M.; Magee, E.; Brooks, D.J.; et al. Methodology of the INVestigating traIning assoCiated blasT pAthology (INVICTA) study. BMC Med Res. Methodol. 2022, 22, 317. [Google Scholar] [CrossRef]
- I Kocik, V.; A Dengler, B.; A Rizzo, J.; Moran, M.M.; Willis, A.M.; April, M.D.; Schauer, S.G. A Narrative Review of Existing and Developing Biomarkers in Acute Traumatic Brain Injury for Potential Military Deployed Use. Mil. Med. 2024, 189, e1374–e1380. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, S.; Liu, Q.; Kranfli, A.A.; Nemes, J.; Sullan, M.; Hoisington, A.; Brenner, L.A.; Skotak, M.; LaValle, C.R.; et al. Impact of prior exposures on biomarkers of blast during military tactical training. Front. Neurol. 2025, 16, 1589742. [Google Scholar] [CrossRef]
- Svetlov, S.I.; Larner, S.F.; Kirk, D.R.; Atkinson, J.; Hayes, R.L.; Wang, K.K. Biomarkers of blast-induced neurotrauma: Profiling molecular and cellular mechanisms of blast brain injury. J. Neurotrauma 2009, 26, 913–921. [Google Scholar] [CrossRef]
- Boutté, A.M.; Thangavelu, B.; Nemes, J.; LaValle, C.R.; Egnoto, M.; Carr, W.; Kamimori, G.H. Neurotrauma Biomarker Levels and Adverse Symptoms Among Military and Law Enforcement Personnel Exposed to Occupational Overpressure Without Diagnosed Traumatic Brain Injury. JAMA Netw. Open 2021, 4, e216445. [Google Scholar] [CrossRef]
- Vorn, R.; Edwards, K.A.; Hentig, J.; Yun, S.; Kim, H.-S.; Lai, C.; Devoto, C.; Yarnell, A.M.; Polejaeva, E.; Dell, K.C.; et al. A Pilot Study of Whole-Blood Transcriptomic Analysis to Identify Genes Associated with Repetitive Low-Level Blast Exposure in Career Breachers. Biomedicines 2022, 10, 690. [Google Scholar] [CrossRef]
- Cheung, T.Y.A. Application of functional magnetic resonance imaging in diagnosing blast-related traumatic brain injuries. Theor. Nat. Sci. 2024, 65, 182–186. [Google Scholar] [CrossRef]
- Carr, W.; Yarnell, A.M.; Ong, R.; Walilko, T.; Kamimori, G.H.; da Silva, U.; McCarron, R.M.; LoPresti, M.L. Ubiquitin carboxy-terminal hydrolase-l1 as a serum neurotrauma biomarker for exposure to occupational low-level blast. Front. Neurol. 2015, 6, 49. [Google Scholar] [CrossRef]
- Edwards, K.A.; Leete, J.J.; Tschiffely, A.E.; Moore, C.Y.; Dell, K.C.; Statz, J.K.; Carr, W.; Walker, P.B.; LoPresti, M.L.; Ahlers, S.T.; et al. Blast exposure results in tau and neurofilament light chain changes in peripheral blood. Brain Inj. 2020, 34, 1213–1221. [Google Scholar] [CrossRef]
- Rhind, S.G.; Shiu, M.Y.; Vartanian, O.; Tenn, C.; Nakashima, A.; Jetly, R.; Yang, Z.; Wang, K.K. Circulating Brain-Reactive Autoantibody Profiles in Military Breachers Exposed to Repetitive Occupational Blast. Int. J. Mol. Sci. 2024, 25, 13683. [Google Scholar] [CrossRef] [PubMed]
- Sergio, L.E.; Gorbet, D.J.; Adams, M.S.; Dobney, D.M. The Effects of Mild Traumatic Brain Injury on Cognitive-Motor Integration for Skilled Performance. Front. Neurol. 2020, 11, 541630. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; DiBattista, A.; Patel, M.A.; Daley, M.; Tenn, C.; Nakashima, A.; Rhind, S.G.; Vartanian, O.; Shiu, M.Y.; Caddy, N.; et al. A Distinct Metabolite Signature in Military Personnel Exposed to Repetitive Low-Level Blasts. Front. Neurol. 2022, 13, 831792. [Google Scholar] [CrossRef] [PubMed]
- Chaganti, S.; Plassard, A.J.; Wilson, L.; Smith, M.A.; Patel, M.B.; Landman, B.A. A Bayesian Framework for Early Risk Prediction in Traumatic Brain Injury. Proc. SPIE Int. Soc. Opt. Eng. 2016, 9784, 978422. [Google Scholar] [CrossRef]
- Mueller, P.M.; Torres-Espín, A.; Haar, C.V. Bayesian Methods: A Means of Improving Statistical Power in Preclinical Neurotrauma? Neurotrauma Rep. 2024, 5, 699–707. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef]
- Smits, M.; Dippel, D.W.; Houston, G.C.; Wielopolski, P.A.; Koudstaal, P.J.; Hunink, M.M.; van der Lugt, A. Postconcussion syndrome after minor head injury: Brain activation of working memory and attention. Hum. Brain Mapp. 2009, 30, 2789–2803. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.A.; Sosa, M.A.G.; De Gasperi, R.; Stone, J.R.; Dickstein, D.L.; Haghighi, F.; Hof, P.R.; Ahlers, S.T. Vascular and Inflammatory Factors in the Pathophysiology of Blast-Induced Brain Injury. Front. Neurol. 2015, 6, 48. [Google Scholar] [CrossRef]
- Basile, E.J.; Syed, F.; Wei, O.C.; Toma, M.; Frankini, E.L.; Ong, C.W. Understanding Traumatic Brain Injuries in Military Personnel: Investigating the Dynamic Interplay of the Cerebrospinal Fluid and Brain During Blasts. Cureus 2023, 15, e46962. [Google Scholar] [CrossRef]
- Agoston, D.V.; Kamnaksh, A. Modeling the Neurobehavioral Consequences of Blast-Induced Traumatic Brain Injury Spectrum Disorder and Identifying Related Biomarkers. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Kamimori, G.H.; LaValle, C.R.; E Eonta, S.; Carr, W.; Tate, C.; Wang, K.K.W. Longitudinal Investigation of Neurotrauma Serum Biomarkers, Behavioral Characterization, and Brain Imaging in Soldiers Following Repeated Low-Level Blast Exposure (New Zealand Breacher Study). Mil. Med. 2018, 183, 28–33. [Google Scholar] [CrossRef]
- Boutté, A.M.; Thangavelu, B.; LaValle, C.R.; Nemes, J.; Gilsdorf, J.; Shear, D.A.; Kamimori, G.H. Brain-related proteins as serum biomarkers of acute, subconcussive blast overpressure exposure: A cohort study of military personnel. PLoS ONE 2019, 14, e0221036. [Google Scholar] [CrossRef]
- Thangavelu, B.; LaValle, C.R.; Egnoto, M.J.; Nemes, J.; Boutté, A.M.; Kamimori, G.H. Overpressure Exposure from .50-Caliber Rifle Training Is Associated with Increased Amyloid Beta Peptides in Serum. Front. Neurol. 2020, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Tschiffely, A.E.; Statz, J.K.; Edwards, K.A.; Goforth, C.; Ahlers, S.T.; Carr, W.S.; Gill, J.M. Assessing a Blast-Related Biomarker in an Operational Community: Glial Fibrillary Acidic Protein in Experienced Breachers. J. Neurotrauma 2020, 37, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Cashion, A.; Osier, N.; Arcurio, L.; Motamedi, V.; Dell, K.C.; Carr, W.; Kim, H.-S.; Yun, S.; Walker, P.; et al. Moderate blast exposure alters gene expression and levels of amyloid precursor protein. Neurol. Genet. 2017, 3, e186. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Motamedi, V.; Osier, N.; Dell, K.; Arcurio, L.; Carr, W.; Walker, P.; Ahlers, S.; LoPresti, M.; Yarnell, A. Moderate blast exposure results in increased IL-6 and TNFα in peripheral blood. Brain. Behav. Immun. 2017, 65, 90–94. [Google Scholar] [CrossRef]
- Gyorgy, A.; Ling, G.; Wingo, D.; Walker, J.; Tong, L.; Parks, S.; Januszkiewicz, A.; Baumann, R.; Agoston, D.V. Time-dependent changes in serum biomarker levels after blast traumatic brain injury. J. Neurotrauma 2011, 28, 1121–1126. [Google Scholar] [CrossRef]
- Muneer, P.A.; Saikia, B.B.; Bhowmick, S. Synergistic effect of mild traumatic brain injury and alcohol aggravates neuroinflammation, amyloidogenesis, tau pathology, neurodegeneration, and blood-brain barrier alterations: Impact on psychological stress. Exp. Neurol. 2022, 358, 114222. [Google Scholar] [CrossRef]
- Edwards, K.A.; Greer, K.; Leete, J.; Lai, C.; Devoto, C.; Qu, B.-X.; Yarnell, A.M.; Polejaeva, E.; Dell, K.C.; LoPresti, M.L.; et al. Neuronally-derived tau is increased in experienced breachers and is associated with neurobehavioral symptoms. Sci. Rep. 2021, 11, 19527. [Google Scholar] [CrossRef]
- Edwards, K.A.; Leete, J.J.; Smith, E.G.; Quick, A.; Modica, C.M.; Wassermann, E.M.; Polejaeva, E.; Dell, K.C.; LoPresti, M.; Walker, P.; et al. Elevations in Tumor Necrosis Factor Alpha and Interleukin 6 From Neuronal-Derived Extracellular Vesicles in Repeated Low-Level Blast Exposed Personnel. Front. Neurol. 2022, 13, 723923. [Google Scholar] [CrossRef]
- Agoston, D.V.; McCullough, J.; Aniceto, R.; Lin, I.-H.; Kamnaksh, A.; Eklund, M.; Graves, W.M., 3rd; Dunbar, C.; Engall, J.; Schneider, E.B.; et al. Blood-Based Biomarkers of Repetitive, Subconcussive Blast Overpressure Exposure in the Training Environment: A Pilot Study. Neurotrauma Rep. 2022, 3, 479–490. [Google Scholar] [CrossRef]
- Graham, N.S.N.; Blissitt, G.; Zimmerman, K.; Friedland, D.; Dumas, M.-E.; Coady, E.; Heslegrave, A.; Zetterberg, H.; Escott-Price, V.; Schofield, S.; et al. ADVANCE-TBI study protocol: Traumatic brain injury outcomes in UK military personnel serving in Afghanistan between 2003 and 2014—A longitudinal cohort study. BMJ Open 2023, 13, e069243. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, D.L.; De Gasperi, R.; Sosa, M.A.G.; Perez-Garcia, G.; Short, J.A.; Sosa, H.; Perez, G.M.; Tschiffely, A.E.; Dams-O’cOnnor, K.; Pullman, M.Y.; et al. Brain and blood biomarkers of tauopathy and neuronal injury in humans and rats with neurobehavioral syndromes following blast exposure. Mol. Psychiatry 2021, 26, 5940–5954. [Google Scholar] [CrossRef] [PubMed]
- Vorn, R.; Naunheim, R.; Lai, C.; Wagner, C.; Gill, J.M. Elevated Axonal Protein Markers Following Repetitive Blast Exposure in Military Personnel. Front. Neurosci. 2022, 16, 853616. [Google Scholar] [CrossRef]
- Gilmore, N.; Tseng, C.-E.J.; Maffei, C.; Tromly, S.L.; Deary, K.B.; McKinney, I.R.; Kelemen, J.N.; Healy, B.C.; Hu, C.G.; Ramos-Llordén, G.; et al. Impact of repeated blast exposure on active-duty United States Special Operations Forces. Proc. Natl. Acad. Sci. USA 2024, 121, e2313568121. [Google Scholar] [CrossRef]
- Barbosa, M.G.d.S.; Francisco, G.G.d.O.A.; de Souza, R.L.V.; de Souza, J.M.A.; Carneiro, R.A.; Rabelo, N.N.; Chaurasia, B. Chronic traumatic encephalopathy in athletes, players, boxers and military: Systematic review. Ann. Med. Surg. 2024, 86, 7238–7247. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Churchill, N.; Schweizer, T.A.; Rhind, S.G.; Richards, D.; Baker, A.J.; Hutchison, M.G. Blood biomarkers are associated with brain function and blood flow following sport concussion. J. Neuroimmunol. 2018, 319, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buonora, J.E.; Yarnell, A.M.; Lazarus, R.C.; Mousseau, M.; Latour, L.L.; Rizoli, S.B.; Baker, A.J.; Rhind, S.G.; Diaz-Arrastia, R.; Mueller, G.P. Multivariate analysis of traumatic brain injury: Development of an assessment score. Front. Neurol. 2015, 6, 68. [Google Scholar] [CrossRef]
- Edlow, B.L.; Bodien, Y.G.; Baxter, T.; Belanger, H.G.; Cali, R.J.; Deary, K.B.; Fischl, B.; Foulkes, A.S.; Gilmore, N.; Greve, D.N.; et al. Long-Term Effects of Repeated Blast Exposure in United States Special Operations Forces Personnel: A Pilot Study Protocol. J. Neurotrauma 2022, 39, 1391–1407. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R.; Avants, B.B.; Tustison, N.J.; Gill, J.; Wilde, E.A.; Neumann, K.D.; Gladney, L.A.; Kilgore, M.O.; Bowling, F.; Wilson, C.M.; et al. Neurological Effects of Repeated Blast Exposure in Special Operations Personnel. J. Neurotrauma 2024, 41, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.B.; Huber, D.L.; Goeckner, B.D.; Gill, J.M.; Pasquina, P.; Broglio, S.P.; McAllister, T.W.; Harezlak, J.; McCrea, M.A.; for CARE Consortium Investigators. Association of Blood Biomarkers of Inflammation with Acute Concussion in Collegiate Athletes and Military Service Academy Cadets. Neurology 2024, 102, e207991. [Google Scholar] [CrossRef]
- Butler, M.L.M.D.; Pervaiz, N.; Breen, K.; Calderazzo, S.; Ypsilantis, P.; Wang, Y.; Breda, J.C.; Mazzilli, S.; Nicks, R.; Spurlock, E.; et al. Repeated head trauma causes neuron loss and inflammation in young athletes. Nature 2025, 647, 228–237. [Google Scholar] [CrossRef]
- Good, I.J. Good Thinking: The Foundations of Probability and its Applications; Courier Corporation: North Chelmsford, MA, USA, 2009. [Google Scholar]
- Betancourt, M. Towards a Principled Bayesian Workflow. 2020. Available online: https://betanalpha.github.io/assets/case_studies/principled_bayesian_workflow.html (accessed on 20 November 2025).
- McElreath, R. Statistical Rethinking: A Bayesian Course with Examples in R and Stan; Chapman and Hall/CRC: New York, NY, USA, 2020. [Google Scholar]
- Nelson, N.W.; Davenport, N.D.; Sponheim, S.R.; Anderson, C.R. Blast-Related Mild Traumatic Brain Injury: Neuropsychological Evaluation and Findings. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bigler, E.D. Systems Biology, Neuroimaging, Neuropsychology, Neuroconnectivity and Traumatic Brain Injury. Front. Syst. Neurosci. 2016, 10, 55. [Google Scholar] [CrossRef]
- Pagulayan, K.F.; Petrie, E.C.; Cook, D.G.; Hendrickson, R.C.; Rau, H.; Reilly, M.; Mayer, C.; Meabon, J.S.; Raskind, M.A.; Peskind, E.R.; et al. Effect of blast-related mTBI on the working memory system: A resting state fMRI study. Brain Imaging Behav. 2018, 14, 949–960. [Google Scholar] [CrossRef]
- Bigler, E.D.; Allder, S.; Victoroff, J. What traditional neuropsychological assessment got wrong about mild traumatic brain injury. II: Limitations in test development, research design, statistical and psychometric issues. Brain Inj. 2024, 38, 1053–1074. [Google Scholar] [CrossRef]
- Bigler, E.D.; Allder, S.; Dunkley, B.T.; Victoroff, J. What traditional neuropsychological assessment got wrong about mild traumatic brain injury. I: Historical perspective, contemporary neuroimaging overview and neuropathology update. Brain Inj. 2025, 39, 1161–1183. [Google Scholar] [CrossRef]
- Lamichhane, B.; Westbrook, A.; Cole, M.W.; Braver, T.S. Exploring brain-behavior relationships in the N-back task. NeuroImage 2020, 212, 116683. [Google Scholar] [CrossRef]
- Woodrow, R.E.; Winzeck, S.; I Luppi, A.; Kelleher-Unger, I.R.; Spindler, L.R.B.; Wilson, J.T.L.; Newcombe, V.F.J.; CENTER-TBI MRI Substudy Participants and Investigators; Coles, J.P.; Menon, D.K.; et al. Acute thalamic connectivity precedes chronic post-concussive symptoms in mild traumatic brain injury. Brain 2023, 146, 3484–3499. [Google Scholar] [CrossRef] [PubMed]
- Jolly, A.E.; Scott, G.T.; Sharp, D.J.; Hampshire, A.H. Distinct patterns of structural damage underlie working memory and reasoning deficits after traumatic brain injury. Brain 2020, 143, 1158–1176. [Google Scholar] [CrossRef]
- Hagen, A.C.; Tracy, B.L.; Stephens, J.A. Altered neural recruitment despite dual task performance recovery in athletes with repeat concussion. medRxiv 2024. [Google Scholar] [CrossRef]
- Dretsch, M.N.; Coldren, R.L.; Kelly, M.P.; Parish, R.V.; Russell, M.L. No Effect of Mild Nonconcussive Injury on Neurocognitive Functioning in U.S. Army Soldiers Deployed to Iraq. Mil. Med. 2012, 177, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Sosa, M.A.G.; De Gasperi, R.; Garcia, G.S.P.; Perez, G.M.; Searcy, C.; Vargas, D.; Spencer, A.; Janssen, P.L.; Tschiffely, A.E.; McCarron, R.M.; et al. Low-level blast exposure disrupts gliovascular and neurovascular connections and induces a chronic vascular pathology in rat brain. Acta Neuropathol. Commun. 2019, 7, 6. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Rhind, S.G.; Tenn, C.; Nakashima, A.; Lam, T.K.; Shiu, M.Y.; King, K.; Ouellet, S.; Vartanian, O. The impact of repetitive exposure to low-level blast on neurocognitive function in Canadian Armed Forces’ breachers, snipers, and military controls. J. Int. Neuropsychol. Soc. 2025, 31, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Friedl, K.; Grate, S.; Proctor, S.; Ness, J.; Lukey, B.; Kane, R. Army research needs for automated neuropsychological tests: Monitoring soldier health and performance status. Arch. Clin. Neuropsychol. 2007, 22, S7–S14. [Google Scholar] [CrossRef]
- Brenner, L.A.; Terrio, H.; Homaifar, B.Y.; Gutierrez, P.M.; Staves, P.J.; Harwood, J.E.F.; Reeves, D.; Adler, L.E.; Ivins, B.J.; Helmick, K.; et al. Neuropsychological test performance in soldiers with blast-related mild TBI. Neuropsychology 2010, 24, 160–167. [Google Scholar] [CrossRef]
- Haran, F.J.; Handy, J.D.; Servatius, R.J.; Rhea, C.K.; Tsao, J.W. Acute neurocognitive deficits in active duty service members following subconcussive blast exposure. Appl. Neuropsychol. Adult 2019, 28, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.S.; Roebuck-Spencer, T.M.; Fuenzalida, E.; Gilliland, K. Test–retest reliability and practice effects for the ANAM General Neuropsychological Screening battery. Clin. Neuropsychol. 2018, 32, 479–494. [Google Scholar] [CrossRef]
- Hayes, J.P.; Morey, R.A.; Tupler, L.A. A case of frontal neuropsychological and neuroimaging signs following multiple primary-blast exposure. Neurocase 2012, 18, 258–269. [Google Scholar] [CrossRef]
- Clark, A.L.; Merritt, V.C.; Bigler, E.D.; Bangen, K.J.; Werhane, M.; Sorg, S.F.; Bondi, M.W.; Schiehser, D.M.; Delano-Wood, L. Blast-Exposed Veterans with Mild Traumatic Brain Injury Show Greater Frontal Cortical Thinning and Poorer Executive Functioning. Front. Neurol. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- A Champagne, A.; Coverdale, N.S.; Ross, A.; Murray, C.; Vallee, I.; Cook, D.J. Characterizing changes in network connectivity following chronic head trauma in special forces military personnel: A combined resting-fMRI and DTI study. Brain Inj. 2021, 35, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.E.; Bellgowan, J.F.; Oakes, T.R.; French, L.M.; Nadar, S.R.; Sham, E.B.; Liu, W.; Riedy, G. Assessing Quantitative Changes in Intrinsic Thalamic Networks in Blast and Nonblast Mild Traumatic Brain Injury: Implications for Mechanisms of Injury. Brain Connect. 2016, 6, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.R.; Hayes, J.P.; Lafleche, G.; Salat, D.H.; Verfaellie, M. Functional Brain Alterations Associated with Cognitive Control in Blast-Related Mild Traumatic Brain Injury. J. Int. Neuropsychol. Soc. 2018, 24, 662–672. [Google Scholar] [CrossRef]
- Lam, T.K.; Rhind, S.G.; Tenn, C.; Nakashima, A.; Shiu, M.; King, K.; Caddy, N.; Vallikanthan, J.; Vartanian, O. Connecting the Dots: Investigating Brain Connectivity Changes in Military Breachers and Snipers Exposed to Repetitive Low-Level Blast; NATO: Brussels, Belgium, 2025; p. 9. [Google Scholar]
- Robinson, M.E.; Clark, D.C.; Milberg, W.P.; McGlinchey, R.E.; Salat, D.H. Characterization of Differences in Functional Connectivity Associated with Close-Range Blast Exposure. J. Neurotrauma 2017, 34, S53–S61. [Google Scholar] [CrossRef]
- Dogra, S.; Arabshahi, S.; Wei, J.; Saidenberg, L.; Kang, S.K.; Chung, S.; Laine, A.; Lui, Y.W. Functional Connectivity Changes on Resting-State fMRI After Mild Traumatic Brain Injury: A Systematic Review. AJNR Am. J. Neuroradiol. 2024, 45, 795–801. [Google Scholar] [CrossRef]
- Diociasi, A.; Iaccarino, M.A.; Sorg, S.; Lubin, E.J.; Wisialowski, C.; Dua, A.; Tan, C.O.; Gupta, R. Distinct Functional MRI Connectivity Patterns and Cortical Volume Variations Associated with Repetitive Blast Exposure in Special Operations Forces Members. Radiology 2025, 315, e233264. [Google Scholar] [CrossRef]
- Hurtubise, J.M.; Gorbet, D.J.; Hynes, L.; Macpherson, A.K.; Sergio, L.E. Cortical and cerebellar structural correlates of cognitive-motor integration performance in females with and without persistent concussion symptoms. Brain Inj. 2023, 37, 397–411. [Google Scholar] [CrossRef]
- Gattoni, C.; Martinez-Gonzalez, B.; Li, C.; Marcora, S.M. Assessing Cognitive-Motor Interference in Military Contexts: Validity and Reliability of Two Dual-Tasking Tests. Mil. Med. 2023, 188, e2900–e2908. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Wang, Y.; Liu, L.; Qian, Q. Changes in effective connectivity during the visual-motor integration tasks: A preliminary f-NIRS study. Behav. Brain Funct. 2024, 20, 4. [Google Scholar] [CrossRef]
- Bodien, Y.G.; Fecchio, M.; Gilmore, N.; Freeman, H.J.; Sanders, W.R.; Meydan, A.; Lawrence, P.K.; Atalay, A.S.; Kirsch, J.; Healy, B.C.; et al. Multimodal Biomarkers of Consciousness in Acute Severe Traumatic Brain Injury. J. Neurotrauma 2025. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Harasym, J.; Miguel-Cruz, A.; Chisholm, S.; Smith-MacDonald, L.; Brémault-Phillips, S. Neurocognitive Assessment Tools for Military Personnel with Mild Traumatic Brain Injury: Scoping Literature Review. JMIR Ment. Health 2021, 8, e26360. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yeh, P.-H.; Ollinger, J.M.; Morris, H.D.; Hood, M.N.; Ho, V.B.; Choi, K.H. Military-related mild traumatic brain injury: Clinical characteristics, advanced neuroimaging, and molecular mechanisms. Transl. Psychiatry 2023, 13, 289. [Google Scholar] [CrossRef]
- Goldstein, L.E.; Fisher, A.M.; Tagge, C.A.; Zhang, X.-L.; Velisek, L.; Sullivan, J.A.; Upreti, C.; Kracht, J.M.; Ericsson, M.; Wojnarowicz, M.W.; et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma Mouse model. Sci. Transl. Med. 2012, 4, 134ra60. [Google Scholar] [CrossRef] [PubMed]
- Tate, D.F.; Wade, B.S.C.; Velez, C.S.; Bigler, E.D.; Davenport, N.D.; Dennis, E.L.; Esopenko, C.; Hinds, S.R.; Kean, J.; Kennedy, E.; et al. Persistent MRI Findings Unique to Blast and Repetitive Mild TBI: Analysis of the CENC/LIMBIC Cohort Injury Characteristics. Mil. Med. 2024, 189, e1938–e1946. [Google Scholar] [CrossRef]
- Scheller, E.; Minkova, L.; Leitner, M.; Kloppel, S. Attempted and successful compensation in preclinical and early manifest neurodegeneration—A review of task FMRI studies. Front. Psychiatry 2014, 5, 132. [Google Scholar] [CrossRef] [PubMed]
- Franke, L.M.; Perera, R.A.; Sponheim, S.R. Long-term resting EEG correlates of repetitive mild traumatic brain injury and loss of consciousness: Alterations in alpha-beta power. Front. Neurol. 2023, 14, 1241481. [Google Scholar] [CrossRef]
- Rowland, J.A.; Stapleton-Kotloski, J.R.; Godwin, D.W.; Hamilton, C.A.; Martindale, S.L. The Functional Connectome and Long-Term Symptom Presentation Associated with Mild Traumatic Brain Injury and Blast Exposure in Combat Veterans. J. Neurotrauma 2024, 41, 2513–2527. [Google Scholar] [CrossRef]
- Bigler, E.D.; Allder, S.; Dunkley, B.T.; Victoroff, J. What traditional neuropsychological assessment got wrong about mild traumatic brain injury. IV: Clinical applications and future directions. Brain Inj. 2025, 39, 703–719. [Google Scholar] [CrossRef]
- Martindale, S.L.; Ord, A.S.; Lad, S.S.; Miskey, H.M.; Taber, K.H.; Rowland, J.A. Differential effects of deployment and nondeployment mild TBI on neuropsychological outcomes. Rehabil. Psychol. 2020, 66, 128–138. [Google Scholar] [CrossRef]
- Merritt, V.C.; Jurick, S.M.; Crocker, L.D.; Sullan, M.J.; Sakamoto, M.S.; Davey, D.K.; Hoffman, S.N.; Keller, A.V.; Jak, A.J. Associations Between Multiple Remote Mild TBIs and Objective Neuropsychological Functioning and Subjective Symptoms in Combat-Exposed Veterans. Arch. Clin. Neuropsychol. 2020, 35, 491–505. [Google Scholar] [CrossRef]
- Lippa, S.M.; Yeh, P.-H.; Gill, J.; French, L.M.; Brickell, T.A.; Lange, R.T. Plasma Tau and Amyloid Are Not Reliably Related to Injury Characteristics, Neuropsychological Performance, or White Matter Integrity in Service Members with a History of Traumatic Brain Injury. J. Neurotrauma 2019, 36, 2190–2199. [Google Scholar] [CrossRef]
- Vedantam, A.; Brennan, J.; Levin, H.S.; McCarthy, J.J.; Dash, P.K.; Redell, J.B.; Yamal, J.-M.; Robertson, C.S. Early versus Late Profiles of Inflammatory Cytokines after Mild Traumatic Brain Injury and Their Association with Neuropsychological Outcomes. J. Neurotrauma 2021, 38, 53–62. [Google Scholar] [CrossRef]
- de Souza, N.L.; Esopenko, C.; Jia, Y.; Parrott, J.S.; Merkley, T.L.; Dennis, E.L.; Hillary, F.G.; Velez, C.M.; Cooper, D.B.; Kennedy, J.E.; et al. Discriminating Mild Traumatic Brain Injury and Posttraumatic Stress Disorder Using Latent Neuroimaging and Neuropsychological Profiles in Active-Duty Military Service Members. J. Head Trauma Rehabil. 2022, 38, E254–E266. [Google Scholar] [CrossRef]
- Bhaumik, D.K.; Wang, Y.; Yen, P.-S.; Ajilore, O.A. Development of a Bayesian multimodal model to detect biomarkers in neuroimaging studies. Front. Neuroimaging 2023, 2, 1147508. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.; Zhang, F.; Burman, C.; Sharples, L. Bayesian Solutions for Assessing Differential Effects in Biomarker Positive and Negative Subgroups. Pharm. Stat. 2025, 24, e2456. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hua, W.; Wang, M.; Xu, Y. A Bayesian semi-parametric model for learning biomarker trajectories and changepoints in the preclinical phase of Alzheimer’s disease. Biometrics 2024, 80, ujae048. [Google Scholar] [CrossRef]
- Hlavac, N.; VandeVord, P.J. Astrocyte Mechano-Activation by High-Rate Overpressure Involves Alterations in Structural and Junctional Proteins. Front. Neurol. 2019, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Norris, C.; Garimella, H.T.; Carr, W.; Boutté, A.M.; Gupta, R.K.; Przekwas, A.J. Modeling biomarker kinetics of Abeta levels in serum following blast. Front. Neurol. 2025, 16, 1548589. [Google Scholar] [CrossRef]
- Agtarap, S.; Hungerford, L.D.; Ettenhofer, M.L. Identifying Unique Symptom Groups Following Mild Traumatic Brain Injury Using the Neurobehavioral Symptom Inventory and PTSD Checklist-5 in Military Personnel: A Bifactor Analysis. J. Head Trauma Rehabil. 2023, 38, E371–E383. [Google Scholar] [CrossRef]
- Belding, J.N.; Bonkowski, J.; Englert, R.; Stanfill, A.G.; Tsao, J.W. Associations between concussion and more severe TBIs, mild cognitive impairment, and early-onset dementia among military retirees over 40 years. Front. Neurol. 2024, 15, 1442715. [Google Scholar] [CrossRef] [PubMed]
- A Wilde, E.; Wanner, I.-B.; Kenney, K.; Gill, J.; Stone, J.R.; Disner, S.; Schnakers, C.; Meyer, R.; Prager, E.M.; Haas, M.; et al. A Framework to Advance Biomarker Development in the Diagnosis, Outcome Prediction, and Treatment of Traumatic Brain Injury. J. Neurotrauma 2022, 39, 436–457. [Google Scholar] [CrossRef]
- Ahlers, S.T.; Beliveau, P.; Bjerke, A.; Bompaire, F.; Borkholder, D.A.; Carr, W.S.; Featherman, S.J.; Flierman, N.; Grobert, S.; Josey, T.; et al. Guidelines to Mitigate Military Occupational Brain Health Risks from Repetitive Blast Exposure; North Atlantic Treaty Organization: Brussels, Belgium, 2025; p. 100. [Google Scholar]
- Vartanian, O.; Blackler, K.; Nakashima, A.; Outelllet, S. Assessment of Blast Overpressure in the Canadian Forces School of Military Engineering (CFSME) Breaching Environment; DRDC-RDDC-2015-L194; DRDC TRC: Toronto, ON, Canada, 2015; p. 6. [Google Scholar]
- Mishra, V.; Skotak, M.; Schuetz, H.; Heller, A.; Haorah, J.; Chandra, N. Primary blast causes mild, moderate, severe and lethal TBI with increasing blast overpressures: Experimental rat injury model. Sci. Rep. 2016, 6, 26992. [Google Scholar] [CrossRef]
- Sloley, S.S.; Turner, S.M. Evaluating evidence supporting the relevancy of 4 psi as a blast overpressure value associated with brain health and performance outcomes following low-level blast overpressure exposure. Shock Waves 2024, 34, 293–302. [Google Scholar] [CrossRef]
- King, N.S.; Crawford, S.; Wenden, F.J.; Moss, N.E.G.; Wade, D.T. The Rivermead Post Concussion Symptoms Questionnaire: A measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 1995, 242, 587–592. [Google Scholar] [CrossRef]
- Eyres, S.; Carey, A.; Gilworth, G.; Neumann, V.; Tennant, A. Construct validity and reliability of the Rivermead Post-Concussion Symptoms Questionnaire. Clin. Rehabil. 2005, 19, 878–887. [Google Scholar] [CrossRef]
- Kane, M.J.; Brown, L.H.; McVay, J.C.; Silvia, P.J.; Myin-Germeys, I.; Kwapil, T.R. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychol. Sci. 2007, 18, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Stanislaw, H.; Todorov, N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999, 31, 137–149. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Rhind, S.G.; Richards, D.; Churchill, N.; Baker, A.J.; Hutchison, M.G. Altered Blood Biomarker Profiles in Athletes with a History of Repetitive Head Impacts. PLoS ONE 2016, 11, e0159929, Correction in PLoS ONE 2016, 11, e0164912. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Moes, K.A.; Shiu, M.Y.; Hutchison, M.G.; Churchill, N.; Thomas, S.G.; Rhind, S.G. High-Intensity Interval Training Is Associated with Alterations in Blood Biomarkers Related to Brain Injury. Front. Physiol. 2018, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.P.; Churchill, N.; Rhind, S.G.; Richards, D.; Hutchison, M.G. The relationship between symptom burden and systemic inflammation differs between male and female athletes following concussion. BMC Immunol. 2020, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Asken, B.M.; Yang, Z.; Xu, H.; Weber, A.G.; Hayes, R.L.; Bauer, R.M.; DeKosky, S.T.; Jaffee, M.S.; Wang, K.K.; Clugston, J.R. Acute Effects of Sport-Related Concussion on Serum Glial Fibrillary Acidic Protein, Ubiquitin C-Terminal Hydrolase L1, Total Tau, and Neurofilament Light Measured by a Multiplex Assay. J. Neurotrauma 2020, 37, 1537–1545. [Google Scholar] [CrossRef]
- Rubenstein, R.; McQuillan, L.; Wang, K.K.; Robertson, C.S.; Chang, B.; Yang, Z.; Xu, H.; Williamson, J.B.; Wagner, A.K. Temporal Profiles of P-Tau, T-Tau, and P-Tau:Tau Ratios in Cerebrospinal Fluid and Blood from Moderate-Severe Traumatic Brain Injury Patients and Relationship to 6–12 Month Global Outcomes. J. Neurotrauma 2024, 41, 369–392. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, M. (Co)variations On a Theme: Variate–Covariate Modeling. Available online: https://betanalpha.github.io/assets/case_studies/variate_covariate_modeling.html (accessed on 20 November 2025).
- Gabry, J.; Češnovar, R. R Interface to CmdStan, R Package Version 0.6.0; R Team: Vienna, Austria, 2023. [Google Scholar]
- R Team. Download RStudio, Version 2022.07.2+576; R Team: Vienna, Austria, 2022. [Google Scholar]
- Betancourt, M. mcmc_visualization_tools: Markov Chain Monte Carlo Visualization Tools. Available online: https://github.com/betanalpha/mcmc_visualization_tools (accessed on 20 November 2025).
- McElreath, R. Rethinking: Statistical Rethinking Book Package, Version 1.96; R Team: Vienna, Austria, 2020. [Google Scholar]
- Iannone, R.; Cheng, J.; Schloerke, B.; Hughes, E.; Lauer, A.; Seo, J.; Brevoort, K.; Roy, O. Gt: Easily Create Presentation-Ready Display Tables, Version 0.2.0; R Team: Vienna, Austria, 2020. [Google Scholar]
- Sjoberg, D.D.; Larmarange, J.; Curry, M.; Lavery, J.; Whiting, K.; Zabor, E.C.; Bai, X.; Drill, E.; Flynn, J.; Hannum, M. Gtsummary: Presentation-Ready Data Summary and Analytic Result Tables, Version 1.8.1; R Team: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H. Dplyr: A Grammar of Data Manipulation, Version 04; R Team: Vienna, Austria, 2015. [Google Scholar]
- Wickham, H.; Vaughan, D.; Girlich, M.; Ushey, K. Tidyr: Tidy Messy Data, Version 2.3.0; R Team: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H. Stringr: Simple, Consistent Wrappers for Common String Operations, Version 1.4.0; R Team: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H. Data analysis. In Ggplot2: Elegant Graphics for Data Analysis; Springer: Cham, Switzerland, 2016; pp. 189–201. [Google Scholar]
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’, Version 1.1.1; R Team: Vienna, Austria, 2025. [Google Scholar]
- Wickham, H. Forcats: Tools for Working with Categorical Variables (Factors), Version 1.1.1; R Team: Vienna, Austria, 2025. [Google Scholar]
- Xie, Y. TinyTeX: A lightweight, cross-platform, and easy-to-maintain LaTeX distribution based on TeX Live. TUGboat 2019, 40, 30–32. [Google Scholar]
- Ooms, J. Magick: Advanced Graphics and Image-Processing in R, Version 2.7.0; R Team: Vienna, Austria, 2025. [Google Scholar]
- Ooms, J. Pdftools: Text Extraction, Rendering and Converting of PDF Documents, Version 2.0; R Team: Vienna, Austria, 2020. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.