Abstract
Alzheimer’s disease (AD) is considered one of the leading causes of death in the United States, and there is no effective cure for it. Understanding the neuropathological mechanisms underlying AD is essential for identifying early, reliable biomarkers and developing effective therapies. In this paper, we report on a comprehensive multimodal study of AD pathology using the 5xFAD mouse model. We employed light-scattering techniques, Partial Wave Spectroscopy (PWS) and Inverse Participation Ratio (IPR), to detect nanoscale structural alterations in brain tissues, nuclear components, and mitochondria. To support the light-scattering experiments, behavior, and histopathological studies were conducted. These analyses revealed significant increases in structural heterogeneity and mass density fluctuations in the brains of 5xFAD mice compared with Non-transgenic controls. Behavioral assessment performed using the Novel Object Recognition test demonstrated memory impairment in 5xFAD mice, reflected by a reduced recognition index. Histopathological analysis further revealed increased amyloid beta plaques and microglia activation in the hippocampus and cortex of 5xFAD mice compared with Non-transgenic controls. An increase in structural disorder within brain tissues can be attributed to higher mass density fluctuations, likely arising from macromolecular rearrangement driven by amyloid beta aggregation and neuroinflammatory responses as the disease progresses. Our findings suggest that PWS and IPR-derived metrics provide sensitive biophysical indicators of early cellular and subcellular disruption, offering potential as quantitative biomarkers for the detection of AD.
1. Introduction
Alzheimer’s Disease (AD) is a progressive neurodegenerative disorder characterized by an impairment in cognitive function and memory, often accompanied by difficulty in performing routine activities [1]. This disease generally affects elderly people who are 65 or older, but younger people can also be affected by AD. Pathologically, AD is linked to the extracellular accumulation of amyloid beta (Aβ) plaques in the cerebral cortex. These plaques impede communication between neurons and impair function in the cerebral region. Secondly, neurofibrillary tangles composed of aberrant tau proteins within neurons and glia have been observed. Together, these pathological hallmarks contribute to synaptic dysfunction and neuronal loss [2,3,4,5]. It has been estimated that nearly 7.2 million Americans older than 65 are currently affected by AD. This number is expected to grow to 14 million by 2060 [2]. More than 57 million people worldwide are suffering from dementia and AD-related disorders [6], and it accounts for an estimated 36.3 million DALYs (Disability Adjusted Life Years) globally [7]. Despite extensive research, AD is rarely diagnosed at its early stages, even though pathological changes occur at subcellular levels long before clinical symptoms become apparent. Moreover, current diagnostic methods typically identify the disease only after significant neuropathology has appeared, limiting the effectiveness of therapeutic intervention. Therefore, there is an urgent need to develop a sensitive and reliable technique for early diagnosis of AD. For that, we need to understand changes in brain tissue better as the disease progresses and identify more promising biomarkers. It is known that Aβ plaques predominantly accumulate in the hippocampus and cortex, which are critically involved in memory and cognition, making them essential targets for studying AD-related neuropathology [8,9]. Therefore, we need to study these brain regions to understand neuropathology better. Nanoscale techniques such as Atomic Force Microscopy and Positron Emission Tomography have been used extensively to study abnormal proteins, including Aβ, tau, and neurofibrillary tangles [10,11]. Here, we used light-scattering techniques to probe nanoscale structural changes induced by AD in mouse brain tissue.
As AD progresses, mass density changes at the tissue to the sub-cellular level [12,13,14,15]. However, these structural changes are not visible under conventional microscopy because these microscopes cannot resolve features below 200 nm. Techniques such as MRI and CT scans [16] detect AD at a later stage, when symptoms have already manifested. Biological cells and tissues are considered elastic scatterers of light, and variations in their refractive index provide insights into the underlying structural organization [17,18,19]. By measuring spatial variations in light scattering due to refractive index fluctuations, this technique reveals how subcellular structures are arranged. Previously, this technique has been effective in detecting different cancer stages [20,21], and also distinguishing different stages of AD in human AD brain tissues [22]. In PWS, backscattered light intensity signals are used to quantify the refractive index variations (dn) and mass density fluctuations from weakly disordered media, including biological cells/tissues. Previous studies have shown that backscattered light intensity is proportional to refractive index fluctuations within tissues/cells, which are further related to mass density fluctuations at the nanometer-to-submicron level [23,24,25]. Using the mesoscopic theory of light, we calculated the structural disorder strength (Ld-PWS) for different cells/tissues and have exploited this parameter as a potential biomarker for PWS in disease studies [26]. Another technique, IPR, quantified as a potential biomarker by Ld-IPR is used to probe molecular-specific structural alterations in nuclear components, including DNA and chromatin, as AD progresses. Fluorescent images of biological samples stained using DAPI are taken, and IPR analysis is performed. The Methods Section (Section 4) discusses detailed information about PWS and IPR techniques.
In this study, we perform spectroscopic analyses of brain tissues/cells from 5xFAD and Non-transgenic (Non-Tg) mice, primarily targeting the hippocampus and cortex regions, which are critically involved in memory and cognition. Using PWS and IPR techniques, we investigated structural alterations at the nanometer-to-submicron scale within these regions. The 5xFAD mouse model effectively mimics several critical features of AD, including the formation of Aβ plaques, synaptic dysfunction, mitochondrial dysfunction, progressive neuronal loss, genotoxic stress, and robust neuroinflammatory responses [27,28]. Our analyses revealed a significant increase in structural disorder in brain tissues and cells of 5xFAD mice compared to Non-Tg mice. We further inspected local structural alterations in hippocampal mitochondria by first scanning them with a Transmission Electron Microscope (TEM) and later applying IPR analysis to detect regional-level changes in AD conditions. Structural disorder was elevated in 5xFAD mitochondria than in Non-Tg mitochondria. We have further correlated these results with Aβ pathology, cognitive function, and mitochondrial DNA copy number, thereby providing an integrated multiscale perspective on AD pathology.
2. Results
2.1. PWS Analysis of Mice Brain Tissues
2.1.1. PWS Analysis of Cortical Tissues
Using the method described in the Methods Section (Section 4) below, we used the PWS setup to analyze thin 5 µm 4-month-old male mouse brain tissue from the cortex region, captured by the Charged-Coupled Device (CCD) in the visible spectrum. We determined the structural disorder strength Ld-PWS at each image pixel. A 2D color map was constructed by calculating Ld-PWS for each pixel, with blue indicating less disorder and red the most. Figure 1a,b shows the bright field images of the Non-transgenic (Non-Tg) and 5xFAD mice tissues, whereas Figure 1a’,b’ are the 2D Ld-PWS color maps of those images. In color maps, there are more yellow and red spots in 5xFAD tissue than in Non-Tg tissue, indicating greater structural disorder, more refractive index fluctuations, and greater mass density changes. We also conducted a statistical analysis to compute the Mean and standard deviation (STD) of Ld-PWS to compare Non-Tg and 5xFAD tissues. Figure 1c,d shows the Mean and STD bar graphs of Ld-PWS comparing Non-Tg and 5xFAD mice brain tissues. There is a significant increase in Mean Ld-PWS of 15.5% in 5xFAD tissue relative to Non-Tg, whereas in STD, there is an increase of 34.3% in STD Ld-PWS in 5xFAD relative to Non-Tg. Hence, 5xFAD has a significant increase in structural disorder compared to Non-Tg, indicating greater mass density fluctuations.
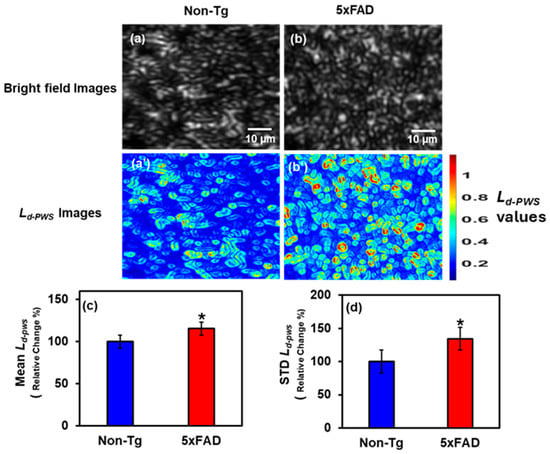
Figure 1.
(a,b) Bright field images of Non-Tg and 5xFAD mice brain tissues (cortex) with their corresponding Ld-PWS colormaps (a’,b’). (c,d) 5xFAD shows increased mean (15.5%), and STD (34.3%) of Ld-PWS compared to Non-Tg. (* Student’s t-test, p < 0.05; n = 4 mice per group).
2.1.2. PWS Analysis of Hippocampus Tissues
Furthermore, we examined hippocampal tissue from 5xFAD and Non-Tg mouse brains to investigate structural changes in the hippocampus associated with AD. Figure 2a,b shows the bright field images of Non-Tg and 5xFAD mice hippocampal tissues, respectively, whereas Figure 2a’,b’ are the respective 2D color maps of Ld-PWS of those images. The statistical comparisons are shown in the bar graphs in Figure 2c,d, which depict the Mean and STD of Ld-PWS Non-Tg and 5xFAD. There is an increase of 7.5% and 6% in Ld-PWS values in 5xFAD brain tissues compared to Non-Tg, respectively. This increase in structural disorder strength indicates greater fluctuations in tissue mass density.
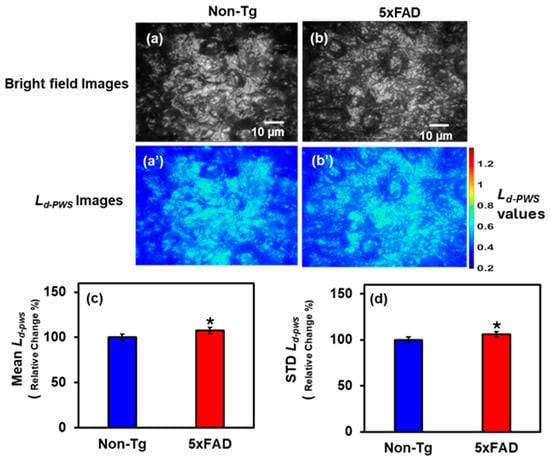
Figure 2.
(a,b) Bright field images of Non-Tg and 5xFAD mice brain tissues (hippocampus) with their corresponding Ld-PWS colormaps (a’,b’). (c,d) 5xFAD shows increased mean (7.5%), and STD (6%) of Ld-PWS compared to Non-Tg. (* Student’s t-test, p < 0.05; n = 4 mice per group).
2.2. IPR Analysis of Mice Brain Tissues
2.2.1. IPR Analysis of Cortex Region
After investigating tissue-level structural changes, we perform the molecular-specific IPR technique to examine nanoscale structural alterations in the nuclear region of 4-month-old male mouse cortical brain cells, targeting DNA/chromatin. We used DAPI-stained confocal imaging targeting nuclear DNA/chromatin. We calculated the Mean and STD of the Ld-IPR after removing the background (non-DNA) region from the confocal micrographs. They were compared between Non-Tg and 5xFAD samples to identify differences in DNA structural disorders.
Figure 3a,b shows the confocal images of DAPI-stained Non-Tg and 5xFAD mice brain tissues (cortex), Figure 3a’,b’ are their corresponding IPR images. We quantified the results by plotting bar graphs of the Mean and STD Ld-IPR values for Non-Tg and 5xFAD mouse brain tissues, shown in Figure 3c and Figure 3d, respectively. There was a 20% increase in the Mean Ld-IPR value in 5xFAD compared to Non-Tg, whereas the rise in STD Ld-IPR was remarkably 82% in 5xFAD compared to its control. This increase reflects structural disorder within DNA/chromatin, leading to changes in the arrangement of macromolecules.
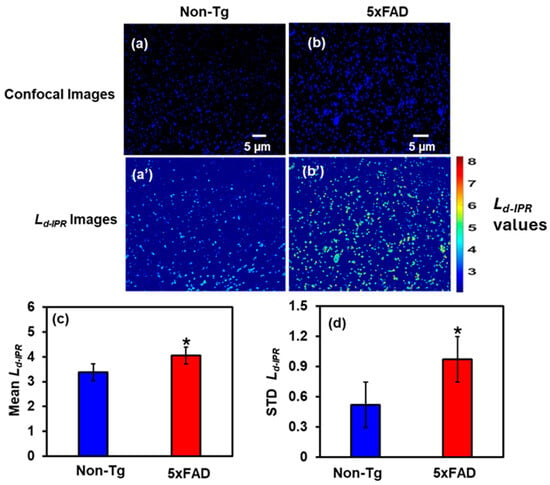
Figure 3.
(a,b) Confocal images of Non-Tg and 5xFAD mice brain tissues (cortex) with their corresponding Ld-IPR colormaps (a’,b’). (c,d) 5xFAD shows increased mean (20%), and STD (82%) of Ld-IPR compared to Non-Tg. (* Student’s t-test, p < 0.05; n = 4 mice per group).
2.2.2. IPR Analysis of Hippocampus Region
The brain tissues from the hippocampus region of the same mice were also studied by staining with DAPI, a nuclear stain. Figure 4a,b shows the representative DAPI-stained confocal images of Non-Tg and 5xFAD mice brain tissues, whereas Figure 4a’,b’ are their corresponding IPR images depicting the concentration of structural disorder in those tissues. To quantify the results, bar graphs were used to better understand alterations in local structural disorder in DNA. An increase of 26% in Mean Ld-IPR in 5xFAD mice DNA compared to their control, as represented in Figure 4c. Similarly, in STD Ld-IPR the fluctuation rose by 19% in 5xFAD mice compared to their Non-Tg controls, as shown in Figure 4d.
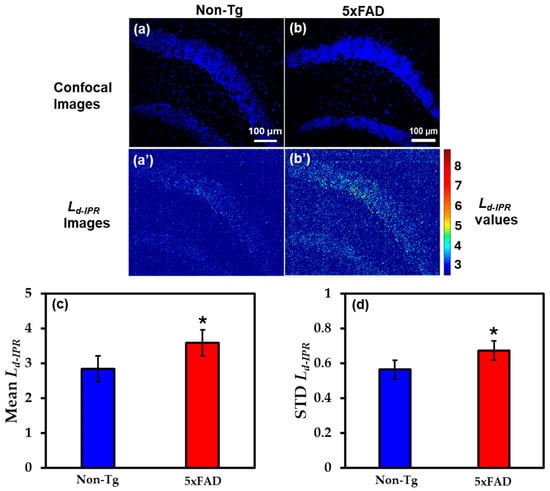
Figure 4.
(a,b) The confocal images of Non-Tg and 5xFAD mice brain tissues (hippocampus) with their corresponding Ld-IPR colormaps (a’,b’). (c,d) 5xFAD shows increased mean (26%), and STD (19%) of Ld-IPR compared to Non-Tg. (* Student’s t-test, p < 0.05; n = 4 mice per group).
AD causes chromatin within the nucleus to misfold, altering local structural disorder. This increases local spatial heterogeneity, leading to greater fluctuations in mass density. An increase in Ld-IPR can also be linked to DNA damage in brain cells caused by AD. In our previous publication, we verified the DNA damage analysis on brain cells due to AD in humans [22].
2.3. Changes in Mitochondria Structure in 5xFAD Mice: TEM Study
We selectively investigated cropped TEM images, focusing solely on mitochondrial structural alterations within hippocampal cells, as shown in Figure 5. Figure 5a,b shows the cropped TEM images of mitochondria of Non-Tg and 5xFAD mice brain cells, whereas Figure 5a’,b’ are their corresponding IPR images. To quantify the results, a bar graph was plotted showing the Mean and STD Ld-IPR for Non-Tg and 5xFAD. We found that the Mean mitochondrial IPR showed an increase of 18% in 5xFAD mice compared with the control, whereas the STD IPR showed an increase of 21% shown in Figure 5c,d. This indicates an increase in mitochondrial structural disorder, which can be linked to an increase in mitochondrial DNA (mtDNA) mutations/damages in AD, leading to mitochondrial fragmentation and ultimately neuronal death [29,30].
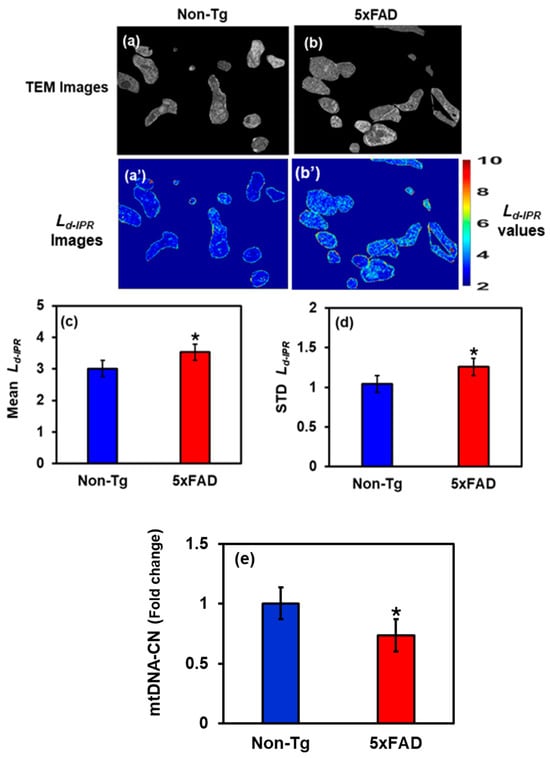
Figure 5.
(a,b) Cropped TEM images of hippocampal mitochondria from Non-Tg and 5xFAD mice with their corresponding Ld-IPR colormaps (a’,b’). (c,d) 5xFAD shows an increase in mean (18%) and STD (21%) Ld-IPR values compared to Non-Tg. (* Student’s t-test, p < 0.05; n = 25–30 mitochondria per group). (e) Relative mtDNA copy number is reduced by 27% in 5xFAD hippocampus, showing impaired mitochondrial maintenance and bioenergetic stress. (* Student’s t-test, p < 0.05; n = 4 mice per group).
2.4. Behavioral Study
To assess cognitive function, 7-month-old male 5xFAD mice and Non-Tg controls were subjected to the Novel Object Recognition (NOR) test. The details of NOR are discussed in Section 4.3. The recognition index (RI), calculated as the proportion of time spent exploring the novel object relative to total object exploration time, was significantly lower in 5xFAD than in their Non-Tg counterparts, as shown in Figure 6, suggesting recognition memory deficits. Non-Tg mice spent more time interacting with the novel object than the familiar one, reflecting intact memory function. In contrast, 5xFAD mice failed to show a marked preference for the novel object, suggesting impaired discrimination. This diminished novelty preference implies disruption in memory encoding or retrieval mechanisms. Our results support the notion that Aβ-related pathology negatively affects brain regions critical for memory, such as the hippocampus and cortex.
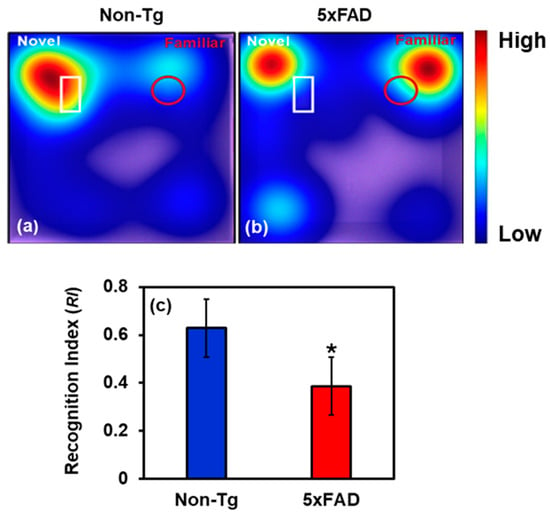
Figure 6.
(a,b) Representative heat maps showing time spent by mice (Non-Tg and Control) near the novel (white-outlined) and familiar (red-outlined) object during the retention phase. (c) The recognition index is reduced by 38.5% in 7-month-old 5xFAD mice compared with Non-Tg, indicating impaired non-spatial recognition memory. (* Student’s t-test, p < 0.05; n = 6 mice per group.).
Microglial Activation and Aβ Accumulation in the Brain of 5xFAD Mice
Immunofluorescence analysis revealed marked differences in Aβ plaque burden and microglial activation between 4-month-old male 5xFAD mice and their Non-Tg littermates, as shown in Figure 7. In the hippocampus, 5xFAD mice exhibited extensive Aβ plaque deposition, visualized by 4G8 immunostaining, accompanied by pronounced microglial clustering, as indicated by increased Iba1-positive cells. These plaques were absent in Non-Tg mice, which also showed a sparse microglial profile, indicative of a homeostatic state. Similarly, cortical sections from 5xFAD mice showed a substantial accumulation of 4G8-positive Aβ plaques. In these regions, Iba1-positive microglia appeared hypertrophic and densely aggregated around the plaques, suggesting a reactive phenotype. In contrast, cortical tissues from Non-Tg controls exhibited no Aβ immunoreactivity and displayed microglia with morphology consistent with a non-activated state. These findings demonstrate significant amyloid pathology and reactive microgliosis in both hippocampal and cortical regions of 5xFAD mice, reflecting hallmark features of AD-like neuropathology. The spatial association between Aβ deposits and activated microglia further supports a potential role for neuroinflammation in disease.
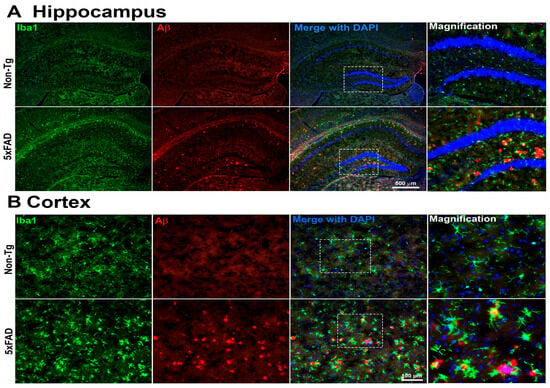
Figure 7.
Representative immunofluorescence images of the hippocampus (A) and cortex (B) from 5xFAD and Non-Tg mouse brains. Sections were stained for microglia (Iba1, green) and Aβ plaques (4G8, red), with nuclei counterstained with DAPI (blue). Merged images are shown for each region. Insets represent higher-magnification views of the areas indicated by white dashed boxes in the corresponding low-magnification images. Compared with Non-Tg controls, 5xFAD brains exhibit increased Aβ plaque burden and enhanced microglial activation in both hippocampal and cortical regions (n = 4 mice per group were examined. Only one representative image per group is presented here.). Hippocampal images were acquired at 5× magnification (scale bar = 500 μm), and cortical images at 20× magnification (scale bar = 100 μm).
2.5. Mitochondrial DNA Analysis: Relative mtDNA
Relative mtDNA copy number was significantly decreased in the hippocampus of 5xFAD mice compared to age-matched Non-Tg controls, as shown in Table 1. This reduction in relative mtDNA indicates impaired mitochondrial maintenance and early bioenergetic stress associated with amyloid pathology, potentially heightening neuronal vulnerability in this Alzheimer’s disease model. The statistics are depicted in the bar graph in Figure 5e.

Table 1.
Primers for mitochondrial DNA copy numbers in mouse.
3. Discussion
In this study, we conducted a comprehensive, systematic investigation of the 5xFAD mouse model of AD, integrating dual-photon imaging, behavioral testing, and histopathological analyses. By combining dual-photonics techniques of PWS and IPR, we identified nanostructural alterations in the mass density of brain cells and tissues within the hippocampus and cortex. These nanoscale alterations closely paralleled Aβ plaque accumulation, microglia activation, and cognitive decline, underscoring a direct link between biophysical nanostructural disorder and AD-related neuropathology. Together, these findings provide a nanoscale perspective on the structural alterations underlying neuronal dysfunction in AD.
Quantitative analysis of PWS measurements revealed pronounced nanoscale structural alterations in 5xFAD mice compared with Non-Tg controls. The observed elevation in Ld-PWS parameters in the 5xFAD mouse brain reflects the emergence of nanoscale structural disorders within cortical and hippocampal neurons, consistent with the progressive disorganization of the cellular microenvironment in AD. Such increases in optical heterogeneity likely stem from pathological protein generation, particularly Aβ and tau, which perturb local refractive index landscapes by altering membrane integrity, cytoskeletal stability, and extracellular matrix composition. The comparatively greater disorder in the cortex suggests a regional sensitivity to early amyloid pathology, in agreement with previous reports identifying cortical regions as initial sites of dense plaque deposition and neuroinflammatory activation [31,32,33]. Greater structural disorder in the cortex further suggests that cortical tissues undergo more pronounced fragmentation or macromolecular reorganization than in the hippocampus, thereby making the cortex more heterogeneous. The smaller change in structural disorder in the hippocampus suggests that macromolecules are more compact and uniform at the nanoscale level of organization than in the cortex. Parallel alterations detected through IPR analysis further highlight the nuclear vulnerability underlying AD pathology. Elevated Ld-IPR values in 5xFAD tissues reflect enhanced nanoscale mass-density fluctuations within chromatin and DNA domains, indicative of oxidative stress, DNA fragmentation, and disrupted chromatin compaction. Such nanoscale nuclear alterations are consistent with extensive DNA damage and chromatin disorganization reported in post-mortem human AD brains, where oxidative lesions, DNA double-strand breaks, and impaired DNA repair have been widely documented [34,35,36]. These changes represent early markers of genomic instability and transcriptional dysregulation, events that precede synaptic loss and neuronal dysfunction. Collectively, the convergence of PWS and IPR findings underscores that nanoscale structural disorder is not merely a secondary consequence of neurodegeneration but a fundamental aspect of the disease cascade. This emerging evidence suggests that biophysical disorganization at the chromatin and cellular levels may play a causal role in driving cognitive decline and the early detection of AD.
To further elucidate the subcellular mechanisms underlying nanoscale structural disorder in AD, we studied hippocampal mitochondria using TEM imaging combined with IPR-based quantitative analysis. Mitochondria from 5xFAD mice exhibited markedly elevated Mean and standard deviation IPR values, indicating enhanced mass-density fluctuations and nanoscale disorganization relative to Non-Tg controls. These changes may reflect alterations in mitochondrial ultrastructure, including disruption, swelling, and fragmentation of cristae, morphological features commonly associated with impaired bioenergetics and oxidative damage. These nanoscale abnormalities likely arise from alterations in mitochondrial DNA (mtDNA), or overwhelming ROS loads, both of which compromise electron transport and ATP synthesis. Mitochondrial dysfunction constitutes a critical nexus in AD pathogenesis, linking early nanoscale instability to downstream events such as energy failure, calcium imbalance, and apoptosis. The elevated IPR values observed here thus provide a quantitative nanoscale signature of mitochondrial distress that may precede overt neuronal degeneration. Reduced mtDNA copy number and impaired mitochondrial biogenesis, well documented in both human AD brains and transgenic models [37,38,39]. This further highlights the essential role of mitochondrial structural integrity in maintaining neuronal homeostasis. Because mtDNA encodes key components of the oxidative phosphorylation machinery, its loss directly diminishes ATP production while heightening oxidative stress [38,40]. In AD, these deficits exacerbate neuronal vulnerability by amplifying ROS production, disrupting calcium signaling, and activating cell death cascades [41,42]. Moreover, declining mtDNA copy number correlates with synaptic loss and cognitive impairment across aging and AD models [38,39,43,44], underscoring its central role in neurodegenerative disease. Taken together, our findings demonstrate that light-scattering–based nanoscale analyses, including PWS and IPR, can sensitively detect mitochondrial and cellular structural abnormalities preceding histopathological lesions. Quantifying structural disorder across spatial scales provides a potential biophysical biomarker for early AD detection and offers mechanistic insights into how nanoscale disorganization of organelles contributes to neurodegeneration.
Behavioral analysis revealed that 5xFAD mice exhibited a significantly reduced recognition index compared with Non-Tg controls, consistent with pronounced cognitive impairment [45,46]. This behavioral deficit coincided with pronounced amyloid pathology and robust microglial activation in the hippocampus and cortex, regions that are fundamental to memory processing. These findings align with prior studies that highlight the 5xFAD model’s fidelity in replicating key features of human amyloid pathology [46,47]. Behavioral and Histological analyses were performed on the same sets of mice to establish a link between AD-associated behavioral symptoms, pathological changes, and measurable nanoscale structural disorder. Histological analyses revealed a marked increase in Aβ plaques, indicative of extensive extracellular aggregation of Aβ peptides. These deposits were consistently encircled by clusters of Iba1-positive microglia exhibiting morphological features of activation, suggesting a phenotypic shift from a homeostatic to a pro-inflammatory state. Iba1 labeling of both resting and activated microglia was used to indicate microglial presence and association with pathology, not to assess activation state. Additional markers and higher-resolution analyses are required to define microglial activation rigorously. This reactive microgliosis is emblematic of AD-associated neuroinflammation and is thought to contribute to synaptic impairment, neuronal dysfunction, and further amplification of Aβ pathology [48,49]. Activated microglia, through the release of pro-inflammatory cytokines and ROS, generate a neurotoxic milieu that exacerbates neurodegeneration [48,50]. Over time, this chronic inflammatory environment is likely to induce structural remodeling of brain tissue, disrupting local cytoarchitecture. Beyond the cellular and tissue level, sustained microglial activation and Aβ deposition may also cause alterations at the nuclear level. Increasing evidence suggests that nuclear architecture is sensitive to chronic oxidative stress and inflammatory cues [51,52]. In neurodegenerative settings, such stressors may initiate chromatin remodeling, histone modifications, and accumulation of genotoxic insults. Our previous work demonstrated elevated neuroinflammation and genotoxic stress in the brains of 5xFAD mice [34,53], supporting this notion. These alterations are reflected in changes detected by PWS and IPR analyses, which capture increased mass density fluctuations and chromatin reorganization at resolutions beyond conventional fluorescence microscopy. These nanoscale architectural changes may serve as early biophysical markers of pathology, as previously reported in human AD brain tissue [22]. Notably, the cognitive deficits observed in 5xFAD mice may stem not only from classical macroscopic hallmarks such as Aβ plaques but also from more subtle nanoscale perturbations in nuclear and chromatin architecture. Together, our findings support the concept that AD pathology reflects a continuum of structural disruptions, ranging from extracellular protein aggregation and neuroinflammation to intranuclear architectural disorganization, that collectively impair cognitive function. The current study was designed to assess the sensitivity of light-scattering techniques for detecting nanoscale structural alterations associated with AD pathology that precede cognitive deficits; therefore, 4-month-old mice were used for PWS/IPR experiments, rather than 7-month-old mice, which were used for behavior analysis when cognitive deficits became apparent. These findings underscore the value of incorporating high-resolution biophysical approaches, including PWS or IPR imaging tools, into preclinical studies.
4. Materials and Methods
Animal model
All mouse experiments were performed in accordance with the National Institutes of Health’s Guidelines for the Care and Use of Laboratory Animals and approved by our Institutional Animal Care and Use Committee. The 5xFAD transgenic mice (Jackson Laboratory, Bar Harbor, ME, USA; stock #006554) express a human amyloid precursor protein (APP) gene carrying three specific point mutations (I716V, V717I, and KM670/671NL), along with a human presenilin 1 (PSEN1) gene that contains two mutations (M146L and L286V). These mutations are co-inherited in the mouse line. All experimental animals were bred in-house and were heterozygous for both mutant transgenes. Genomic DNA extracted from tail biopsies was analyzed by PCR before weaning to verify the presence of the APP and PSEN1 transgenes in all mice. Only male mice were used for all experiments to avoid potential sex-related variability. PWS, IPR, and histological analyses were performed at 4 months of age, whereas behavioral analysis was performed at 7 months of age for both groups. Four mice were used per group for all experiments, except the behavioral study, which used 6.
4.1. Partial Wave Spectroscopy Experiment
4.1.1. Optical Setup
A detailed picture of the setup can be found in our previous works [20,21,26]. However, a brief overview of the setup is provided here for completeness. The setup consists of a broadband white-light Xe lamp (Newport Corporation, Irvine, CA, USA) source, followed by a set of converging lenses forming a 4f system to collimate the light with Kohler illumination. The light then goes onto a right-angled prism (BRP), which directs the light to a beamsplitter, then to a 40× objective lens (NA = 0.65), where the sample is held by a XYZ motorized stage (x − y = 40 nm, z = 100 nm; Zaber Technologies, Vancouver, BC, Canada). The reflected backscattered light travels to a liquid crystal tunable filter (LCTF) (Thorlabs, Newton, NJ, USA) and then to a CCD (Teledyne Photometrics, AZ, USA), where the images are acquired in the visible range of 450–700 nm. With the motorized stage, the setup can measure the strength of structural disorder (Ld-PWS) at each pixel, with XY accuracy of 40 nm and Z accuracy of 100 nm.
4.1.2. Measurement of Structural Disorder Strength
The fluctuating reflectance spectra R(x,y,λ) from the sample are found to be proportional to refractive index fluctuations (dn) in the sample, which change as the mass density of cells/tissues in the cells/tissues changes at the nano- to sub-micron level. This has been demonstrated in our previous works [54,55,56]. We were able to quantify these mass density changes in terms of structural disorder strength Ld-PWS using the mesoscopic theory of light transport [55,57,58], assuming these RI fluctuations are within its correlation length, Lc, as Ld = <dn2> × Lc, where <dn2> is the variance in RI. Reflectance spectra are recorded of a 3D sample as a 3D data cube with two spatial positions (x, y) and wavelength as an additional parameter, spanning the visible range from 450 to 700 nm, with reflectance averaged over the z direction.
Mesoscopic light transport theory can be applied to the transport of electrons and light in dielectric media [58,59]. Biological 3D samples are considered as several interconnected 1D weakly disordered parallel media, allowing the quasi-1D theory to be applied to them. The backscattered light I(x,y,λ) is processed with a fifth-order polynomial to remove variations from the light source. Once we obtain the processed reflected spectra, the light is virtually divided into many 1D parallel channels by a quasi-1D approximation with a 200 × 200 nm (within the diffraction limit) pixel size. <R(k)> rms value of reflected spectra is calculated in the range of 450–700 nm, with auto correlation length C(∆k) [56,60,61].
In the above equation, B is a calibration constant, n0 = 1. (∆k)2/ln(<C(∆k)>) can be found by performing a linear fit of ln(<C(∆k)>) vs. (∆k)2.
4.1.3. Sample Preparation for PWS Experiment
The University of Tennessee Health Science Center (UTHSC) provided all samples with IACUC approval. 4-month-old male mice samples were used for both groups in this study. The mouse tissue samples were sectioned into 5 µm slices using a microtome, embedded in paraffin according to standard protocol, and placed on a glass slide for light-scattering experiments.
4.2. Inverse Participation Ratio Quantification Using Confocal Microscopy and Transmission Electron Microscopy
4.2.1. Confocal Imaging
Confocal images targeting the cell’s nucleus were captured using a Zeiss 710 confocal microscope (Carl Zeiss Microscopy, Jena, Germany), where we used Z-stack mode above and below a cell’s nucleus. Pictures captured in the Z-stack mode were selected based on the most significant changes observed in the stack, providing the best coverage of the nuclear area. To analyze nuclear components such as DNA/chromatin, each cell type was identified based on its characteristics. Various software, such as MATLAB R2025b and ImageJ v1.54c (National Institutes of Health, Bethesda, MD, USA), were used to process the images. Similarly, tissue images from different groups were captured for further processing and calculations.
4.2.2. Measurement of IPR
The light intensity in the confocal images of biological samples is due to multiple scattering within cells and tissues. Scattering within cells and tissues occurs due to structural disorder, especially from components such as DNA, lipids, and proteins. Image intensity changes as the refractive index within cells and tissues varies. A change in refractive index can be linked to a change in mass density in a voxel of the cell. I(x, y) ∝ n(x, y) ∝ M(x, y) [62]. Similarly, the optical potential can be defined as
In Equation (2), dn is the refractive index fluctuation, n0 is the average refractive index of the medium, dI is the variation in light intensity, and I0 is the average light intensity coming from the cell voxel.
The Hamiltonian of the system is defined using the Anderson tight-binding model [63,64],
where |i> and |j> are the optical wave functions at the ith and jth lattice sites, t is the inter-lattice hopping energy, and is the optical potential at the ith state. Mean IPR can be derived by substituting the optical potential in the Hamiltonian for sample length L,
In Equation (4), Ei is the ith eigenfunction of the optical lattice of size L × L, p is the 2D pixel size of the confocal micrograph and N is the total number of eigenfunctions in the refractive index matrix (N = (L/dx)2).
We have shown in our previous works that Mean and STD <IPR>p is proportional to the structural disorder strength Ld of similar cells, where Ld-IPR = <∆n> × lc [65].
4.2.3. Sample Preparation for IPR Experiment
4-month-old male mice samples were used for this study. A fluorescent dye, DAPI, was used to stain nuclear structures, such as DNA and chromatin, allowing them to be visualized under fluorescence microscopy and helping us differentiate nuclei from the rest of the cell. The following steps were taken to stain the samples with dye. Firstly, the slides were cleansed in Phosphate-Buffered Saline (PBS) with 2–4% paraformaldehyde for 5 min. This step was repeated 3 times before moving further. Later, the slides were stained with blue dye to label nuclear structures, such as DNA, using Prolong Diamond antifade mountant containing DAPI.
4.2.4. TEM Imaging of Mitochondria
Transmission electron microscopy was performed using a JEM-2000EX II microscope (JEOL Co., Tokyo, Japan) equipped with a side-mounted digital camera (Advanced Microscopy Techniques, Woburn, MA, USA) operated at 60 kV, as described by us [66]. Hippocampal tissues from 5xFAD and Non-Tg mice were fixed in a solution containing 4% paraformaldehyde and 2% glutaraldehyde in 0.13 M sodium cacodylate buffer (pH 7.2). This fixation process can introduce minor alterations in tissue morphology; such effects are generally minimal and do not obscure relative differences when samples are processed altogether, as those changes would be systematic across all groups. After several washes in the same buffer, samples were post-fixed with 1% osmium tetroxide in 0.13 M sodium cacodylate buffer for 2 h, rinsed sequentially in buffer and distilled water, and then dehydrated through a graded ethanol series. Dehydrated tissues were infiltrated overnight at room temperature with a 1:1 mixture of Embed-812 resin and acetone, followed by three incubations in 100% Embed-812 resin for 2 h each. Samples were embedded in resin and polymerized at 65 °C overnight. Ultrathin sections (60–65 nm) were cut using a Leica EM UC7 ultramicrotome and mounted on 200-mesh copper grids. Sections were stained with Uranyless and lead citrate before imaging at 60 kV. For quantitative analysis of structural disorders using IPR-based molecular-specific mass density fluctuations, a total of 25–30 mitochondria per group was analyzed.
4.2.5. IPR Quantification Using TEM Images
Mitochondria are called the powerhouse of the cell as they produce most of the energy required for cells/neurons. We wanted to study mitochondrial subcellular structural changes in AD. The IPR analysis is described in the sections above. A similar analysis was used to study mitochondrial structural disorder in the brains of AD mice. For this analysis, TEM images of Non-Tg and 5xFAD mice brain tissues from the hippocampus region were processed in ImageJ to isolate mitochondria, with only the mitochondria and background regions turned black, as shown in Figure 8. In the IPR analysis, we considered only IPR values arising from mitochondria. Interestingly, both the Mean and the standard deviation of IPR values increased in the AD-affected mouse.
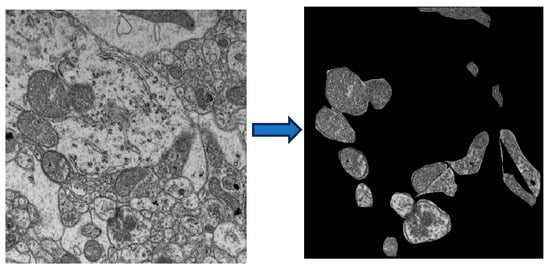
Figure 8.
Transmission electron microscopy (TEM) image of mitochondria from the 5xFAD mouse model (left). The image was processed with ImageJ to isolate individual mitochondria, and the background was further separated for analysis (right).
For TEM imaging, the refractive index/optical properties are indirectly related to the TEM images through mass density. The TEM intensity is proportional to the charge density, which in turn is proportional to the mass density. As it has been discussed in detail [21]:
Based on this, the TEM intensity can be converted to the tissue’s refractive index, and the same IPR formalism can be applied.
4.3. Novel Object Recognition
7-month-old male mice were used in this study because behavioral symptoms in 5xFAD mice first appear at this age. The novel object recognition assay was employed to evaluate recognition memory, which is a component of learning and memory, based on the natural inclination of mice to investigate novel stimuli [67,68]. The test was conducted in an open-field arena (40 cm × 40 cm × 30 cm) containing two objects as described by us [66]. During the habituation phase (Day 1), Non-Tg and 5xFAD mice were individually allowed to explore the empty arena freely. To minimize olfactory interference, the chamber was cleaned with 70% ethanol between trials to eliminate olfactory cues. On Day 2, animals were initially presented with two identical, familiar objects (red outlined) positioned along adjacent walls. After a 2 h retention interval following this acquisition phase, one of the familiar objects was replaced with a novel object (white outline), while the other remained unchanged. Each trial lasted 5 min, and all sessions were video recorded and analyzed using EthoVision XT 18 software (Noldus, Wageningen, The Netherlands) for automated tracking of object exploration time. The time spent interacting with the novel versus the familiar object was recorded, with increased exploration of the novel object interpreted as an indication of intact recognition memory.
4.4. Immunofluorescence Staining
4-month-old male mice samples were used for this study. Immunofluorescent labeling was carried out following previously established protocols by our group [22,34,69]. In brief, mice were anesthetized using isoflurane, and brains were rapidly extracted, post-fixed in 4% paraformaldehyde, and cryoprotected in 30% sucrose prepared in 0.1 M phosphate-buffered saline (PBS) at 4 °C for 48–72 h. Coronal brain sections (25 μm thick) were prepared using a cryostat. Sections were rinsed in PBS, then blocked in a solution containing 5% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) and 0.3% Triton X-100. Subsequently, sections were incubated overnight at 4 °C with the primary antibodies ionized calcium-binding adaptor molecule 1 (Iba-1; 1:500, Synaptic System, Gottingen, Germany) and the 4G8 anti-Aβ antibody (BioLegend, San Diego, CA, USA). After primary antibody incubation, sections were treated with fluorescent secondary antibodies—either Alexa Fluor 488 anti-chicken or Alexa Fluor 555-anti-mouse (1:500, Invitrogen, Waltham, MA, USA). Following washes, sections were counterstained with DAPI (Vector Laboratories, Newark, CA, USA) and coverslipped. Imaging was performed with a fluorescence microscope at 50× and 200× magnification, using 5× and 20× objectives, respectively. Image acquisition parameters (laser intensity, gain, and magnification) were kept consistent across all experimental groups.
4.5. Quantification for Mitochondrial DNA Copy Number
Mitochondrial DNA (mtDNA) copy number was quantified to assess mitochondrial abundance in hippocampal tissue of 5xFAD and Non-Tg mice. Total DNA was isolated using a commercial extraction kit (Bio-Rad, Hercules, CA, USA), ensuring high-quality nucleic acid suitable for downstream amplification. Quantitative PCR (qPCR) was performed using primer sets specific for mtDNA and nuclear DNA (nDNA) sequences. The ratio of mtDNA to nDNA amplification served as an index of relative mtDNA copy number. The primer sequences used in this analysis are provided in Section 2 above.
5. Conclusions
This study offers a multimodal, multiparameter view of AD in the 5xFAD mouse model by combining dual-photon imaging with histopathological, ultrastructural, and behavioral analyses. All the results are summarized in Figure 9. Our findings reveal that nanoscale structural disorder arises early in AD and correlates with overt neuropathological changes and cognitive impairment. Elevated Ld-PWS and Ld-IPR values reflect increased mass density fluctuations within mitochondrial and nuclear compartments, suggesting that amyloid beta deposition, neuroinflammation, and oxidative stress collectively destabilize brain architecture across multiple spatial scales. These results demonstrate that optical biophysical approaches such as PWS and IPR can sensitively detect subcellular alterations that are invisible to conventional imaging techniques. Quantifying such nanoscale disorder provides a robust, powerful framework for understanding the structural underpinnings of neurodegeneration and identifying potential early biomarkers of AD and related dementia. The limitations of our study include the use of a single transgenic model, age-mismatch between mice for behavioral and other light-scattering experiments, and the lack of longitudinal in vivo validation. Expanding these approaches to additional AD models that constitute both amyloid and tau pathology will be critical for establishing a more comprehensive framework linking nanoscale structural disorder with behavioral and molecular outcomes across various stages of disease progression. For future studies, we aim to examine distinct subregional structural changes in the hippocampus and cortex using the 5xFAD model, along with a larger cohort of AD mice at multiple time points and disease stages, to identify the age at which nanoscale structural changes first occur and to link nanoscale structural changes with functional outcomes directly. This approach will advance the translational potential of nanoscale biophysical metrics as early biomarkers of AD and possibly other neurodegenerative disorders.
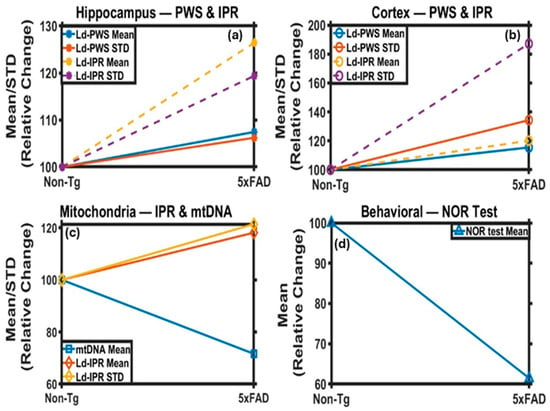
Figure 9.
Summary Charts of changes in 5xFAD tissues with respect to Non-Tg: (a) Ld-PWS and (b) Ld-IPR results from the hippocampus and cortex tissue of the mouse brain, respectively, for Non-Tg and 5xFAD mice. The Mean and STD of Ld-PWS and Ld-IPR values are increasing for hippocampus and cortex for 5xFAD vs. Non-Tg. (c) The Mean and STD of Ld-IPR(TEM) values are also increasing, while mtDNA is decreasing for 5xFAD vs. Non-Tg, for the hippocampus region. (d) Behavioral study results from the NOR analysis indicate that the value decreases in 5xFAD relative to Non-Tg.
Author Contributions
P.P. and M.M.K. conceived the study and designed the project. M.M.K. and S.K. provided the mouse brain tissues. D.S., F.A., M.A.P. and F.S. performed the PWS optical experiments on hippocampus and cortex brain tissues. I.A., D.S. and S.M. performed the IPR-confocal experiments and analyses. S.K. performed the behavioral study and edited the manuscript. S.M. performs TEM structural analysis of mitochondria. S.N. performed histopathology experiments and edited the manuscript. J.X. performed the RT-qPCR analysis and edited the manuscript. D.S., I.A., S.M., S.K., M.M.K. and P.P. wrote the first draft, and all authors contributed to the final version of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the National Institutes of Health under grant R21 CA260147 and ORED, Mississippi State, to P.P. M.M.K.’s works on Alzheimer’s disease and related dementia are supported by NIH grant R03AG075597, Alzheimer’s Association Award AARG-NTF-22-972518, and Department of Defense Award Number HT9425-23-1-0043.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Tennessee Health Science Center (Protocol Code: 24-0575 and date of approval: 24 October 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We thank MS state and UTHSC imaging facilities for imaging.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Alzheimer’s disease |
| PWS | Partial Wave Spectroscopy |
| IPR | Inverse Participation Ratio |
| Aβ | Amyloid beta |
| TEM | Transmission Electron Microscope |
| Non-Tg | Non-transgenic |
| mtDNA | Mitochondrial DNA |
| NOR | Novel Object Recognition |
| RI | Refractive Index |
References
- Bush, A.I. The Metallobiology of Alzheimer’s Disease. Trends Neurosci. 2003, 26, 207–214. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2025 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2025, 21, e70235. [Google Scholar] [CrossRef]
- Murphy, M.P.; LeVine, H. Alzheimer’s Disease and the β-Amyloid Peptide. J. Alzheimers Dis. 2010, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Joachim, C.L.; Mori, H.; Selkoe, D.J. Amyloid β-Protein Deposition in Tissues Other than Brain in Alzheimer’s Disease. Nature 1989, 341, 226–230. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s Disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 5 December 2025).
- Li, J.; Li, J. Trends, Inequalities, and Cross-Location Similarities in Global Dementia Burden and Attributable Risk Factors across 204 Countries and Territories: A Systematic Analysis for the Global Burden of Disease Study 2021. Int. J. Surg. 2025, 111, 5298–5310. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of A Beta-Deposition in the Human Brain and Its Relevance for the Development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Marcuello, C.; Lim, K.; Nisini, G.; Pokrovsky, V.S.; Conde, J.; Ruggeri, F.S. Nanoscale Analysis beyond Imaging by Atomic Force Microscopy: Molecular Perspectives on Oncology and Neurodegeneration. Small Sci. 2025, 5, 2500351. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, W.; Wang, L.; Li, J.; Huang, D.; Cheng, W.; Tian, J.; Luan, P. Advance and Prospect of Positron Emission Tomography in Alzheimer’s Disease Research. Mol. Psychiatry 2025, 30, 4899–4909. [Google Scholar] [CrossRef]
- Pradhan, P.; Damania, D.; Joshi, H.M.; Turzhitsky, V.; Subramanian, H.; Roy, H.K.; Taflove, A.; Dravid, V.P.; Backman, V. Quantification of Nanoscale Density Fluctuations by Electron Microscopy: Probing Cellular Alterations in Early Carcinogenesis. Phys. Biol. 2011, 8, 026012. [Google Scholar] [CrossRef]
- Thompson, P.M.; Hayashi, K.M.; de Zubicaray, G.; Janke, A.L.; Rose, S.E.; Semple, J.; Herman, D.; Hong, M.S.; Dittmer, S.S.; Doddrell, D.M.; et al. Dynamics of Gray Matter Loss in Alzheimer’s Disease. J. Neurosci. 2003, 23, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Ribarič, S. Detecting Early Cognitive Decline in Alzheimer’s Disease with Brain Synaptic Structural and Functional Evaluation. Biomedicines 2023, 11, 355. [Google Scholar] [CrossRef]
- Apachigawo, I.; Solanki, D.; Tate, R.; Singh, H.; Khan, M.M.; Pradhan, P. Fractal Dimension Analyses to Detect Alzheimer’s and Parkinson’s Diseases Using Their Thin Brain Tissue Samples via Transmission Optical Microscopy. Biophysica 2023, 3, 569–581. [Google Scholar] [CrossRef]
- Drayer, B.P.; Heyman, A.; Wilkinson, W.; Barrett, L.; Weinberg, T. Early-Onset Alzheimer’s Disease: An Analysis of CT Findings. Ann. Neurol. 1985, 17, 407–410. [Google Scholar] [CrossRef]
- Popp, A.K.; Valentine, M.T.; Kaplan, P.D.; Weitz, D.A. Microscopic Origin of Light Scattering in Tissue. Appl. Opt. 2003, 42, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Boustany, N.N.; Thakor, N.V. Light Scatter Spectroscopy and Imaging of Cellular and Subcellular Events. In Biomedical Photonics: Handbook; CRC Press: Boca Raton, FL, USA, 2003; pp. 437–460. [Google Scholar]
- Drezek, R.; Dunn, A.; Richards-Kortum, R. Light Scattering from Cells: Finite-Difference Time-Domain Simulations and Goniometric Measurements. Appl. Opt. 1999, 38, 3651–3661. [Google Scholar] [CrossRef]
- Adhikari, P.; Alharthi, F.; Pradhan, P. Partial Wave Spectroscopy Detection of Cancer Stages Using Tissue Microarrays (TMA) Samples. In Proceedings of the Frontiers in Optics + Laser Science APS/DLS (2019), Washington, DC, USA, 15–19 September 2019; Optica Publishing Group: Washington, DC, USA, 2019; p. JW4A.89. [Google Scholar]
- Adhikari, P.; Hasan, M.; Sridhar, V.; Roy, D.; Pradhan, P. Studying Nanoscale Structural Alterations in Cancer Cells to Evaluate Ovarian Cancer Drug Treatment, Using Transmission Electron Microscopy Imaging. Phys. Biol. 2020, 17, 036005. [Google Scholar] [CrossRef]
- Alharthi, F.; Apachigawo, I.; Solanki, D.; Khan, S.; Singh, H.; Khan, M.M.; Pradhan, P. Dual Photonics Probing of Nano- to Submicron-Scale Structural Alterations in Human Brain Tissues/Cells and Chromatin/DNA with the Progression of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 12211. [Google Scholar] [CrossRef]
- Liu, P.Y.; Chin, L.K.; Ser, W.; Chen, H.F.; Hsieh, C.-M.; Lee, C.-H.; Sung, K.-B.; Ayi, T.C.; Yap, P.H.; Liedberg, B.; et al. Cell Refractive Index for Cell Biology and Disease Diagnosis: Past, Present and Future. Lab Chip 2016, 16, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Gladstein, S.; Damania, D.; Almassalha, L.M.; Smith, L.T.; Gupta, V.; Subramanian, H.; Rex, D.K.; Roy, H.K.; Backman, V. Correlating Colorectal Cancer Risk with Field Carcinogenesis Progression Using Partial Wave Spectroscopic Microscopy. Cancer Med. 2018, 7, 2109–2120. [Google Scholar] [CrossRef]
- Backman, V.; Roy, H.K. Light-Scattering Technologies for Field Carcinogenesis Detection: A Modality for Endoscopic Prescreening. Gastroenterology 2011, 140, 35–41.e5. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Pradhan, P.; Liu, Y.; Capoglu, I.R.; Li, X.; Rogers, J.D.; Heifetz, A.; Kunte, D.; Roy, H.K.; Taflove, A.; et al. Optical Methodology for Detecting Histologically Unapparent Nanoscale Consequences of Genetic Alterations in Biological Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 20118–20123. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Wisniewski, T. Alzheimer’s Disease: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- New Genetically Modified Mouse Model Mimics Multiple Aspects of Human Alzheimer’s Disease. Available online: https://www.nia.nih.gov/news/new-genetically-modified-mouse-model-mimics-multiple-aspects-human-alzheimers-disease (accessed on 2 December 2024).
- Hoekstra, J.G.; Hipp, M.J.; Montine, T.J.; Kennedy, S.R. Mitochondrial DNA Mutations Increase in Early Stage Alzheimer’s Disease and Are Inconsistent with Oxidative Damage. Ann. Neurol. 2016, 80, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H. Mitochondrial DNA–Related Mitochondrial Dysfunction in Neurodegenerative Diseases. Arch. Pathol. Lab Med. 2002, 126, 271–280. [Google Scholar] [CrossRef]
- Tsering, W.; Prokop, S. Neuritic Plaques—Gateways to Understanding Alzheimer’s Disease. Mol. Neurobiol. 2024, 61, 2808–2821. [Google Scholar] [CrossRef]
- Gazestani, V.; Kamath, T.; Nadaf, N.M.; Dougalis, A.; Burris, S.J.; Rooney, B.; Junkkari, A.; Vanderburg, C.; Pelkonen, A.; Gomez-Budia, M.; et al. Early Alzheimer’s Disease Pathology in Human Cortex Involves Transient Cell States. Cell 2023, 186, 4438–4453.e23. [Google Scholar] [CrossRef]
- Kobro-Flatmoen, A.; Lagartos-Donate, M.J.; Aman, Y.; Edison, P.; Witter, M.P.; Fang, E.F. Re-Emphasizing Early Alzheimer’s Disease Pathology Starting in Select Entorhinal Neurons, with a Special Focus on Mitophagy. Ageing Res. Rev. 2021, 67, 101307. [Google Scholar] [CrossRef]
- Thadathil, N.; Delotterie, D.F.; Xiao, J.; Hori, R.; McDonald, M.P.; Khan, M.M. DNA Double-Strand Break Accumulation in Alzheimer’s Disease: Evidence from Experimental Models and Postmortem Human Brains. Mol. Neurobiol. 2021, 58, 118–131. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Huang, M.; Gunewardena, S.; Haeri, M.; Swerdlow, R.H.; Wang, N. Landscape of Double-Stranded DNA Breaks in Postmortem Brains from Alzheimer’s Disease and Non-Demented Individuals. J. Alzheimers Dis. 2023, 94, 519–535. [Google Scholar] [CrossRef]
- Dileep, V.; Boix, C.A.; Mathys, H.; Marco, A.; Welch, G.M.; Meharena, H.S.; Loon, A.; Jeloka, R.; Peng, Z.; Bennett, D.A.; et al. Neuronal DNA Double-Strand Breaks Lead to Genome Structural Variations and 3D Genome Disruption in Neurodegeneration. Cell 2023, 186, 4404–4421.e20. [Google Scholar] [CrossRef] [PubMed]
- Mengel-From, J.; Thinggaard, M.; Dalgård, C.; Kyvik, K.O.; Christensen, K.; Christiansen, L. Mitochondrial DNA Copy Number in Peripheral Blood Cells Declines with Age and Is Associated with General Health among Elderly. Hum. Genet. 2014, 133, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N.-G. Mitochondrial DNA Copy Number in Human Disease: The More the Better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef] [PubMed]
- Cerantonio, A.; Citrigno, L.; Greco, B.M.; De Benedittis, S.; Passarino, G.; Maletta, R.; Qualtieri, A.; Montesanto, A.; Spadafora, P.; Cavalcanti, F. The Role of Mitochondrial Copy Number in Neurodegenerative Diseases: Present Insights and Future Directions. Int. J. Mol. Sci. 2024, 25, 6062. [Google Scholar] [CrossRef]
- Castellani, C.A.; Longchamps, R.J.; Sun, J.; Guallar, E.; Arking, D.E. Thinking Outside the Nucleus: Mitochondrial DNA Copy Number in Health and Disease. Mitochondrion 2020, 53, 214–223. [Google Scholar] [CrossRef]
- D’Alessandro, M.C.B.; Kanaan, S.; Geller, M.; Praticò, D.; Daher, J.P.L. Mitochondrial Dysfunction in Alzheimer’s Disease. Ageing Res. Rev. 2025, 107, 102713. [Google Scholar] [CrossRef]
- Perez Ortiz, J.M.; Swerdlow, R.H. Mitochondrial Dysfunction in Alzheimer’s Disease: Role in Pathogenesis and Novel Therapeutic Opportunities. Br. J. Pharmacol. 2019, 176, 3489–3507. [Google Scholar] [CrossRef]
- Harerimana, N.V.; Paliwali, D.; Romero-Molina, C.; Bennett, D.A.; Pa, J.; Goate, A.; Swerdlow, R.H.; Andrews, S.J. The Role of Mitochondrial Genome Abundance in Alzheimer’s Disease. Alzheimers Dement. 2023, 19, 2069–2083. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wiggins, K.L.; Kurniansyah, N.; Guo, X.; Rodrigue, A.L.; Zhao, W.; Yanek, L.R.; Ratliff, S.M.; Pitsillides, A.; et al. Association of Mitochondrial DNA Copy Number With Brain MRI Markers and Cognitive Function: A Meta-Analysis of Community-Based Cohorts. Neurology 2023, 100, e1930–e1943. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, H.-A.; Han, Y.S.; Jeon, W.K.; Han, J.-S. Recognition Memory Impairments and Amyloid-Beta Deposition of the Retrosplenial Cortex at the Early Stage of 5XFAD Mice. Physiol. Behav. 2020, 222, 112891. [Google Scholar] [CrossRef]
- Pádua, M.S.; Guil-Guerrero, J.L.; Lopes, P.A. Behaviour Hallmarks in Alzheimer’s Disease 5xFAD Mouse Model. Int. J. Mol. Sci. 2024, 25, 6766. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal Beta-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Rajendran, L.; Paolicelli, R.C. Microglia-Mediated Synapse Loss in Alzheimer’s Disease. J. Neurosci. 2018, 38, 2911–2919. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of Pro-Inflammatory Cytokines Released from Microglia in Neurodegenerative Diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Romero-Bueno, R.; de la Cruz Ruiz, P.; Artal-Sanz, M.; Askjaer, P.; Dobrzynska, A. Nuclear Organization in Stress and Aging. Cells 2019, 8, 664. [Google Scholar] [CrossRef]
- Gauthier, B.R.; Comaills, V. Nuclear Envelope Integrity in Health and Disease: Consequences on Genome Instability and Inflammation. Int. J. Mol. Sci. 2021, 22, 7281. [Google Scholar] [CrossRef]
- Shukla, P.K.; Delotterie, D.F.; Xiao, J.; Pierre, J.F.; Rao, R.; McDonald, M.P.; Khan, M.M. Alterations in the Gut-Microbial-Inflammasome-Brain Axis in a Mouse Model of Alzheimer’s Disease. Cells 2021, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Shukla, P.; Almabadi, H.; Sahay, P.; Rao, R.; Pradhan, P. Optical Study of Stress Hormone-Induced Nanoscale Structural Alteration in Brain Using Partial Wave Spectroscopic (PWS) Microscopy. J. Biophotonics 2018, 12, e201800002. [Google Scholar] [CrossRef]
- Pradhan, P.; Subramanian, H.; Liu, Y.; Kim, Y.; Roy, H.; Backman, V. Application of Mesoscopic Light Transport Theory to Ultra-Early Detection of Cancer in a Single Biological Cell. In Proceedings of the 2007 APS March Meeting, Denver, CO, USA, 5–9 March 2007; p. B41.013. [Google Scholar]
- Pradhan, P. Phase Statistics of Light Wave Reflected from One-Dimensional Optical Disordered Media and Its Effects on Light Transport Properties. Photonics 2021, 8, 485. [Google Scholar] [CrossRef]
- Pradhan, P.; Liu, Y.; Kim, Y.; Li, X.; Wali, R.K.; Roy, H.K.; Backman, V. Mesoscopic Light Transport Properties of a Single Biological Cell: Early Detection of Cancer. In Proceedings of the 2006 APS March Meeting, Baltimore, MD, USA, 13–17 March 2006; p. Q1.326. [Google Scholar]
- Pradhan, P.; Subramanian, H.; Damania, D.; Roy, H.; Backman, V. Mesoscopic Light Reflection Spectroscopy of Weakly Disordered Dielectric Media: Nanoscopic to Mesoscopic Light Transport Properties of a Single Biological Cell and Ultra-Early Detection of Cancer. In Proceedings of the 2009 APS March Meeting, Pittsburgh, PA, USA, 16–20 March 2009; p. A28.001. [Google Scholar]
- Pradhan, P.; Kumar, N. Localization of Light in Coherently Amplifying Random Media. Phys. Rev. B 1994, 50, 9644–9647. [Google Scholar] [CrossRef]
- Roy, H.K.; Subramanian, H.; Damania, D.; Hensing, T.A.; Rom, W.N.; Pass, H.I.; Ray, D.; Rogers, J.D.; Bogojevic, A.; Shah, M.; et al. Optical Detection of Buccal Epithelial Nanoarchitectural Alterations in Patients Harboring Lung Cancer: Implications for Screening. Cancer Res. 2010, 70, 7748–7754. [Google Scholar] [CrossRef]
- Subramanian, H.; Pradhan, P.; Kunte, D.; Deep, N.; Roy, H.; Backman, V. Single-Cell Partial Wave Spectroscopic Microscopy. In Proceedings of the Biomedical Optics (2008), St. Petersburg, FL, USA, 16–19 March 2008; Optica Publishing Group: Washington, DC, USA, 2008; p. BTuC5. [Google Scholar]
- Pradhan, P.; Damania, D.; Joshi, H.M.; Turzhitsky, V.; Subramanian, H.; Roy, H.K.; Taflove, A.; Dravid, V.P.; Backman, V. Quantification of Nanoscale Density Fluctuations Using Electron Microscopy: Light-Localization Properties of Biological Cells. Appl. Phys. Lett. 2010, 97, 243704. [Google Scholar] [CrossRef]
- Lee, P.A.; Fisher, D.S. Anderson Localization in Two Dimensions. Phys. Rev. Lett. 1981, 47, 882–885. [Google Scholar] [CrossRef]
- Mafi, A. Transverse Anderson Localization of Light: A Tutorial. Adv. Opt. Photon. 2015, 7, 459–515. [Google Scholar] [CrossRef]
- Adhikari, P.; Shukla, P.K.; Rao, R.; Pradhan, P. Quantification of Light Localization Properties to Study the Effect of Probiotic on Chronic Alcoholic Brain Cells via Confocal Imaging. In Proceedings of the Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues XIX, Online Only, USA, 6–11 March 2021; Leary, J.F., Tarnok, A., Georgakoudi, I., Eds.; SPIE: Bellingham, WA, USA, 2021; p. 41. [Google Scholar]
- Singh, H.; Khan, S.; Xiao, J.; Nguyen, N.; Das, A.; Johnson, D.; Fang-Liao, F.; Frautschy, S.A.; McDonald, M.P.; Pourmotabbed, T.; et al. Harnessing cGAS-STING Signaling to Counteract the Genotoxic-Immune Nexus in Tauopathy. bioRxiv 2025. [Google Scholar] [CrossRef]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 126, 55718. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, X. Behavioral and Pathological Characteristics of 5xFAD Female Mice in the Early Stage. Sci. Rep. 2025, 15, 6924. [Google Scholar] [CrossRef]
- Khan, S.; Delotterie, D.F.; Xiao, J.; Thangavel, R.; Hori, R.; Koprich, J.; Alway, S.E.; McDonald, M.P.; Khan, M.M. Crosstalk between DNA Damage and cGAS-STING Immune Pathway Drives Neuroinflammation and Dopaminergic Neurodegeneration in Parkinson’s Disease. Brain Behav. Immun. 2025, 130, 106065. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.