Transcriptional Regulators in the Cerebellum in Chronic Schizophrenia: Novel Possible Targets for Pharmacological Interventions
Abstract
1. Introduction
2. Results
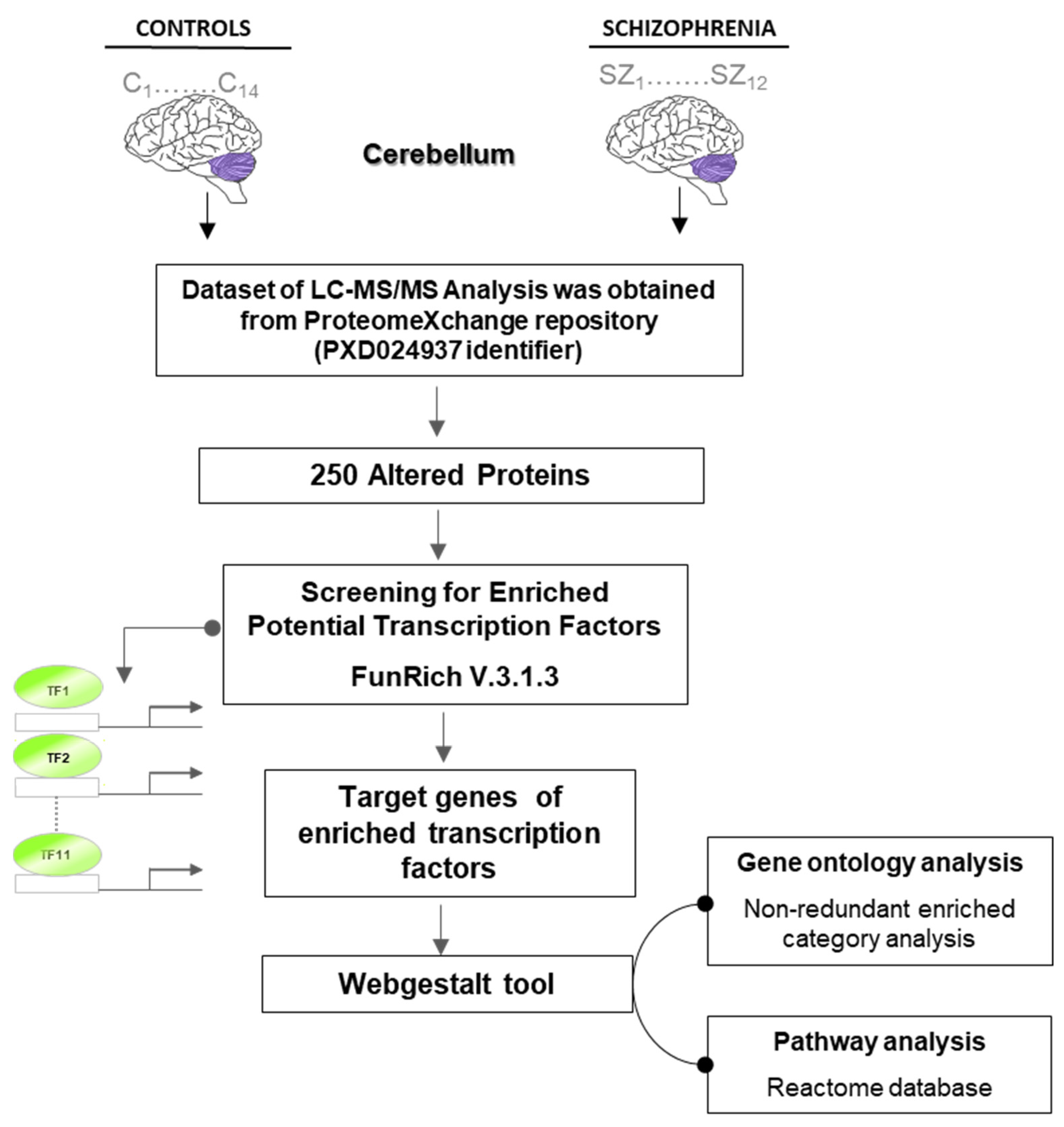
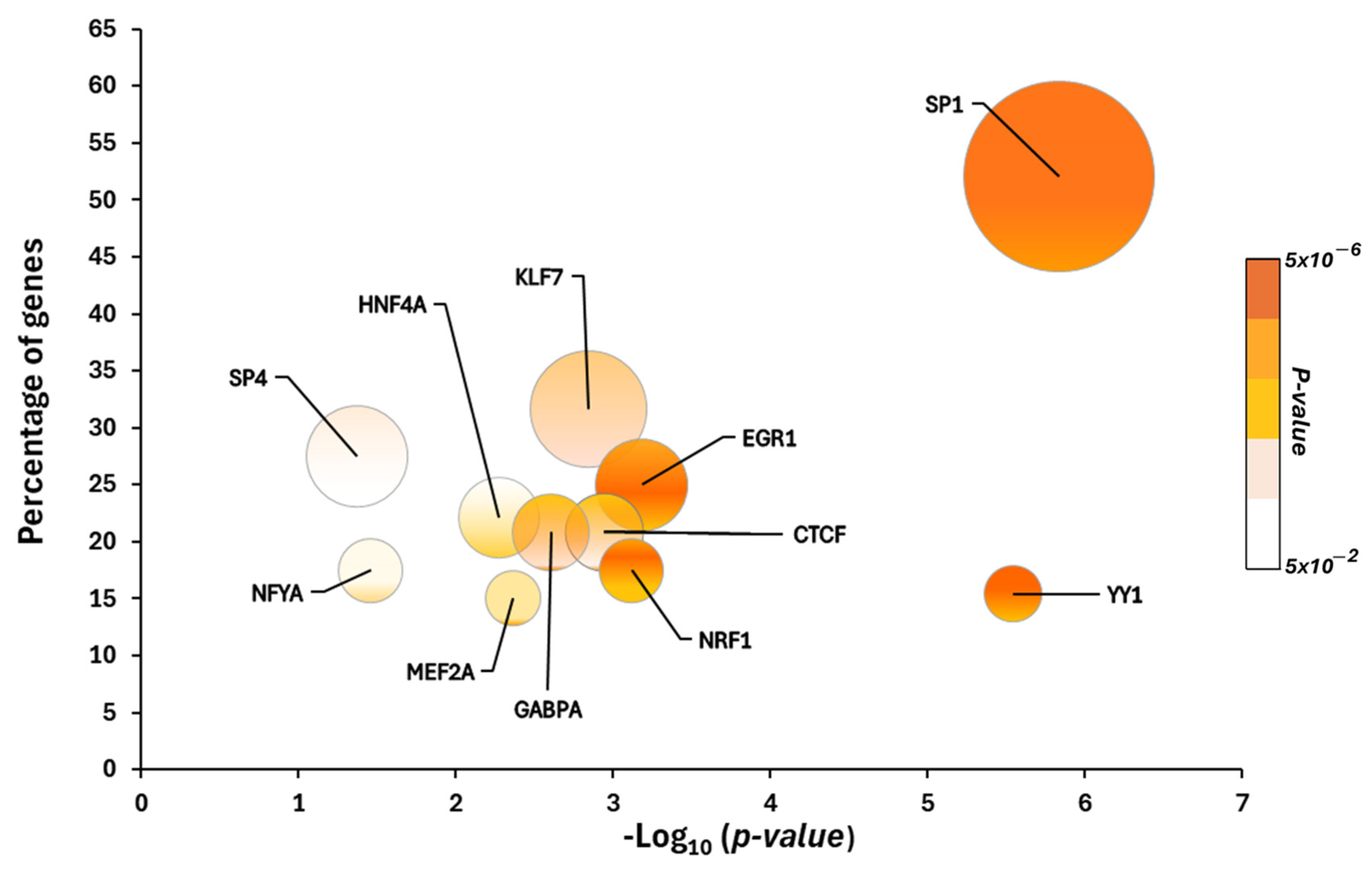
2.1. Putative Transcriptional Programs Responsible for Changes in the Proteomic Profile in the Cerebellum
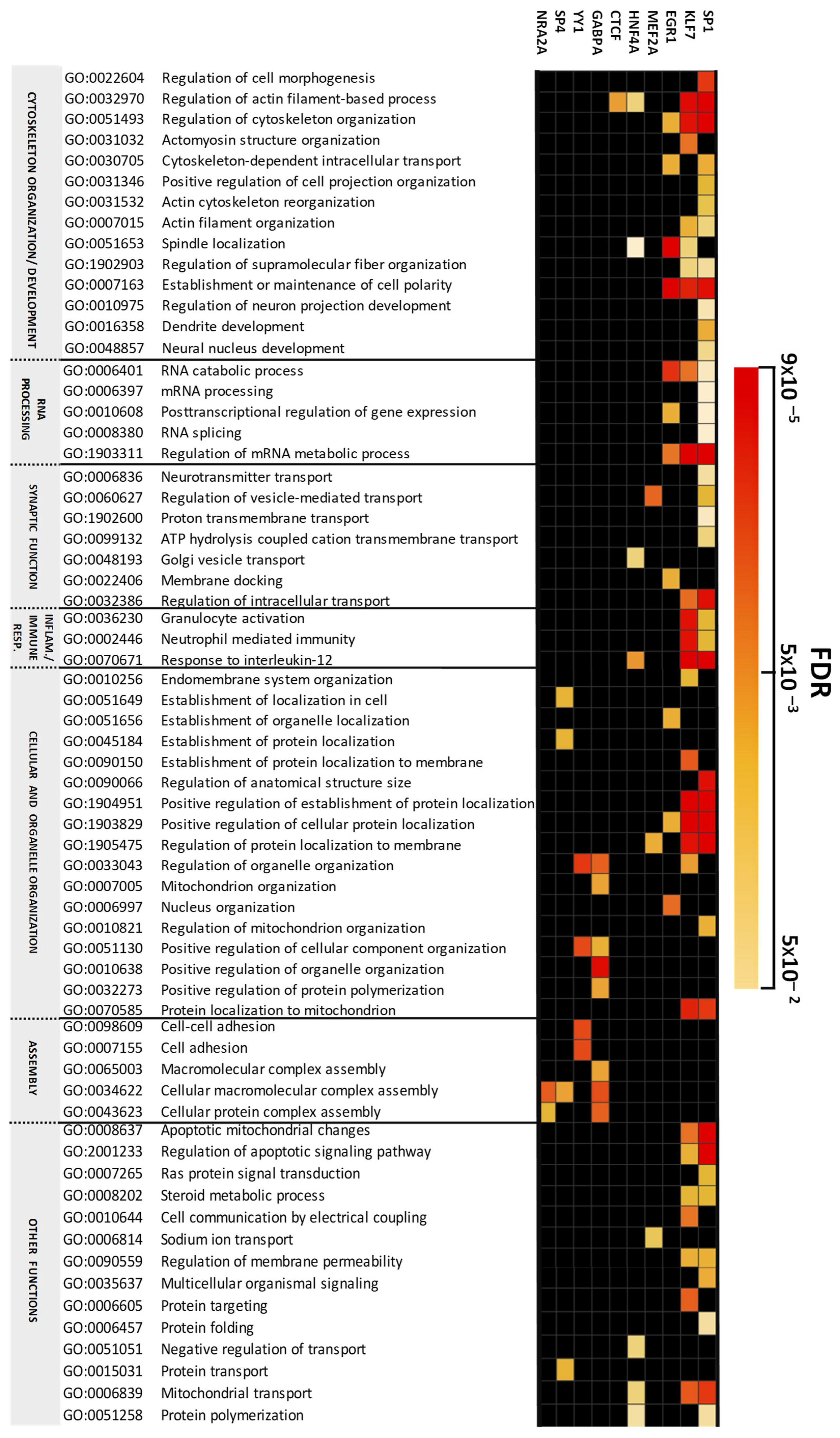
2.2. Altered Biological Processes Controlled by Transcriptional Programs in the Cerebellum in Chronic Schizophrenia
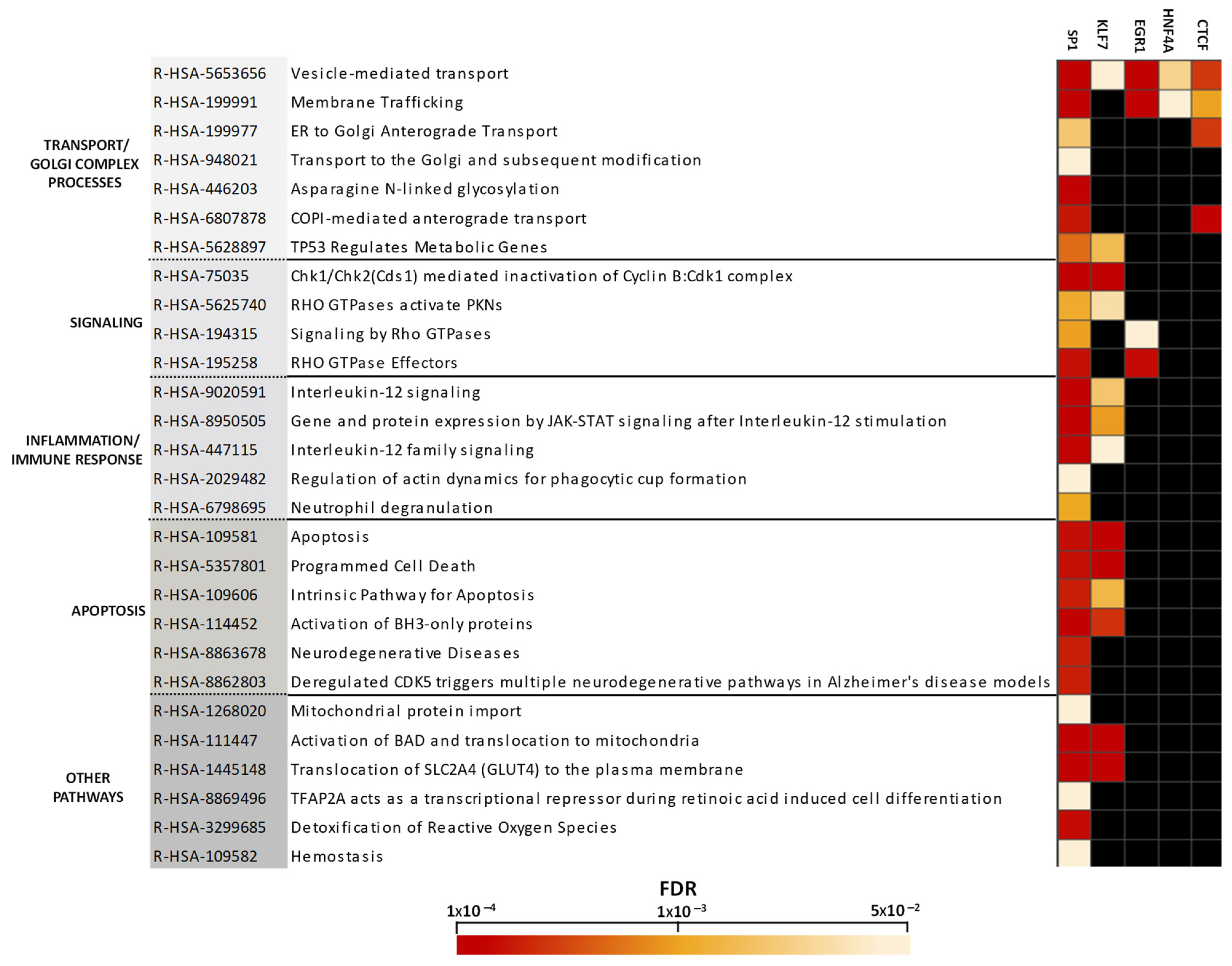
2.3. Altered Pathway Analysis Controlled by Transcriptional Programs in the Cerebellum in Chronic Schizophrenia
3. Discussion
3.1. Transcription Factor-Dependently Enriched Biological Processes
3.1.1. Cytoskeleton and Organelle Organization
3.1.2. mRNA Processing and Splicing
3.1.3. Synaptic Function
3.2. Transcription Factor-Dependently Enriched Pathways
3.2.1. Transport and Golgi Complex
3.2.2. Immune Response and Inflammatory Processes
3.2.3. Apoptotic Events
3.2.4. Limitations of the Study
4. Materials and Methods
4.1. Postmortem Human Brain Tissue
4.2. Bioinformatic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SZ | Schizophrenia |
| CB | Cerebellum |
| CCTC | Cortico-thalamo-cerebellar circuit |
| CNS | Central nervous system |
| TFs | Transcription factors |
| NKX2-1 | Homeobox protein Nkx-2.1 |
| SP1 | Transcription factor Specificity Protein 1 |
| SP4 | Transcription factor Specificity Protein 4 |
| KLF7 | Krüppel-like factor 7 |
| EGR1 | Early growth response protein 1 |
| HNF4A | Hepatocyte nuclear factor 4-alpha |
| CTCF | Transcriptional repressor CTCFL |
| GABPA | GA-binding protein alpha chain |
| NRF1 | Endoplasmic reticulum membrane sensor NFE2L1 |
| NFYA | Nuclear transcription factor Y subunit alpha |
| MEF2A | Myocyte-specific enhancer factor 2A |
| YY1 | Transcriptional repressor protein YY1 |
References
- Murray, R.M.; Bhavsar, V.; Tripoli, G.; Howes, O. 30 Years on: How the Neurodevelopmental Hypothesis of Schizophrenia Morphed into the Developmental Risk Factor Model of Psychosis. Schizophr. Bull. 2017, 43, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Eyre, H.; Jacka, F.N.; Dodd, S.; Dean, O.; McEwen, S.; Debnath, M.; McGrath, J.; Maes, M.; Amminger, P.; et al. A review of vulnerability and risks for schizophrenia: Beyond the two hit hypothesis. Neurosci. Biobehav. Rev. 2016, 65, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, J.L.; Giedd, J.N.; Gogtay, N. Neurodevelopmental model of schizophrenia: Update 2012. Mol. Psychiatry 2012, 17, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Guerrin, C.G.; Doorduin, J.; Sommer, I.E.; de Vries, E.F. The dual hit hypothesis of schizophrenia: Evidence from animal models. Neurosci. Biobehav. Rev. 2021, 131, 1150–1168. [Google Scholar] [CrossRef]
- Andreasen, N.C.; Daniel, S.; Leary, S.O.; Paradiso, S. Cognitive of “Cognitive Dysmetria“ as an Integrative Theory of Dysfunction in Cortical- Schizophrenia: A Dysfunction Subcortical—Cerebellar Circuitry? Network 2018, 24, 203–218. [Google Scholar] [CrossRef]
- Andreasen, N.C.; Nopoulos, P.; O’Leary, D.S.; Miller, D.D.; Wassink, T.; Flaum, M. Defining the phenotype of schizophrenia: Cognitive dysmetria and its neural mechanisms. Biol. Psychiatry 1999, 46, 908–920. [Google Scholar] [CrossRef]
- Bernard, J.A.; Orr, J.M.; Mittal, V.A. Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. NeuroImage Clin. 2017, 14, 622–628. [Google Scholar] [CrossRef]
- Kim, S.E.; Jung, S.; Sung, G.; Bang, M.; Lee, S.-H. Impaired cerebro-cerebellar white matter connectivity and its associations with cognitive function in patients with schizophrenia. Schizophrenia 2021, 7, 38. [Google Scholar] [CrossRef]
- Chen, P.; Ye, E.; Jin, X.; Zhu, Y.; Wang, L. Association between Thalamocortical Functional Connectivity Abnormalities and Cognitive Deficits in Schizophrenia. Sci. Rep. 2019, 9, 2952. [Google Scholar] [CrossRef]
- Pfaff, D.W. Neuroscience in the 21st century: From basic to clinical. In Neuroscience in the 21st Century: From Basic to Clinical; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–3111. [Google Scholar]
- Leto, K.; Arancillo, M.; Becker, E.B.E.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.B.; Dusart, I.; Haldipur, P.; Hatten, M.E.; et al. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef]
- Badowska, D.M.; Brzózka, M.M.; Kannaiyan, N.; Thomas, C.; Dibaj, P.; Chowdhury, A.; Steffens, H.; Turck, C.W.; Falkai, P.; Schmitt, A.; et al. Modulation of cognition and neuronal plasticity in gain- and loss-of-function mouse models of the schizophrenia risk gene Tcf4. Transl. Psychiatry 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Mesman, S.; Bakker, R.; Smidt, M.P. Tcf4 is required for correct brain development during embryogenesis. Mol. Cell. Neurosci. 2020, 106, 103502. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.; Gaudillière, B.; Bonni, A.; Gill, G. Transcription factor Sp4 regulates dendritic patterning during cerebellar maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 9882–9887. [Google Scholar] [CrossRef] [PubMed]
- Pinacho, R.; Valdizán, E.M.; Pilar-Cuellar, F.; Prades, R.; Tarragó, T.; Haro, J.M.; Ferrer, I.; Ramos, B. Increased SP4 and SP1 transcription factor expression in the postmortem hippocampus of chronic schizophrenia. J. Psychiatr. Res. 2014, 58, 189–196. [Google Scholar] [CrossRef]
- Ye, B.; Kim, J.H.; Yang, L.; McLachlan, I.; Younger, S.; Jan, L.Y.; Jan, Y.N. Differential regulation of dendritic and axonal development by the novel Krüppel-like factor dar1. J. Neurosci. 2011, 31, 3309–3319. [Google Scholar] [CrossRef]
- Hsueh, Y.P.; Hirata, Y.; Simmen, F.A.; Denver, R.J.; Ávila-Mendoza, J.; Subramani, A. Krüppel-Like Factors 9 and 13 Block Axon Growth by Transcriptional Repression of Key Components of the cAMP Signaling Pathway. Front. Mol. Neurosci. 2020, 13, 602638. [Google Scholar]
- Malt, E.A.; Juhasz, K.; Malt, U.F.; Naumann, T. A role for the transcription factor Nk2 homeobox 1 in schizophrenia: Convergent evidence from animal and human studies. Front. Behav. Neurosci. 2016, 10, 59. [Google Scholar] [CrossRef]
- Vera-Montecinos, A.; Rodríguez-Mias, R.; MacDowell, K.S.; García-Bueno, B.; Bris, Á.G.; Caso, J.R.; Villén, J.; Ramos, B. Analysis of Molecular Networks in the Cerebellum in Chronic Schizophrenia: Modulation by Early Postnatal Life Stressors in Murine Models. Int. J. Mol. Sci. 2021, 22, 10076. [Google Scholar] [CrossRef]
- Ben-Shachar, D. The interplay between mitochondrial complex I, dopamine and Sp1 in schizophrenia. J Neural. Transm. 2009, 116, 1383–1396. [Google Scholar] [CrossRef]
- Fusté, M.; Meléndez-Pérez, I.; Villalta-Gil, V.; Pinacho, R.; Villalmanzo, N.; Cardoner, N.; Menchón, J.M.; Haro, J.M.; Soriano-Mas, C.; Ramos, B. Specificity proteins 1 and 4, hippocampal volume and first-episode psychosis. Br. J. Psychiatry 2016, 208, 591–592. [Google Scholar] [CrossRef]
- Fusté, M.; Pinacho, R.; Meléndez-Pérez, I.; Villalmanzo, N.; Villalta-Gil, V.; Haro, J.M.; Ramos, B. Reduced expression of SP1 and SP4 transcription factors in peripheral blood mononuclear cells in first-episode psychosis. J. Psychiatr. Res. 2013, 47, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, K.; Wang, Q.; Li, Z.; Shen, J.; Li, T.; Wang, M.; Wen, Z.; Li, W.; Qiang, Y.; et al. Role played by the SP4 gene in schizophrenia and major depressive disorder in the Han Chinese population. Br. J. Psychiatry 2016, 208, 441–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinacho, R.; Villalmanzo, N.; Roca, M.; Iniesta, R.; Monje, A.; Haro, J.M.; Meana, J.J.; Ferrer, I.; Gill, G.; Ramos, B. Analysis of Sp transcription factors in the postmortem brain of chronic schizophrenia: A pilot study of relationship to negative symptoms. J. Psychiatry Res. 2013, 47, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Pinacho, R.; Saia, G.; Meana, J.J.; Gill, G.; Ramos, B. Transcription factor SP4 phosphorylation is altered in the postmortem cerebellum of bipolar disorder and schizophrenia subjects. Eur. Neuropsychopharmacol. 2015, 25, 1650–1660. [Google Scholar] [CrossRef][Green Version]
- Saia, G.; Lalonde, J.; Sun, X.; Ramos, B.; Gill, G. Phosphorylation of the transcription factor Sp4 is reduced by NMDA receptor signaling. J. Neurochem. 2014, 129, 743–752. [Google Scholar] [CrossRef]
- Pinacho, R.; Saia, G.; Fusté, M.; Meléndez-Pérez, I.; Villalta-Gil, V.; Haro, J.M.; Gill, G.; Ramos, B. Phosphorylation of transcription factor specificity protein 4 is increased in peripheral blood mononuclear cells of first-episode psychosis. PLoS ONE 2015, 10, e0125115. [Google Scholar] [CrossRef][Green Version]
- Zhou, X. Over-representation of potential SP4 target genes within schizophrenia-risk genes. Mol. Psychiatry 2022, 27, 849–854. [Google Scholar] [CrossRef]
- Hu, T.-M.; Chen, S.-J.; Hsu, S.-H.; Cheng, M.-C. Functional analyses and effect of DNA methylation on the EGR1 gene in patients with schizophrenia. Psychiatry Res. 2019, 275, 276–282. [Google Scholar] [CrossRef]
- Ramaker, R.C.; Bowling, K.M.; Lasseigne, B.N.; Hagenauer, M.H.; Hardigan, A.A.; Davis, N.S.; Gertz, J.; Cartagena, P.M.; Walsh, D.M.; Vawter, M.P.; et al. Post-mortem molecular profiling of three psychiatric disorders. Genome Med. 2017, 9, 72. [Google Scholar] [CrossRef]
- Iwakura, Y.; Kawahara-Miki, R.; Kida, S.; Sotoyama, H.; Gabdulkhaev, R.; Takahashi, H.; Kunii, Y.; Hino, M.; Nagaoka, A.; Izumi, R.; et al. Elevation of EGR1/zif268, a Neural Activity Marker, in the Auditory Cortex of Patients with Schizophrenia and its Animal Model. Neurochem. Res. 2022, 47, 2715–2727. [Google Scholar] [CrossRef]
- Vawter, M.P.; Mamdani, F.; Macciardi, F. An integrative functional genomics approach for discovering biomarkers in Schizophrenia. Brief. Funct. Genom. 2011, 10, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Juraeva, D.; Haenisch, B.; Zapatka, M.; Frank, J.; GROUP Investigators; PSYCH-GEMS SCZ Working Group; Witt, S.H.; Mühleisen, T.W.; Treutlein, J.; Strohmaier, J.; et al. Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia. PLoS Genet. 2014, 10, e1004345. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Li, S.; Liu, J.; Li, X.; Luo, X.-J. Functional genomics reveal gene regulatory mechanisms underlying schizophrenia risk. Nat. Commun. 2019, 10, 670. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Liu, J.; Wang, J.; Li, X.; Huo, Y.; Li, Y.; Liu, Y.; Li, M.; Xiao, X.; et al. Regulatory variants at 2q33.1 confer schizophrenia risk by modulating distal gene TYW5 expression. Brain 2022, 145, 770–786. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, L.J.; Lucas, E.K.; Meador-Woodruff, J.H.; McCullumsmith, R.E.; Hendrickson, R.C.; Gamble, K.L.; Cowell, R.M. Cortical PGC-1α-Dependent Transcripts Are Reduced in Postmortem Tissue from Patients with Schizophrenia. Schizophr. Bull. 2016, 42, 1009–1017. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Ma, X.; Long, Q.; Yu, L.; Chen, Y.; Wu, W.; Guo, Z.; Teng, Z.; Zeng, Y. The NRF1/miR-4514/SOCS3 Pathway Is Associated with Schizophrenia Pathogenesis. Clin. Neurol. Neurosci. 2021, 5, 82. [Google Scholar] [CrossRef]
- Smeland, O.B.; Frei, O.; Kauppi, K.; Hill, W.D.; Li, W.; Wang, Y.; Krull, F.; Bettella, F.; Eriksen, J.A.; Witoelar, A.; et al. Identification of genetic loci jointly influencing schizophrenia risk and the cognitive traits of verbal-numerical reasoning, reaction time, and general cognitive function. JAMA Psychiatry 2017, 74, 1065–1075. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Javidfar, B.; Pothula, V.; Ibi, D.; Shen, E.Y.; Peter, C.J.; Bicks, L.K.; Fehr, T.; Jiang, Y.; Brennand, K.J.; et al. MEF2C transcription factor is associated with the genetic and epigenetic risk architecture of schizophrenia and improves cognition in mice. Mol. Psychiatry 2018, 23, 123–132. [Google Scholar] [CrossRef]
- Kimoto, S.; Bazmi, H.H.; Lewis, D.A. Lower Expression of Glutamic Acid Decarboxylase 67 in the Prefrontal Cortex in Schizophrenia: Contribution of Altered Regulation by Zif268. Am. J. Psychiatry 2014, 171, 969–978. [Google Scholar] [CrossRef]
- Yamada, K.; Gerber, D.J.; Iwayama, Y.; Ohnishi, T.; Ohba, H.; Toyota, T.; Arugaet, J.; Minabe, Y.; Tonegawa, S.; Yoshikawa, T. Genetic analysis of the calcineurin pathway identifies members of the EGR gene family, specifically EGR3, as potential susceptibility candidates in schizophrenia. Proc. Natl. Acad. Sci. USA 2007, 104, 2815–2820. [Google Scholar] [CrossRef]
- van Vliet, J.; Crofts, L.A.; Quinlan, K.G.; Czolij, R.; Perkins, A.C.; Crossley, M. Human KLF17 is a new member of the Sp/KLF family of transcription factors. Genomics 2006, 87, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xu, J.; Chen, J.; Kim, S.; Reimers, M.; Bacanu, S.-A.; Yu, H.; Liu, C.; Sun, J.; Wang, Q.; et al. Transcriptome sequencing and genome-wide association analyses reveal lysosomal function and actin cytoskeleton remodeling in schizophrenia and bipolar disorder. Mol. Psychiatry 2015, 20, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-Y.; Hsu, T.-I.; Chuang, J.-Y.; Su, T.-P.; Chang, W.-C.; Hung, J.-J. Sp1 in Astrocyte Is Important for Neurite Outgrowth and Synaptogenesis. Mol. Neurobiol. 2020, 57, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.; Valín, A.; Sun, X.; Gill, G. Sp4-dependent repression of neurotrophin-3 limits dendritic branching. Mol. Cell. Neurosci. 2009, 42, 152–159. [Google Scholar] [CrossRef]
- Laub, F.; Lei, L.; Sumiyoshi, H.; Kajimura, D.; Dragomir, C.; Smaldone, S.; Puche, A.C.; Petros, T.J.; Mason, C.; Parada, L.F.; et al. Transcription Factor KLF7 Is Important for Neuronal Morphogenesis in Selected Regions of the Nervous System. Mol. Cell. Biol. 2005, 25, 5699–5711. [Google Scholar] [CrossRef]
- Laub, F.; Aldabe, R.; Friedrich, V.; Ohnishi, S.; Yoshida, T.; Ramirez, F. Developmental expression of mouse Krüppel-like transcription factor KLF7 suggests a potential role in neurogenesis. Dev. Biol. 2001, 233, 305–318. [Google Scholar] [CrossRef]
- Barry, G.; A Briggs, J.; Vanichkina, D.P.; Poth, E.M.; Beveridge, N.J.; Ratnu, V.S.; Nayler, S.P.; Nones, K.; Hu, J.; Bredy, T.W.; et al. The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol. Psychiatry 2014, 19, 486–494. [Google Scholar] [CrossRef]
- Saia-Cereda, V.M.; Santana, A.G.; Schmitt, A.; Falkai, P.; Martins-De-Souza, D. The Nuclear Proteome of White and Gray Matter from Schizophrenia Postmortem Brains. Complex Psychiatry 2017, 3, 37–52. [Google Scholar] [CrossRef]
- Nakata, K.; Lipska, B.K.; Hyde, T.M.; Ye, T.; Newburn, E.N.; Morita, Y.; Vakkalanka, R.; Barenboim, M.; Sei, Y.; Weinberger, D.R.; et al. DISC1 splice variants are upregulated in schizophrenia and associated with risk polymorphisms. Proc. Natl. Acad. Sci. USA 2009, 106, 15873–15878. [Google Scholar] [CrossRef]
- Law, A.J.; Kleinman, J.E.; Weinberger, D.R.; Weickert, C.S. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum. Mol. Genet. 2007, 16, 129–141. [Google Scholar] [CrossRef]
- Alberstein, M.; Amit, M.; Vaknin, K.; O’Donnell, A.; Farhy, C.; Lerenthal, Y.; Shomron, N.; Shaham, O.; Sharrocks, A.D.; Ashery-Padan, R.; et al. Regulation of transcription of the RNA splicing factor hSlu7 by Elk-1 and Sp1 affects alternative splicing. RNA 2007, 13, 1988–1999. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shalizi, A.; Gaudillière, B.; Yuan, Z.; Stegmüller, J.; Shirogane, T.; Ge, Q.; Tan, Y.; Schulman, B.; Harper, J.W.; Bonni, A. A Calcium-Regulated MEF2 Sumoylation Switch Controls Postsynaptic Differentiation. Science 2006, 311, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Lisek, M.; Przybyszewski, O.; Zylinska, L.; Guo, F.; Boczek, T. The Role of MEF2 Transcription Factor Family in Neuronal Survival and Degeneration. Int. J. Mol. Sci. 2023, 24, 3120. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, C.; Drago, A.; Calabrò, M.; Spina, E.; Serretti, A. A molecular pathway analysis informs the genetic background at risk for schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 59, 21–30. [Google Scholar] [CrossRef]
- Thygesen, J.H.; Zambach, S.K.; Ingason, A.; Lundin, P.; Hansen, T.; Bertalan, M.; Rosengren, A.; Bjerre, D.; Ferrero-Miliani, L.; Rasmussen, H.B.; et al. Linkage and whole genome sequencing identify a locus on 6q25–26 for formal thought disorder and implicate MEF2A regulation. Schizophr. Res. 2015, 169, 441–446. [Google Scholar] [CrossRef]
- Carmichael, R.E.; Wilkinson, K.A.; Craig, T.J.; Ashby, M.C.; Henley, J.M. MEF2A regulates mGluR-dependent AMPA receptor trafficking independently of Arc/Arg3.1. Sci. Rep. 2018, 8, 5263. [Google Scholar] [CrossRef]
- Chi, X.; Wang, S.; Huang, Y.; Stamnes, M.; Chen, J.-L. Roles of Rho GTPases in intracellular transport and cellular transformation. Int. J. Mol. Sci. 2013, 14, 7089–7108. [Google Scholar] [CrossRef]
- Mo, J.; Kim, C.H.; Lee, D.; Sun, W.; Lee, H.W.; Kim, H. Early growth response 1 (Egr-1) directly regulates GABAA receptor α2, α4, and θ subunits in the hippocampus. J. Neurochemistry. 2015, 133, 489–500. [Google Scholar] [CrossRef]
- Duclot, F.; Kabbaj, M. The role of early growth response 1 (EGR1) in brain plasticity and neuropsychiatric disorders. Front. Behav. Neurosci. 2017, 11, 35. [Google Scholar] [CrossRef]
- Watson, P.; Stephens, D.J. ER-to-Golgi transport: Form and formation of vesicular and tubular carriers. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2005, 1744, 304–315. [Google Scholar] [CrossRef]
- Qin, F.; Chen, G.; Yu, K.N.; Yang, M.; Cao, W.; Kong, P.; Peng, S.; Sun, M.; Nie, L.; Han, W. Golgi Phosphoprotein 3 Mediates Radiation-Induced Bystander Effect via ERK/EGR1/TNF-α Signal Axis. Antioxidants 2022, 11, 2172. [Google Scholar] [CrossRef] [PubMed]
- Etemadik, M.; Nia, A.; Wetter, L.; Feuk, L. Transcriptome analysis of fibroblasts from schizophrenia patients reveals differential expression of schizophrenia-related genes. Sci. Rep. 2020, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Altamura, A.C.; Pozzoli, S.; Fiorentini, A.; Dell’Osso, B. Neurodevelopment and inflammatory patterns in schizophrenia in relation to pathophysiology. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 42, 63–70. [Google Scholar] [CrossRef]
- Meyer, J.M.; McEvoy, J.P.; Davis, V.G.; Goff, D.C.; Nasrallah, H.A.; Davis, S.M.; Hsiao, J.K.; Swartz, M.S.; Stroup, T.S.; Lieberman, J.A. Inflammatory Markers in Schizophrenia: Comparing Antipsychotic Effects in Phase 1 of the Clinical Antipsychotic Trials of Intervention Effectiveness Study. Biol. Psychiatry 2009, 66, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.S.; Phillips, M.R.; Yang, L.H.; Kegeles, L.S.; Susser, E.S.; Lieberman, J.A. Neurodegenerative model of schizophrenia: Growing evidence to support a revisit. Schizophr. Res. 2022, 243, 154–162. [Google Scholar] [CrossRef]
- Comer, A.L.; Carrier, M.; Tremblay, M.È.; Cruz-Martín, A. The Inflamed Brain in Schizophrenia: The Convergence of Genetic and Environmental Risk Factors That Lead to Uncontrolled Neuroinflammation. Front. Cell. Neurosci. 2020, 14, 274. [Google Scholar] [CrossRef]
- Gober, R.; Dallmeier, J.; Davis, D.; Brzostowicki, D.; Vaccari, J.P.d.R.; Cyr, B.; Barreda, A.; Sun, X.; Gultekin, S.H.; Garamszegi, S.; et al. Increased inflammasome protein expression identified in microglia from postmortem brains with schizophrenia. J. Neuropathol. Exp. Neurol. 2024, 83, 951–966. [Google Scholar] [CrossRef]
- Miller, B.J.; Goldsmith, D.R. Evaluating the Hypothesis That Schizophrenia Is an Inflammatory Disorder. Focus 2020, 18, 391–401. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: A reconceptualization. Transl. Psychiatry 2017, 7, e1024. [Google Scholar] [CrossRef]
- Hughes, H.; Ashwood, P. Overlapping evidence of innate immune dysfunction in psychotic and affective disorders. Brain Behav. Immun. Health 2020, 2, 100038. [Google Scholar] [CrossRef]
- Fond, G.; Lançon, C.; Korchia, T.; Auquier, P.; Boyer, L. The Role of Inflammation in the Treatment of Schizophrenia. Front. Psychiatry 2020, 11, 160. [Google Scholar] [CrossRef]
- Carril Pardo, C.; Oyarce Merino, K.; Vera-Montecinos, A. Neuroinflammatory Loop in Schizophrenia, Is There a Relationship with Symptoms or Cognition Decline? Int. J. Mol. Sci. 2025, 26, 310. [Google Scholar] [CrossRef]
- Cui, L.-B.; Wang, X.-Y.; Fu, Y.-F.; Liu, X.-F.; Wei, Y.; Zhao, S.-W.; Gu, Y.-W.; Fan, J.-W.; Wu, W.-J.; Gong, H.; et al. Transcriptional level of inflammation markers associates with short-term brain structural changes in first-episode schizophrenia. BMC Med. 2023, 21, 250. [Google Scholar] [CrossRef]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef]
- Yang, X.; Liang, M.; Tang, Y.; Ma, D.; Li, M.; Yuan, C.; Hou, Y.; Sun, C.; Liu, J.; Wei, Q.; et al. KLF7 promotes adipocyte inflammation and glucose metabolism disorder by activating the PKCζ/NF-κB pathway. FASEB J. 2023, 37, e23033. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wu, J.; Ha, X.; Deng, Y.; Zhang, X.; Wang, J.; Chen, K.; Feng, J.; Zhu, J.; et al. The effect and mechanism of KLF7 in the TLR4/NF-κB/IL-6 inflammatory signal pathway of adipocytes. Mediat. Inflamm. 2018, 2018, 1756494. [Google Scholar] [CrossRef]
- Borovac, J.; Bosch, M.; Okamoto, K. Regulation of actin dynamics during structural plasticity of dendritic spines: Signaling messengers and actin-binding proteins. Mol. Cell. Neurosci. 2018, 91, 122–130. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, B.; Han, H.-B. Serum interleukin-6 in schizophrenia: A system review and meta-analysis. Cytokine 2021, 141, 155441. [Google Scholar] [CrossRef]
- Nayak, L.; Goduni, L.; Takami, Y.; Sharma, N.; Kapil, P.; Jain, M.K.; Mahabeleshwar, G.H. Kruppel-like factor 2 is a transcriptional regulator of chronic and acute inflammation. Am. J. Pathol. 2013, 182, 1696–1704. [Google Scholar] [CrossRef]
- Luo, W.-W.; Lian, H.; Zhong, B.; Shu, H.-B.; Li, S. Krüppel-like factor 4 negatively regulates cellular antiviral immune response. Cell. Mol. Immunol. 2016, 13, 65–72. [Google Scholar] [CrossRef]
- Date, D.; Das, R.; Narla, G.; Simon, D.I.; Jain, M.K.; Mahabeleshwar, G.H. Kruppel-like transcription factor 6 regulates inflammatory macrophage polarization. J. Biol. Chem. 2014, 289, 10318–10329. [Google Scholar] [CrossRef]
- Wu, Z.; Kim, H.-P.; Xue, H.-H.; Liu, H.; Zhao, K.; Leonard, W.J. Interleukin-21 Receptor Gene Induction in Human T Cells Is Mediated by T-Cell Receptor-Induced Sp1 Activity. Mol. Cell. Biol. 2005, 25, 9741–9752. [Google Scholar] [CrossRef]
- El-Said, H.; Fayyad-Kazan, M.; Aoun, R.; Borghol, N.; Skafi, N.; Rouas, R.; Vanhamme, L.; Mourtada, M.; Ezzeddine, M.; Burny, A.; et al. MiR302c, Sp1, and NFATc2 regulate interleukin-21 expression in human CD4+CD45RO+ T lymphocytes. J. Cell. Physiology 2019, 234, 5998–6011. [Google Scholar] [CrossRef]
- Shbeer, A.M.; Robadi, I.A. The role of Interleukin-21 in autoimmune Diseases: Mechanisms, therapeutic Implications, and future directions. Cytokine 2024, 173, 156437. [Google Scholar] [CrossRef]
- Sun, H.-J.; Xu, X.; Wang, X.-L.; Wei, L.; Li, F.; Lu, J.; Huang, B.-Q. Transcription factors Ets2 and Sp1 act synergistically with histone acetyltransferase p300 in activating human interleukin-12 p40 promoter. Acta Biochim. Biophys. Sin. 2006, 38, 194–200. [Google Scholar] [CrossRef]
- Powell, M.D.; Read, K.A.; Sreekumar, B.K.; Jones, D.M.; Oestreich, K.J. IL-12 signaling drives the differentiation and function of a TH1-derived TFH1-like cell population. Sci. Rep. 2019, 9, 13991. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Suh, I.B.; Kim, H.; Han, C.S.; Lim, C.S.; Choi, S.H.; Licinio, J. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: Effects of psychotropic drugs. Mol. Psychiatry 2002, 7, 1107–1114. [Google Scholar] [CrossRef]
- Ozbey, U.; Tug, E.; Kara, M.; Namli, M. The value of interleukin-12B (p40) gene promoter polymorphism in patients with schizophrenia in a region of East Turkey. Psychiatry Clin. Neurosci. 2008, 62, 307–312. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, Y.; Kwon, H.J.; Kim, D.S. Sp1-associated activation of macrophage inflammatory protein-2 promoter by CpG-oligodeoxynucleotide and lipopolysaccharide. Cell. Mol. Life Sci. 2005, 62, 188–198. [Google Scholar] [CrossRef]
- Iwanaszko, M.; Kimmel, M. NF-κB and IRF pathways: Cross-regulation on target genes promoter level. BMC Genom. 2015, 16, 307. [Google Scholar] [CrossRef]
- MacDowell, K.S.; Pinacho, R.; Leza, J.C.; Costa, J.; Ramos, B.; García-Bueno, B. Differential regulation of the TLR4 signalling pathway in post-mortem prefrontal cortex and cerebellum in chronic schizophrenia: Relationship with SP transcription factors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 481–492. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, B.F.; Faux, S.F.; McCarley, R.W.; Kimble, M.O.; Salisbury, D.F.; Nestor, P.G.; Kikinis, R.; Jolesz, F.A.; Shenton, M.E. Increased Rate of P300 Latency Prolongation with Age in Schizophrenia: Electrophysiological Evidence for a Neurodegenerative Process. Arch Gen Psychiatry 1995, 52, 544–549. [Google Scholar] [CrossRef]
- Morén, C.; Treder, N.; Martínez-Pinteño, A.; Rodríguez, N.; Arbelo, N.; Madero, S.; Gómez, M.; Mas, S.; Gassó, P.; Parellada, E. Systematic Review of the Therapeutic Role of Apoptotic Inhibitors in Neurodegeneration and Their Potential Use in Schizophrenia. Antioxidants 2022, 11, 2275. [Google Scholar] [CrossRef]
- Glantz, L.A.; Gilmore, J.H.; Lieberman, J.A.; Jarskog, L.F. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr. Res. 2005, 81, 47–63. [Google Scholar] [CrossRef]
- Parellada, E.; Gassó, P. Glutamate and microglia activation as a driver of dendritic apoptosis: A core pathophysiological mechanism to understand schizophrenia. Transl. Psychiatry 2021, 11, 271. [Google Scholar] [CrossRef]
- Deniaud, E.; Baguet, J.; Mathieu, A.-L.; Pagès, G.; Marvel, J.; Leverrier, Y. Overexpression of Sp1 transcription factor induces apoptosis. Oncogene 2006, 25, 7096–7105. [Google Scholar] [CrossRef]
- Torabi, B.; Flashner, S.; Beishline, K.; Sowash, A.; Donovan, K.; Bassett, G.; Azizkhan-Clifford, J. Caspase cleavage of transcription factor Sp1 enhances apoptosis. Apoptosis 2018, 23, 65–78. [Google Scholar] [CrossRef]
- Deniaud, E.; Baguet, J.; Chalard, R.; Blanquier, B.; Brinza, L.; Meunier, J.; Michallet, M.-C.; Laugraud, A.; Ah-Soon, C.; Wierinckx, A.; et al. Overexpression of Transcription Factor Sp1 Leads to Gene Expression Perturbations and Cell Cycle Inhibition. PLoS ONE 2009, 4, e7035. [Google Scholar] [CrossRef]
- Lei, L.; Laub, F.; Lush, M.; Romero, M.; Zhou, J.; Luikart, B.; Klesse, L.; Ramirez, F.; Parada, L.F. The zinc finger transcription factor Klf7 is required for TrkA gene expression and development of nociceptive sensory neurons. Genes Dev. 2005, 19, 1354–1364. [Google Scholar] [CrossRef]
- Mallipattu, S.K.; Horne, S.J.; D’agati, V.; Narla, G.; Liu, R.; Frohman, M.A.; Dickman, K.; Chen, E.Y.; Ma’ayan, A.; Bialkowska, A.B.; et al. Krüppel-like factor 6 regulates mitochondrial function in the kidney. J. Clin. Investig. 2015, 125, 1347–1361. [Google Scholar] [CrossRef]
- Piret, S.E.; Guo, Y.; Attallah, A.A.; Horne, S.J.; Zollman, A.; Owusu, D.; Henein, J.; Sidorenko, V.S.; Revelo, M.P.; Hato, T.; et al. Krüppel-like factor 6–mediated loss of BCAA catabolism contributes to kidney injury in mice and humans. Proc. Natl. Acad. Sci. USA 2021, 118, e2024414118. [Google Scholar] [CrossRef]
- Xu, Q.; Kong, F.; Zhao, G.; Jin, J.; Feng, S.; Li, M. USP7 alleviates neuronal inflammation and apoptosis in spinal cord injury via deubiquitinating NRF1/KLF7 axis. Neurol. Res. 2024, 46, 1008–1017. [Google Scholar] [CrossRef]
- Jarskog, L.F.; Glantz, L.A.; Gilmore, J.H.; Lieberman, J.A. Apoptotic mechanisms in the pathophysiology of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 846–858. [Google Scholar] [CrossRef]
- Lidow, M.S.; Song, Z.-M.; A Castner, S.; Allen, P.B.; Greengard, P.; Goldman-Rakic, P.S. Antipsychotic treatment induces alterations in dendrite- and spine-associated proteins in dopamine-rich areas of the primate cerebral cortex. Biol. Psychiatry 2001, 49, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Duncan, D.; Shi, Z.; Zhang, B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): Update 2013. Nucleic Acids Res. 2013, 41, W77–W83. [Google Scholar] [CrossRef]
- Gardner, D.M.; Murphy, A.L.; O’Donnell, H.; Centorrino, F.; Baldessarini, R.J. International consensus study of antipsychotic dosing. Am. J. Psychiatry 2010, 167, 686–693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Montecinos, A.; Ramos, B. Transcriptional Regulators in the Cerebellum in Chronic Schizophrenia: Novel Possible Targets for Pharmacological Interventions. Int. J. Mol. Sci. 2025, 26, 3653. https://doi.org/10.3390/ijms26083653
Vera-Montecinos A, Ramos B. Transcriptional Regulators in the Cerebellum in Chronic Schizophrenia: Novel Possible Targets for Pharmacological Interventions. International Journal of Molecular Sciences. 2025; 26(8):3653. https://doi.org/10.3390/ijms26083653
Chicago/Turabian StyleVera-Montecinos, América, and Belén Ramos. 2025. "Transcriptional Regulators in the Cerebellum in Chronic Schizophrenia: Novel Possible Targets for Pharmacological Interventions" International Journal of Molecular Sciences 26, no. 8: 3653. https://doi.org/10.3390/ijms26083653
APA StyleVera-Montecinos, A., & Ramos, B. (2025). Transcriptional Regulators in the Cerebellum in Chronic Schizophrenia: Novel Possible Targets for Pharmacological Interventions. International Journal of Molecular Sciences, 26(8), 3653. https://doi.org/10.3390/ijms26083653




