Short-Chain Fatty Acids (SCFAs) Modulate the Hepatic Glucose and Lipid Metabolism of Coilia nasus via the FFAR/AMPK Signaling Pathway In Vitro
Abstract
1. Introduction
2. Results
2.1. Effects of High Glucose on the Hepatocytes of C. nasus
2.2. Effects of High Lipid on the Hepatocytes of C. nasus
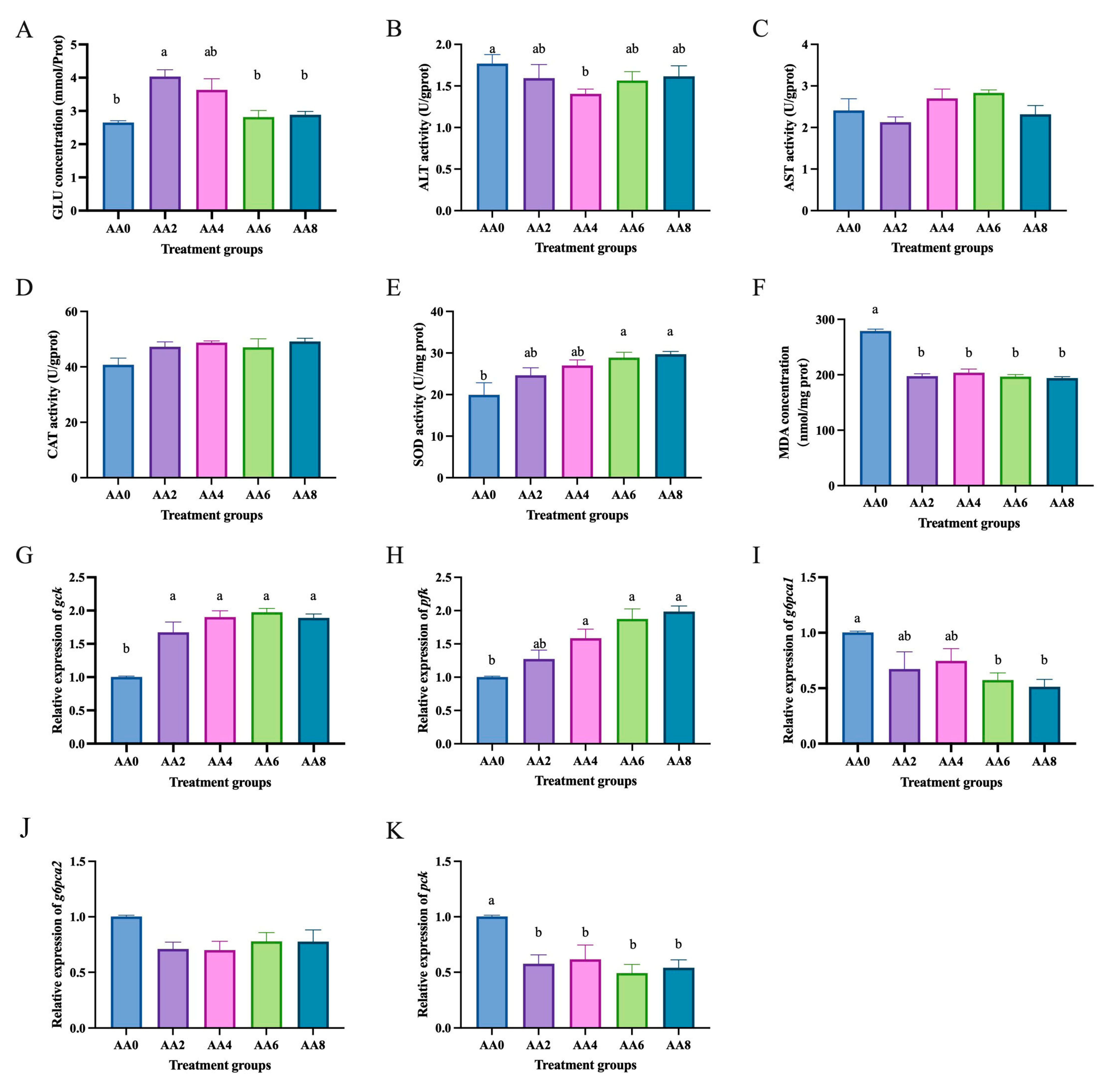
2.3. Effects of Short-Chain Fatty Acids on Hepatocytes of C. nasus Treated with High Glucose
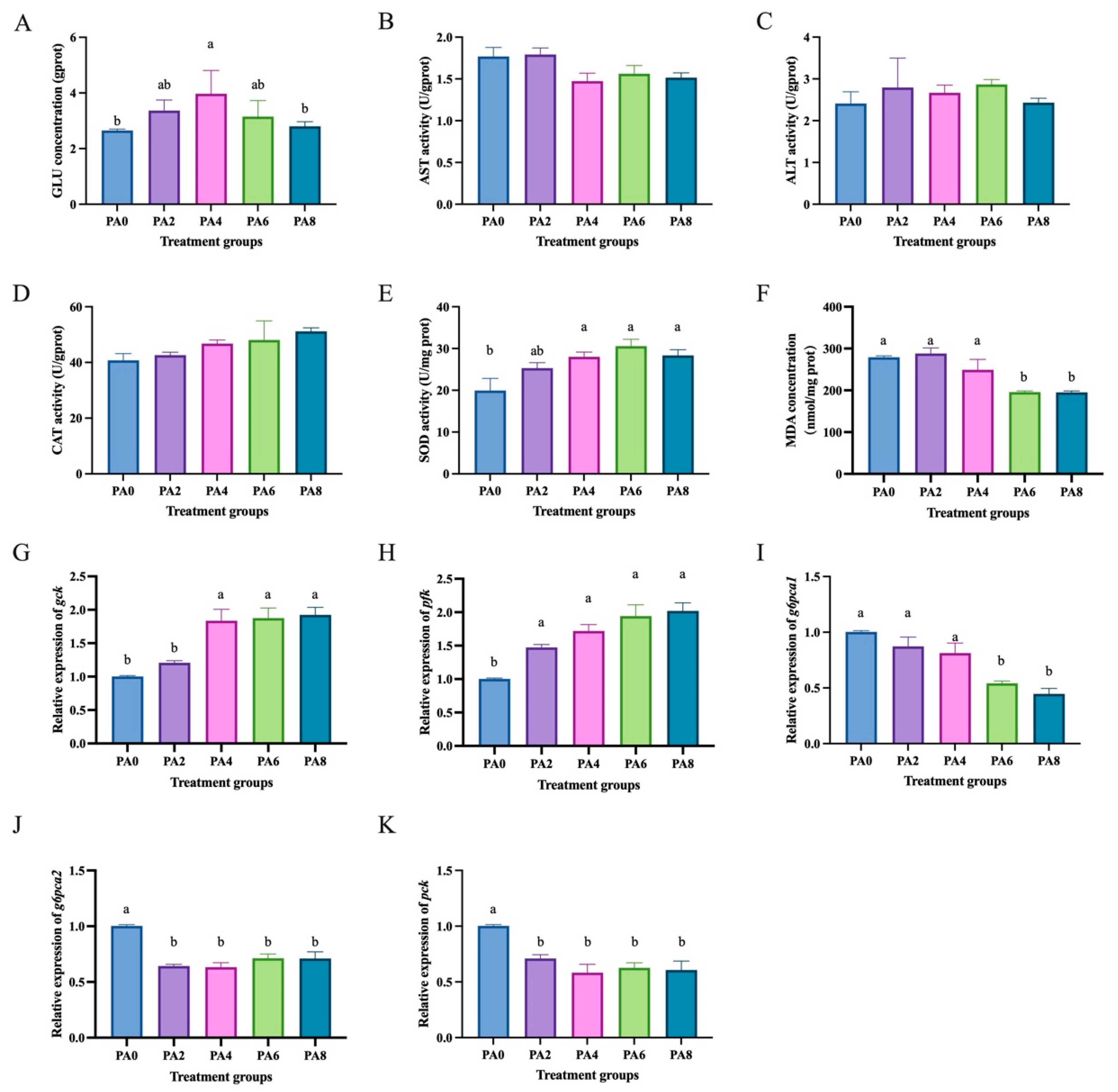
2.4. Effects of Short-Chain Fatty Acids on Hepatocytes of C. nasus Treated with High Lipids
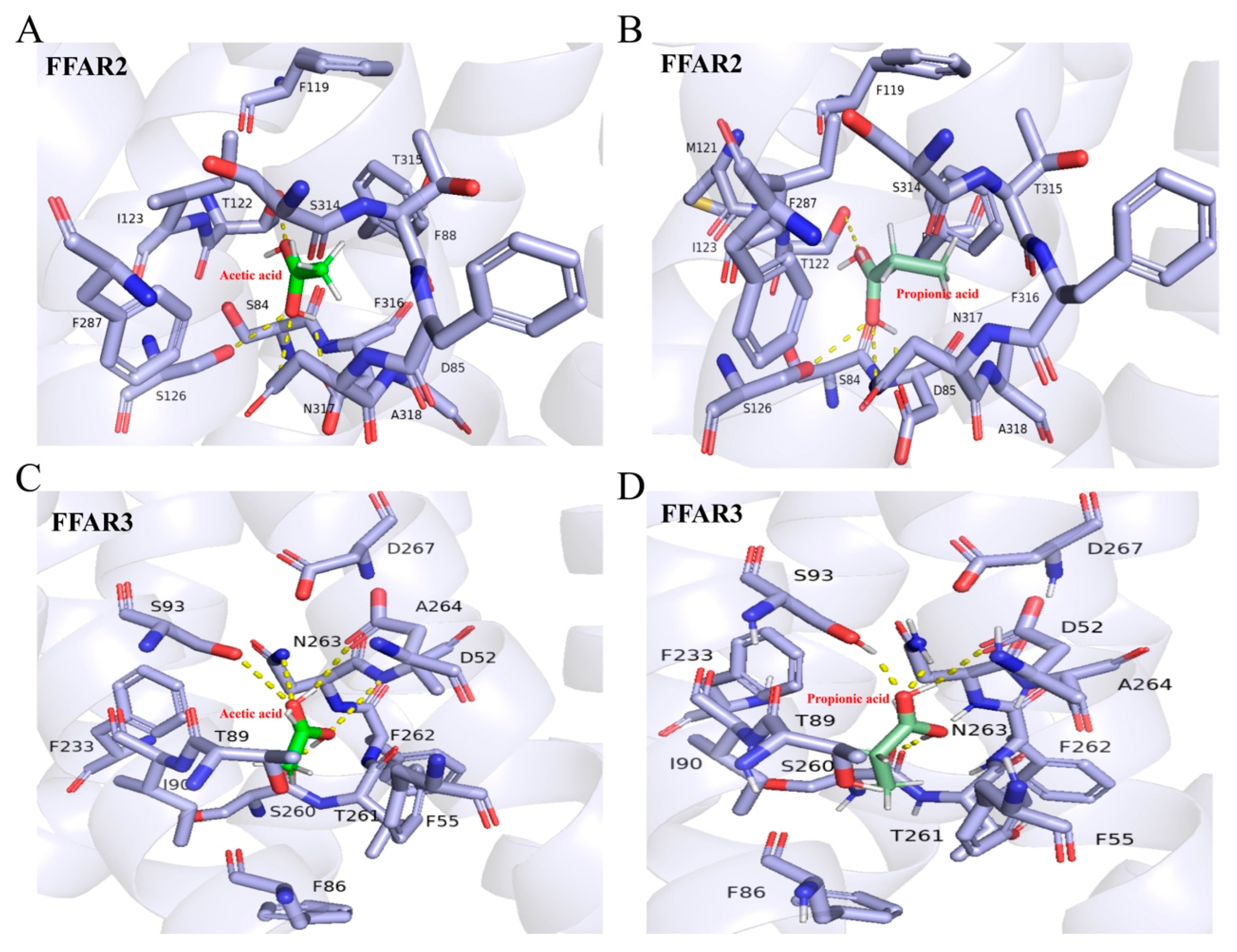
2.5. Molecular Docking Results of SCFAs with Free Fatty Acid Receptor (FFAR)
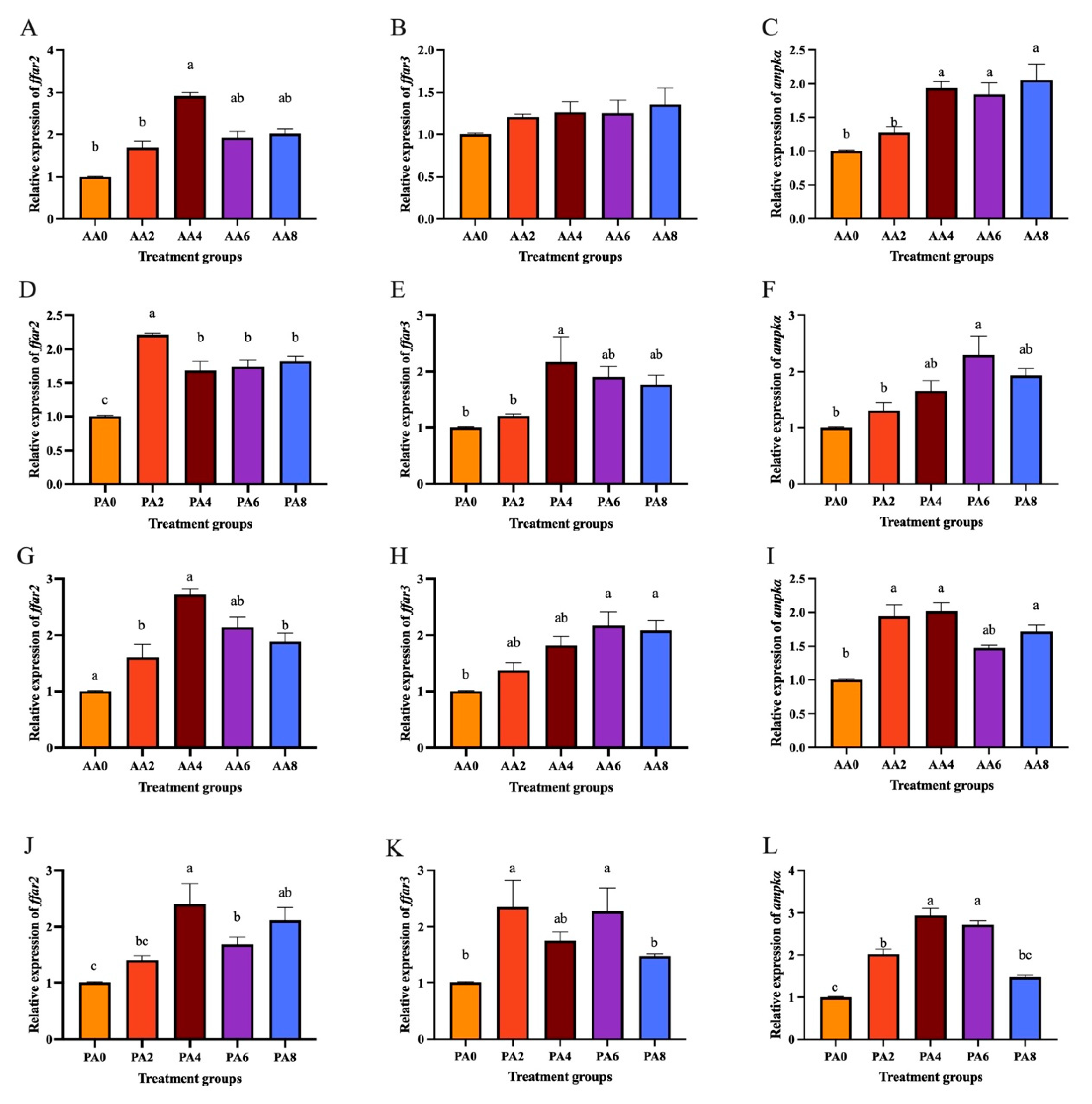
2.6. Expression of ffar2, ffar3, and ampkα Treated with SCFAs and High Glucose and Lipid in Hepatocytes of C. nasus
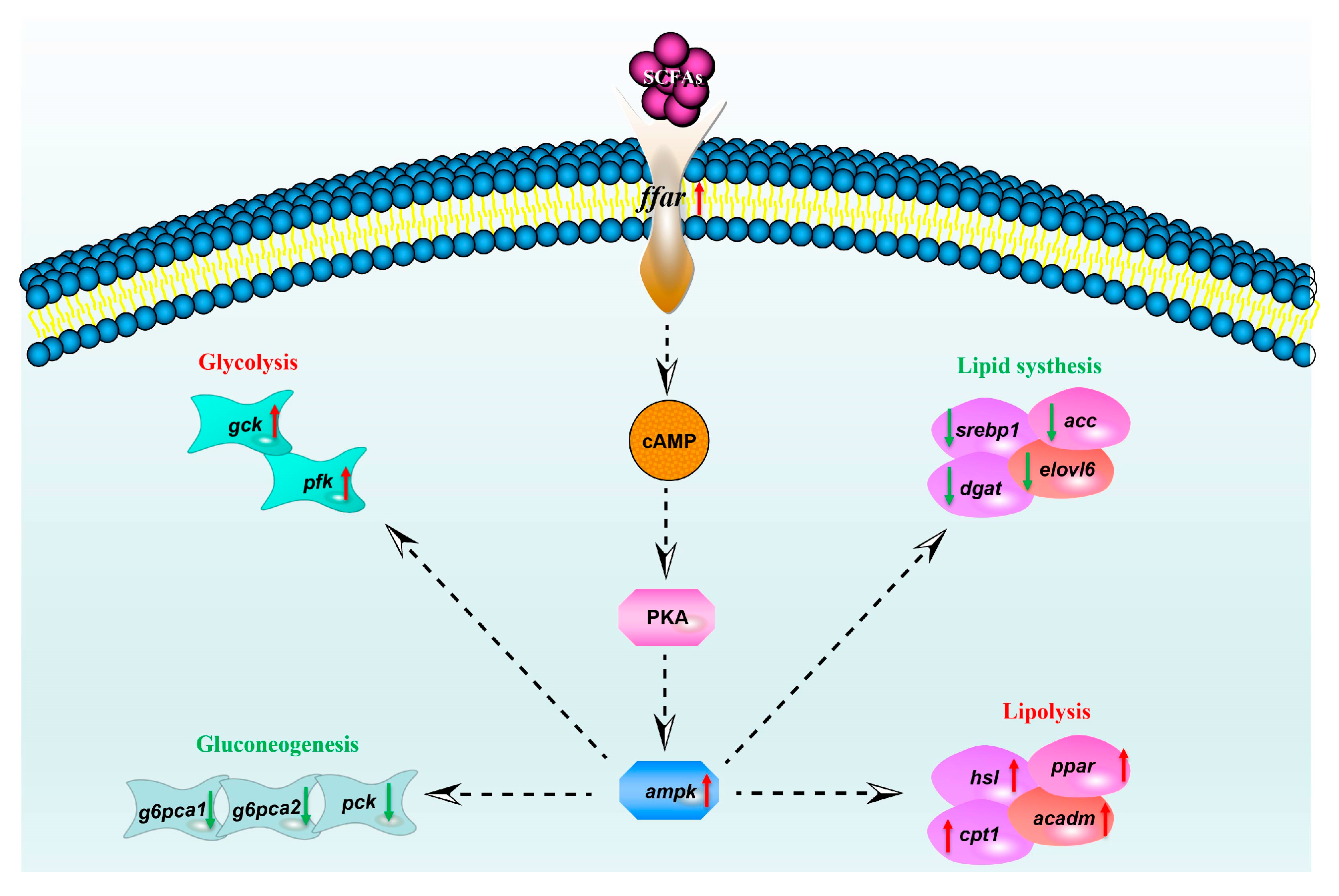
3. Discussion
4. Materials and Methods
4.1. Primary Culture of C. nasus Hepatocytes
4.2. Construction of High Glucose and High Lipid Models
4.3. SCFA Incubation Treatment
4.4. Cell Viability Assay
4.5. Detection of Glucose, Triglycerides, Cholesterol, and Immunoenzyme Activity
4.6. RT-qPCR Analysis of Hepatic Glucose and Lipid Metabolism Gene Expression
4.7. Data Analysis
4.8. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Fei, S.; Chen, Z.; Liu, H.; Jin, J.; Yang, Y.; Han, D.; Zhu, X.; Xie, S. Dietary carbohydrate to lipid ratio affects growth, reproductive performance and health of female yellow catfish (Pelteobagrus fulvidragrus): A lipidomics analysis. Anim. Nutr. 2024, 19, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-X.; Li, W.-J.; Shan, C.-J.; Zhang, Z.-Y.; Lv, H.-B.; Qiao, F.; Du, Z.-Y.; Zhang, M.-L. Oligosaccharides improve the flesh quality and nutrition value of Nile tilapia fed with high carbohydrate diet. Food Chem. Mol. Sci. 2021, 3, 100040. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Eslami, M.; Kalhor, N.; Zaretabar, A.; Mohammadzadeh, S.; Moghadam, M.S.; Yousefi, M.; Ahmadifar, M.; Hoseinifar, S.H.; Pusadee, T.; et al. Effect of a diet enriched with sodium propionate on growth performance, antioxidant property, innate-adaptive immune response, and growth-related genes expression in critically endangered beluga sturgeon (Huso huso). Fish Shellfish Immunol. 2022, 125, 101–108. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Zoheiri, F.; Caipang, C.M. Dietary sodium propionate improved performance, mucosal and humoral immune responses in Caspian white fish (Rutilus frisii kutum) fry. Fish Shellfish Immunol. 2016, 55, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, W.; Wu, F.; Qu, Q.; Tan, Q.; Gong, W. Dietary supplementation of sodium butyrate may benefit growth performance and intestinal function in juvenile grass carp (Ctenopharyngodon idellus). Aquac. Res. 2017, 48, 4102–4111. [Google Scholar] [CrossRef]
- Hong, J.; Jia, Y.; Pan, S.; Jia, L.; Li, H.; Han, Z.; Cai, D.; Zhao, R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 2016, 7, 56071–56082. [Google Scholar] [CrossRef]
- den Besten, G.; Gerding, A.; van Dijk, T.H.; Ciapaite, J.; Bleeker, A.; van Eunen, K.; Havinga, R.; Groen, A.K.; Reijngoud, D.-J.; Bakker, B.M. Protection against the Metabolic Syndrome by Guar Gum-Derived Short-Chain Fatty Acids Depends on Peroxisome Proliferator-Activated Receptor γ and Glucagon-Like Peptide-1. PLoS ONE 2015, 10, e0136364. [Google Scholar] [CrossRef]
- Merrill, G.F.; Kurth, E.J.; Hardie, D.G.; Winder, W.W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol.-Endocrinol. Metab. 1997, 273, E1107–E1112. [Google Scholar] [CrossRef]
- Sakakibara, S.; Yamauchi, T.; Oshima, Y.; Tsukamoto, Y.; Kadowaki, T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem. Biophys. Res. Commun. 2006, 344, 597–604. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986. [Google Scholar] [CrossRef]
- Kim, D.; Beck, B.R.; Heo, S.-B.; Kim, J.; Kim, H.D.; Lee, S.-M.; Kim, Y.; Oh, S.Y.; Lee, K.; Do, H.; et al. Lactococcus lactis BFE920 activates the innate immune system of olive flounder (Paralichthys olivaceus), resulting in protection against Streptococcus iniae infection and enhancing feed efficiency and weight gain in large-scale field studies. Fish Shellfish Immunol. 2013, 35, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, J.; Bu, X.; Zhang, H.; Zhang, J.; Zhang, X.; He, Y.; Wang, D.; Zhang, Z.; Meng, F. Effects of Polygonum cuspidatum on AMPK-FOXO3α Signaling Pathway in Rat Model of Uric Acid-Induced Renal Damage. Chin. J. Integr. Med. 2019, 25, 182–189. [Google Scholar] [CrossRef]
- Zambell, K.L.; Fitch, M.D.; Fleming, S.E. Acetate and butyrate are the major substrates for de novo lipogenesis in rat colonic epithelial cells. J. Nutr. 2003, 133, 3509–3515. [Google Scholar] [CrossRef] [PubMed]
- Kindt, A.; Liebisch, G.; Clavel, T.; Haller, D.; Hörmannsperger, G.; Yoon, H.; Kolmeder, D.; Sigruener, A.; Krautbauer, S.; Seeliger, C.; et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat. Commun. 2018, 9, 3760. [Google Scholar] [CrossRef]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269. [Google Scholar] [CrossRef]
- Huang, M.-Q.; Zhou, C.-J.; Zhang, Y.-P.; Zhang, X.-Q.; Xu, W.; Lin, J.; Wang, P.-J. Salvianolic Acid B Ameliorates Hyperglycemia and Dyslipidemia in db/db Mice through the AMPK Pathway. Cell. Physiol. Biochem. 2016, 40, 933–943. [Google Scholar] [CrossRef]
- Guo, L.; Kang, J.S.; Park, Y.H.; Je, B.I.; Lee, Y.J.; Kang, N.J.; Park, S.Y.; Hwang, D.Y.; Choi, Y.W. S-petasin inhibits lipid accumulation in oleic acid-induced HepG2 cells through activation of the AMPK signaling pathway. Food Funct. 2020, 11, 5664–5673. [Google Scholar] [CrossRef]
- Tang, T.; Song, J.; Li, J.; Wang, H.; Zhang, Y.; Suo, H. A synbiotic consisting of Lactobacillus plantarum S58 and hull-less barley β-glucan ameliorates lipid accumulation in mice fed with a high-fat diet by activating AMPK signaling and modulating the gut microbiota. Carbohydr. Polym. 2020, 243, 116398. [Google Scholar] [CrossRef]
- Yap, F.; Craddock, L.; Yang, J. Mechanism of AMPK Suppression of LXR-dependent Srebp-1c Transcription. Int. J. Biol. Sci. 2011, 7, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Mang, Q.; Gao, J.; Li, Q.; Sun, Y.; Xu, G.; Xu, P. Probiotics Enhance Coilia nasus Growth Performance and Nutritional Value by Regulating Glucolipid Metabolism via the Gut–Liver Axis. Int. J. Mol. Sci. 2024, 25, 2196. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Shan, Z.; Shi, L.; Duan, Y.; An, Y.; He, C.; Lyu, Y.; Zhao, Y.; Wang, M.; Du, Y.; et al. Mulberry leaf polysaccharides ameliorate glucose and lipid metabolism disorders via the gut microbiota-bile acids metabolic pathway. Int. J. Biol. Macromol. 2024, 282, 136876. [Google Scholar] [CrossRef]
- Jia, R.; Hou, Y.; Zhang, L.; Li, B.; Zhu, J. Effects of Berberine on Lipid Metabolism, Antioxidant Status, and Immune Response in Liver of Tilapia (Oreochromis niloticus) under a High-Fat Diet Feeding. Antioxidants 2024, 13, 548. [Google Scholar] [CrossRef]
- Li, L.; Pan, L.; Lin, Z.; Wen, J.; Tan, B.; Liu, H.; Hu, Y. Metformin improves insulin resistance, liver healthy and abnormal hepatic glucolipid metabolism via IR/PI3K/AKT pathway in Ctenopharyngodon idella fed a high-carbohydrate diet. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2024, 283, 109976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.-H.; Wu, C.-C.; Limbu, S.M.; Li, R.-X.; Chen, L.-Q.; Qiao, F.; Luo, Y.; Zhang, M.-L.; Han, T.; Du, Z.-Y. More simple more worse: Simple carbohydrate diets cause alterations in glucose and lipid metabolism in Nile tilapia (Oreochromis niloticus). Aquaculture 2022, 550, 737857. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, H.; Maulu, S.; Ge, X.; Ren, M.; Xie, J.; Xi, B. Dietary phosphorus affects growth, glucolipid metabolism, antioxidant activity and immune status of juvenile blunt snout bream (Megalobrama amblycephala). Anim. Feed. Sci. Technol. 2021, 274, 114896. [Google Scholar] [CrossRef]
- Zhu, X.; Ding, G.; Ren, S.; Xi, J.; Liu, K. The bioavailability, absorption, metabolism, and regulation of glucolipid metabolism disorders by quercetin and its important glycosides: A review. Food Chem. 2024, 458, 140262. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, L.; Jiang, L. Dynamic regulation of gut Clostridium-derived short-chain fatty acids. Trends Biotechnol. 2022, 40, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Mountfort, D.O.; Campbell, J.; Clements, K.D. Hindgut fermentation in three species of marine herbivorous fish. Appl. Environ. Microbiol. 2002, 68, 1374–1380. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Abo-Al-Ela, H.G.; Hasan, M.T. Modulation of transcriptomic profile in aquatic animals: Probiotics, prebiotics and synbiotics scenarios. Fish Shellfish Immunol. 2020, 97, 268–282. [Google Scholar] [CrossRef]
- Albaladejo-Riad, N.; El Qendouci, M.; Cuesta, A.; Esteban, M.Á. Ability of short-chain fatty acids to reduce inflammation and attract leucocytes to the inflamed skin of gilthead seabream (Sparus aurata L.). Sci. Rep. 2024, 14, 31404. [Google Scholar] [CrossRef]
- Ostrowska, J.; Samborowska, E.; Jaworski, M.; Toczyłowska, K.; Szostak-Węgierek, D. The Potential Role of SCFAs in Modulating Cardiometabolic Risk by Interacting with Adiposity Parameters and Diet. Nutrients 2024, 16, 100350. [Google Scholar] [CrossRef] [PubMed]
- Cherta-Murillo, A.; Pugh, J.E.; Alaraj-Alshehhi, S.; Hajjar, D.; Chambers, E.S.; Frost, G.S. The effects of SCFAs on glycemic control in humans: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2022, 116, 335–361. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Hoseinifar, S.H.; Kavandi, M. Modulation of antioxidant defense and immune response in zebra fish (Danio rerio) using dietary sodium propionate. Fish. Physiol. Biochem. 2016, 42, 1733–1739. [Google Scholar] [CrossRef]
- Mir, M.M.; Mir, R.; Alghamdi, M.A.A.; Wani, J.I.; Elfaki, I.; Sabah, Z.U.; Alhujaily, M.; Jeelani, M.; Marakala, V.; Alharthi, M.H.; et al. Potential impact of GCK, MIR-196A-2 and MIR-423 gene abnormalities on the development and progression of type 2 diabetes mellitus in Asir and Tabuk regions of Saudi Arabia. Mol. Med. Rep. 2022, 25, 162. [Google Scholar] [CrossRef]
- Chan, C.-Y.; Dominguez, D.; Parra, K.J. Regulation of Vacuolar H+-ATPase (V-ATPase) Reassembly by Glycolysis Flow in 6-Phosphofructo-1-kinase (PFK-1)-deficient Yeast Cells. J. Biol. Chem. 2016, 291, 15820–15829. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, C. Gluconeogenesis in Cancer: Function and Regulation of PEPCK, FBPase, and G6Pase. Trends Cancer 2019, 5, 30–45. [Google Scholar] [CrossRef]
- He, Q.; Ji, L.; Wang, Y.; Zhang, Y.; Wang, H.; Wang, J.; Zhu, Q.; Xie, M.; Ou, W.; Liu, J.; et al. Acetate enables metabolic fitness and cognitive performance during sleep disruption. Cell Metab. 2024, 36, 1998–2014.e15. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, J.; Tian, C.; Dong, L.; Kang, Z.; Wang, J.; Zhao, S.; Li, M.; Tong, X. Mechanisms of regulation of glycolipid metabolism by natural compounds in plants: Effects on short-chain fatty acids. Nutr. Metab. 2024, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Bertolio, R.; Napoletano, F.; Mano, M.; Maurer-Stroh, S.; Fantuz, M.; Zannini, A.; Bicciato, S.; Sorrentino, G.; Del Sal, G. Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism. Nat. Commun. 2019, 10, 1326. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Xu, X.; Xiang, X.; Li, Y.; Zhao, Z.; Li, J.; Gao, S.; Liu, Q.; Mai, K.; Ai, Q. High Fat Activates O-GlcNAcylation and Affects AMPK/ACC Pathway to Regulate Lipid Metabolism. Nutrients 2021, 13, 1740. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, L.; Du, Y.; Qin, L.; Lu, Y.; Zhang, Q.; He, Y.; Tan, D. Gypenosides ameliorate high-fat diet-induced nonalcoholic fatty liver disease in mice by regulating lipid metabolism. PeerJ 2023, 11, e15225. [Google Scholar] [CrossRef]
- Fougerat, A.; Schoiswohl, G.; Polizzi, A.; Régnier, M.; Wagner, C.; Smati, S.; Fougeray, T.; Lippi, Y.; Lasserre, F.; Raho, I.; et al. ATGL-dependent white adipose tissue lipolysis controls hepatocyte PPARα activity. Cell Rep. 2022, 39, 110910. [Google Scholar] [CrossRef]
- Idrees, M.; Xu, L.; El Sheikh, M.; Sidrat, T.; Song, S.-H.; Joo, M.-D.; Lee, K.-L.; Kong, I.-K. The PPARδ Agonist GW501516 Improves Lipolytic/Lipogenic Balance through CPT1 and PEPCK during the Development of Pre-Implantation Bovine Embryos. Int. J. Mol. Sci. 2019, 20, 6066. [Google Scholar] [CrossRef]
- Cook, K.M. Sodium Propionate and Sodium Butyrate Promote Fatty Acid Oxidation in HepG2 Cells under Oxidative Stress. Available online: https://api.semanticscholar.org/CorpusID:249581884 (accessed on 24 December 2024).
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Kotlo, K.U.; Dudeja, P.K.; Layden, B.T. Role of Short Chain Fatty Acid Receptors in Intestinal Physiology and Pathophysiology. Compr. Physiol. 2018, 8, 1091. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Shackley, M.; Ma, Y.; Tate, E.W.; Brown, A.J.H.; Frost, G.; Hanyaloglu, A.C. Short Chain Fatty Acids Enhance Expression and Activity of the Umami Taste Receptor in Enteroendocrine Cells via a Gαi/o Pathway. Front. Nutr. 2020, 7, 568991. [Google Scholar] [CrossRef] [PubMed]
- Schlatterer, K.; Peschel, A.; Kretschmer, D. Short-Chain Fatty Acid and FFAR2 Activation—A New Option for Treating Infections? Front. Cell Infect. Microbiol. 2021, 11, 785833. [Google Scholar] [CrossRef]
- Ragavan, M.L.; Hemalatha, S. The functional roles of short chain fatty acids as postbiotics in human gut: Future perspectives. Food Sci. Biotechnol. 2024, 33, 275–285. [Google Scholar] [CrossRef]
- Li, F.; Tai, L.; Sun, X.; Lv, Z.; Tang, W.; Wang, T.; Zhao, Z.; Gong, D.; Ma, S.; Tang, S.; et al. Molecular recognition and activation mechanism of short-chain fatty acid receptors FFAR2/3. Cell Res. 2024, 34, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.; Zhang, Y.; Zhong, Y.; Kan, Y.; Jawad, M.; Gui, L.; Ren, M.; Xu, G.; Liu, D.; Li, M. Establishment of a Coilia nasus Spermatogonial Stem Cell Line Capable of Spermatogenesis In Vitro. Biology 2023, 12, 1175. [Google Scholar] [CrossRef]
- Kan, Y.; Zhong, Y.; Jawad, M.; Chen, X.; Liu, D.; Ren, M.; Xu, G.; Gui, L.; Li, M. Establishment of a Coilia nasus Gonadal Somatic Cell Line Capable of Sperm Induction In Vitro. Biology 2022, 11, 1049. [Google Scholar] [CrossRef]
- Thompson, E.; Hensley, J.; Taylor, R.S. Effect of High Glucose on Embryological Development of Zebrafish, Brachyodanio, Rerio through Wnt Pathway. Int. J. Mol. Sci. 2024, 25, 9443. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Chu, L.W.; Chen, J.Y.; Hsieh, S.L.; Chang, Y.C.; Dai, Z.K.; Wu, B.N. Loganin attenuates high glucose-induced schwann cells pyroptosis by inhibiting ros generation and nlrp3 inflammasome activation. Cells 2020, 9, 1948. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, X.; Li, X.; Hu, C.; Zhang, Y.; Yin, H. Epigallocatechin gallate improves oleic acid-induced hepatic steatosis in laying hen hepatocytes via the MAPK pathway. Poult. Sci. 2024, 103, 104204. [Google Scholar] [CrossRef]
- Qi, Y.; Zheng, T.; Liu, X.; Yang, S.; Li, Q.; Shao, J.; Zeng, X.; Guan, W.; Zhang, S. Sodium acetate regulates milk fat synthesis through the activation of GPR41/GPR43 signaling pathway. Front. Nutr. 2023, 10, 1098715. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Mang, Q.; Sun, Y.; Xu, G. Short-Chain Fatty Acids (SCFAs) Modulate the Hepatic Glucose and Lipid Metabolism of Coilia nasus via the FFAR/AMPK Signaling Pathway In Vitro. Int. J. Mol. Sci. 2025, 26, 3654. https://doi.org/10.3390/ijms26083654
Gao J, Mang Q, Sun Y, Xu G. Short-Chain Fatty Acids (SCFAs) Modulate the Hepatic Glucose and Lipid Metabolism of Coilia nasus via the FFAR/AMPK Signaling Pathway In Vitro. International Journal of Molecular Sciences. 2025; 26(8):3654. https://doi.org/10.3390/ijms26083654
Chicago/Turabian StyleGao, Jun, Qi Mang, Yi Sun, and Gangchun Xu. 2025. "Short-Chain Fatty Acids (SCFAs) Modulate the Hepatic Glucose and Lipid Metabolism of Coilia nasus via the FFAR/AMPK Signaling Pathway In Vitro" International Journal of Molecular Sciences 26, no. 8: 3654. https://doi.org/10.3390/ijms26083654
APA StyleGao, J., Mang, Q., Sun, Y., & Xu, G. (2025). Short-Chain Fatty Acids (SCFAs) Modulate the Hepatic Glucose and Lipid Metabolism of Coilia nasus via the FFAR/AMPK Signaling Pathway In Vitro. International Journal of Molecular Sciences, 26(8), 3654. https://doi.org/10.3390/ijms26083654




