Oxidative Stress and Antioxidant Status in Pregnant Women with Gestational Diabetes Mellitus and Late-Onset Complication of Pre-Eclampsia
Abstract
1. Introduction
2. Results
2.1. Antioxidant Enzyme Activities (SOD, CAT, and GPx)
2.2. Gestational Diabetes Mellitus Biomarkers
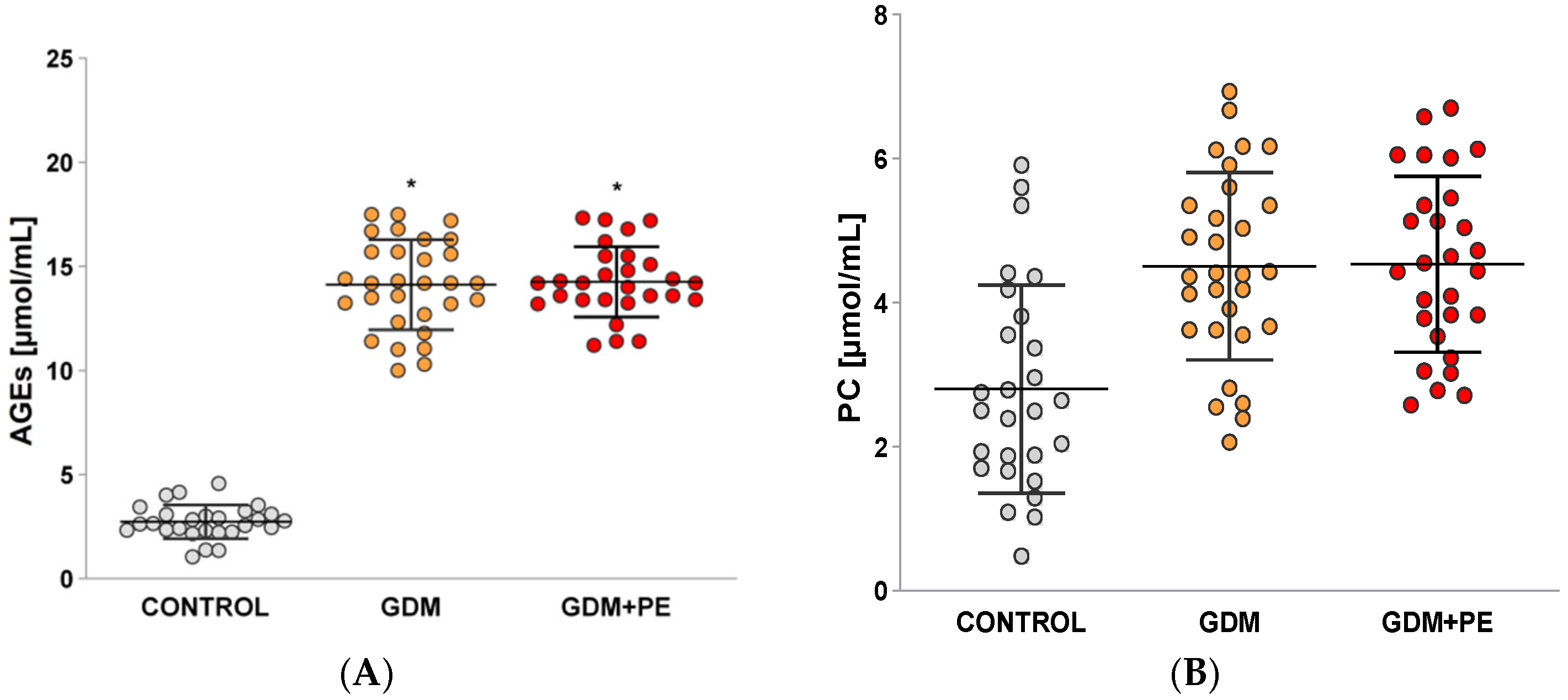
2.3. Levels of Protein Carbonyls (PCs) and Advanced Glycation End Products (AGEs)
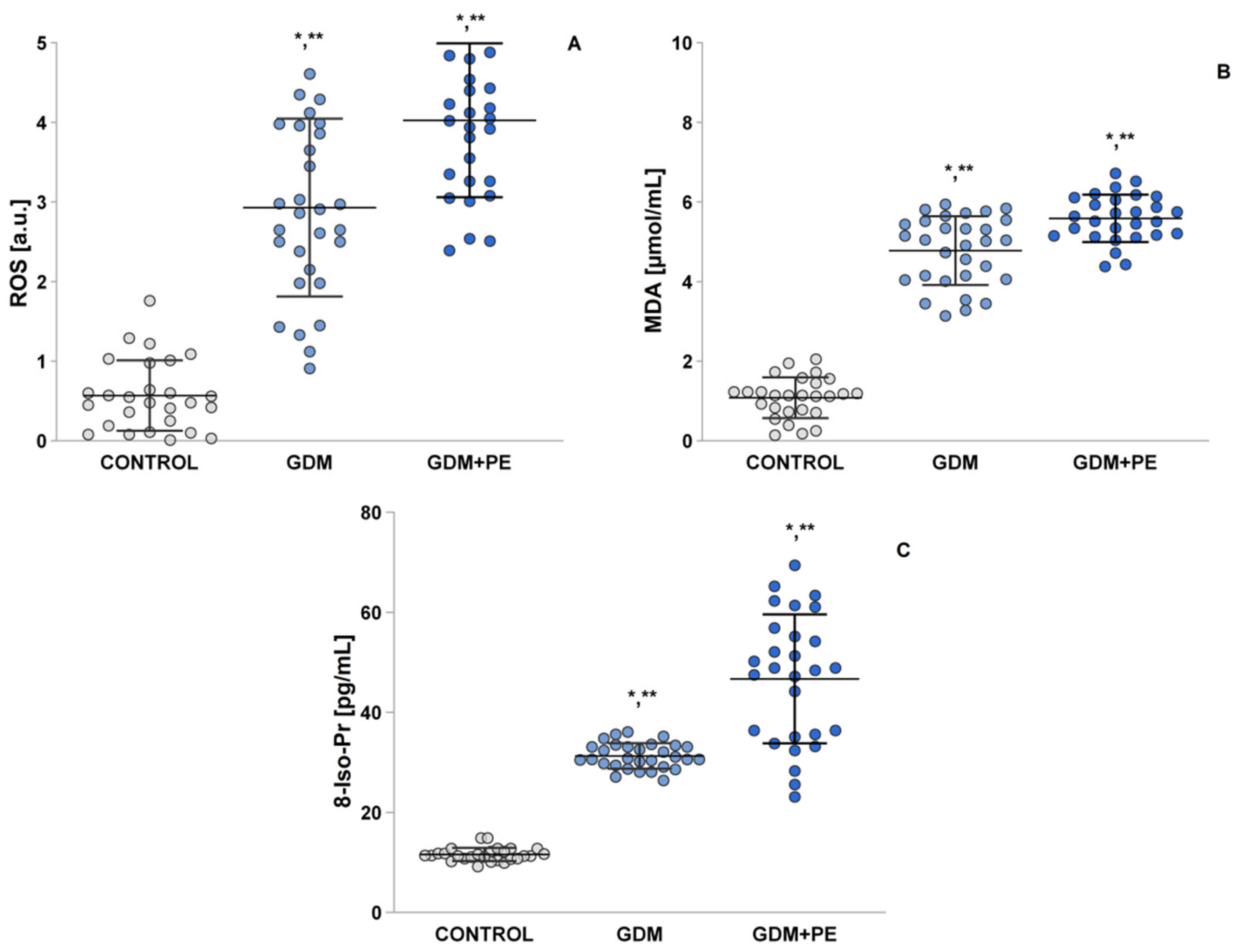
2.4. Assessment of Oxidative Stress by Measuring the ROS, MDA, and 8-Iso-Pr
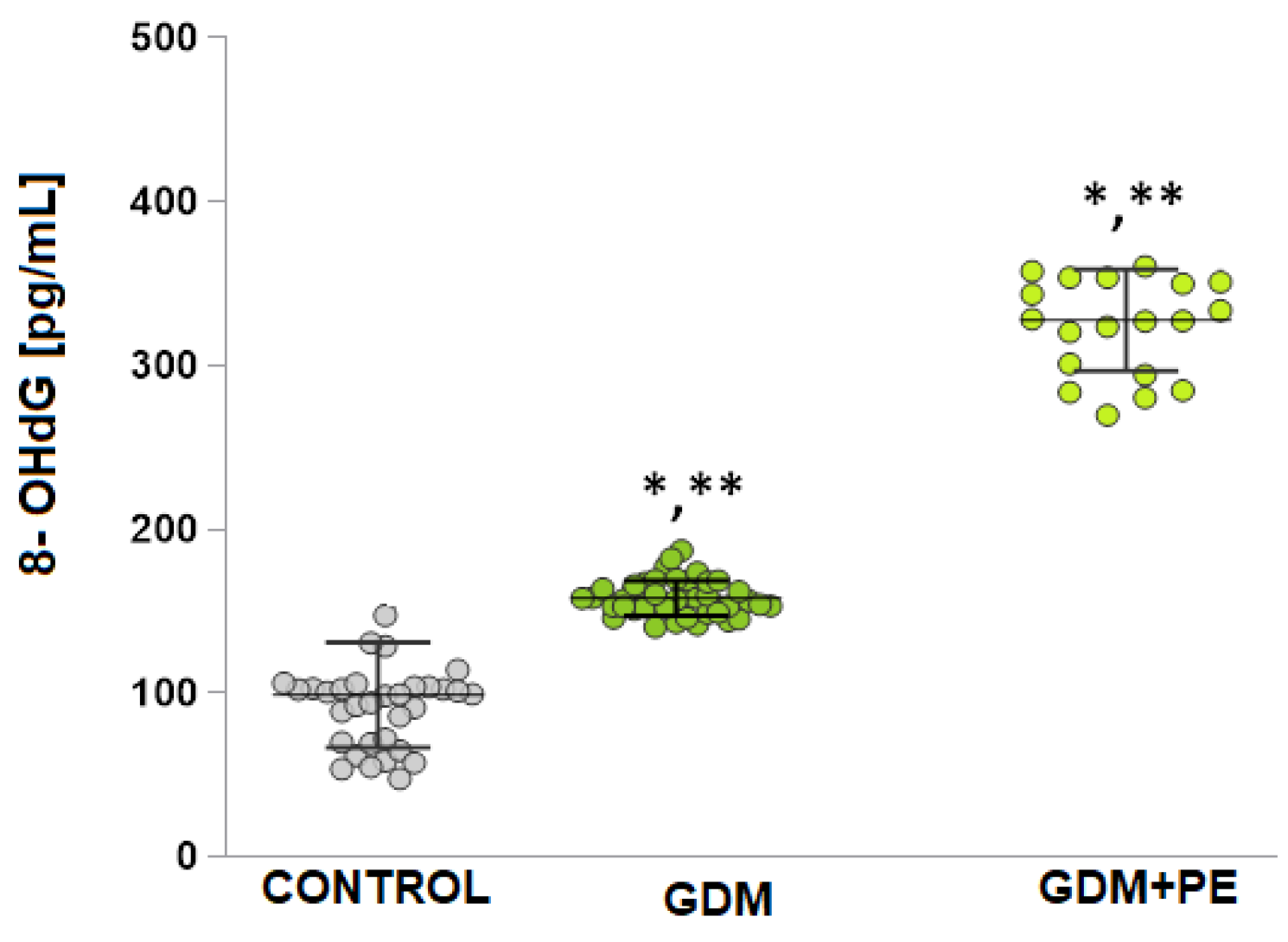
2.5. The Levels of DNA Damage
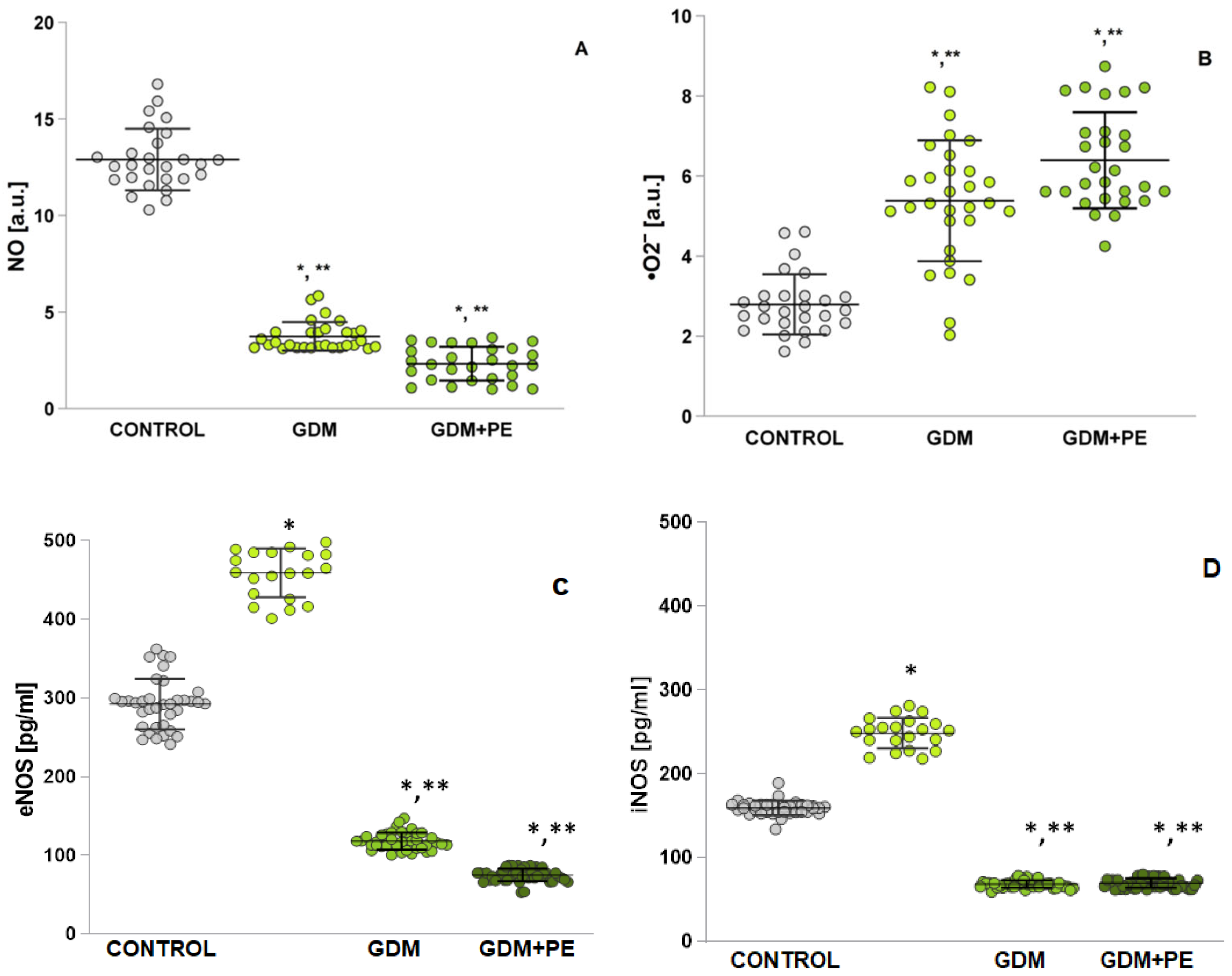
2.6. Assessment of Oxidative Stress by Measuring Nitrosative Stress
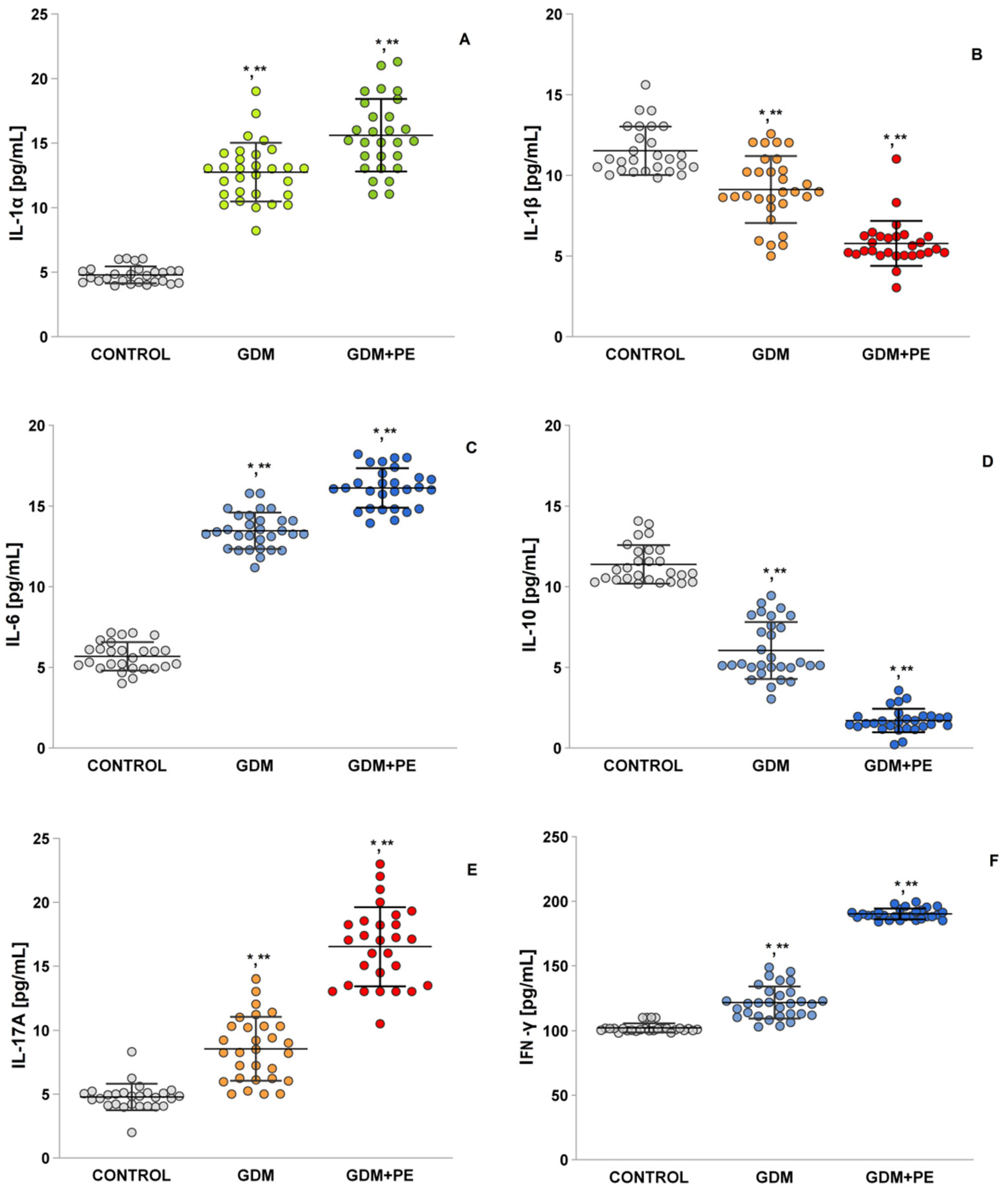
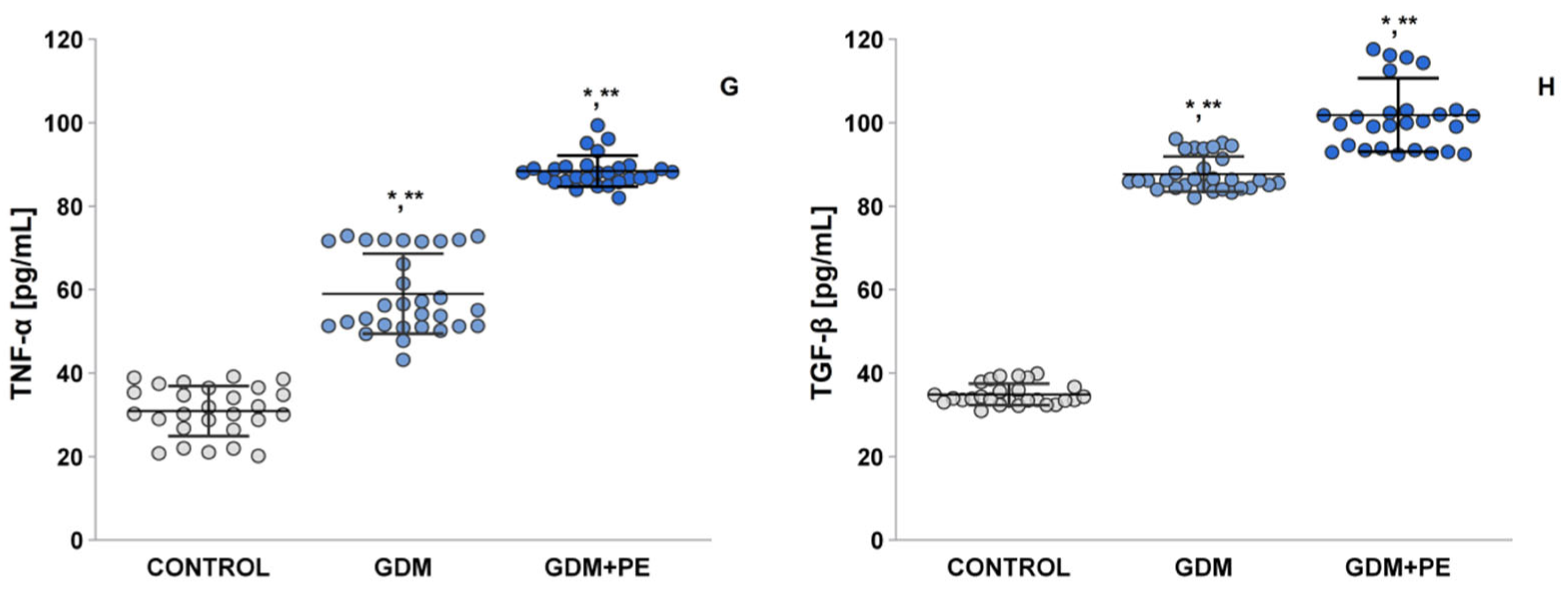
2.7. The Levels of Pro-Inflammatory Cytokines
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Diagnostic Criteria for Outcomes
4.3. Patients
4.4. Blood Samples Preparation
4.5. Electron Paramagnetic Resonance (EPR) Study
4.5.1. EPR Evaluation of ROS Product
4.5.2. EPR Evaluation of •NO Radical
4.5.3. EPR Evaluation of Superoxide Anion Radical
4.6. Enzyme-Linked Immunosorbent Assay
4.7. Statistical Analysis
5. Conclusions
- Comprehensive profiling of oxidative/inflammatory pathways in the comorbidity of gestational diabetes with pre-eclampsia. While oxidative stress and inflammation have been studied separately in GDM + PE, the present study is the first to systematically analyze these pathways in their co-occurrence. The study provides new insights into how oxidative stress and inflammation may interact to worsen pregnancy outcomes;
- Identification of mechanistic links between oxidative stress, GDM severity, and the onset of complications with subsequent PE. The study investigates whether oxidative stress in GDM contributes to the development of PE, filling a gap in the understanding of their shared pathophysiology. An analysis of the results provides a multiparametric assessment, not previously carried out in comorbid GDM-PE cases, by measuring the reactive oxygen/nitrogen species (ROS/RNS), lipid/protein oxidation, antioxidant enzyme activity, and cytokines;
- Potential for new therapeutic strategies. The current management of GDM focuses on glucose control, ignoring oxidative stress and inflammation. The findings presented could support antioxidant-based therapies (e.g., α-lipoic acid, vitamins C/E, and N-acetylcysteine), together with glycemic control, suggesting a new paradigm for the treatment of high-risk pregnancies;
- Bridging clinical and molecular gaps. Previous studies have linked GDM + PE epidemiologically, but few have profiled the biomarkers at different stages of the disease. By comparing patients with GDM alone and patients with GDM+PE, the study elucidates whether oxidative/inflammatory markers predict the risk of PE in women with GDM;
- Potential for early risk stratification. If specific oxidative/inflammatory signatures are associated with worse outcomes, they could serve as early diagnostic or prognostic markers to improve prenatal care. The present study may change clinical management by highlighting oxidative stress as a modifiable risk factor in high-risk pregnancies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joo, E.H.; Kim, Y.R.; Kim, N.; Jung, J.E.; Han, S.H.; Cho, H.Y. Effect of Endogenic and Exogenic Oxidative Stress Triggers on Adverse Pregnancy Outcomes: Preeclampsia, Fetal Growth Restriction, Gestational Diabetes Mellitus and Preterm Birth. Int. J. Mol. Sci. 2021, 22, 10122. [Google Scholar] [CrossRef] [PubMed]
- Tsikouras, P.; Nikolettos, K.; Kotanidou, S.; Kritsotaki, N.; Oikonomou, E.; Bothou, A.; Andreou, S.; Nalmpanti, T.; Chalkia, K.; Spanakis, V.; et al. Renal Function and the Role of the Renin-Angiotensin-Aldosterone System (RAAS) in Normal Pregnancy and Pre-Eclampsia. J. Clin. Med. 2025, 14, 892. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.; et al. The Pivotal Role of the Placenta in Normal and Pathological Pregnancies: A Focus on Preeclampsia, Fetal Growth Restriction, and Maternal Chronic Venous Disease. Cells 2022, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Funes Moñux, R.M.; Rodriguez-Martín, S.; Bravo, C.; De Leon-Luis, J.A.; Saz, J.V.; Saez, M.A.; Guijarro, L.G.; et al. The Placentas of Women Who Suffer an Episode of Psychosis during Pregnancy Have Increased Lipid Peroxidation with Evidence of Ferroptosis. Biomolecules 2023, 13, 120. [Google Scholar] [CrossRef]
- Ortega, M.A.; Garcia-Puente, L.M.; Fraile-Martinez, O.; Pekarek, T.; García-Montero, C.; Bujan, J.; Pekarek, L.; Barrena-Blázquez, S.; Gragera, R.; Rodríguez-Rojo, I.C.; et al. Oxidative Stress, Lipid Peroxidation and Ferroptosis Are Major Pathophysiological Signatures in the Placental Tissue of Women with Late-Onset Preeclampsia. Antioxidants 2024, 13, 591. [Google Scholar] [CrossRef]
- Vornic, I.; Buciu, V.; Furau, C.G.; Gaje, P.N.; Ceausu, R.A.; Dumitru, C.-S.; Barb, A.C.; Novacescu, D.; Cumpanas, A.A.; Latcu, S.C.; et al. Oxidative Stress and Placental Pathogenesis: A Contemporary Overview of Potential Biomarkers and Emerging Therapeutics. Int. J. Mol. Sci. 2024, 25, 12195. [Google Scholar] [CrossRef]
- Karcz, K.; Królak-Olejnik, B. Impact of Gestational Diabetes Mellitus on Fetal Growth and Nutritional Status in Newborns. Nutrients 2024, 16, 4093. [Google Scholar] [CrossRef]
- Torres-Torres, J.; Monroy-Muñoz, I.E.; Perez-Duran, J.; Solis-Paredes, J.M.; Camacho-Martinez, Z.A.; Baca, D.; Espino-y-Sosa, S.; Martinez-Portilla, R.; Rojas-Zepeda, L.; Borboa-Olivares, H.; et al. Cellular and Molecular Pathophysiology of Gestational Diabetes. Int. J. Mol. Sci. 2024, 25, 11641. [Google Scholar] [CrossRef]
- Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. The Influence of Lifestyle and Treatment on Oxidative Stress and Inflammation in Diabetes. Int. J. Mol. Sci. 2022, 23, 15743. [Google Scholar] [CrossRef]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int. J. Mol. Sci. 2021, 22, 2965. [Google Scholar] [CrossRef]
- Afrose, D.; Chen, H.; Ranashinghe, A.; Liu, C.C.; Henessy, A.; Hansbro, P.M.; McClements, L. The diagnostic potential of oxidative stress biomarkers for preeclampsia: Systematic review and meta-analysis. Biol. Sex Differ. 2022, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Osorio, A.S.; Carreón-Torres, E.; Correa-Solís, E.; Ángel-García, J.; Arias-Rico, J.; Jiménez-Garza, O.; Morales-Castillejos, L.; Díaz-Zuleta, H.A.; Baltazar-Tellez, R.M.; Sánchez-Padilla, M.L.; et al. Inflammation and Oxidative Stress Induced by Obesity, Gestational Diabetes, and Preeclampsia in Pregnancy: Role of High-Density Lipoproteins as Vectors for Bioactive Compounds. Antioxidants 2023, 12, 1894. [Google Scholar] [CrossRef] [PubMed]
- Vanli Tonyali, N.; Karabay, G.; Arslan, B.; Aktemur, G.; Tokgoz Cakir, B.; Seyhanli, Z.; Demir Çendek, B.; Yilmaz Ergani, S.; Eroglu, H.; Mermi, S.; et al. Maternal Serum Catestatin Levels in Gestational Diabetes Mellitus: A Potential Biomarker for Risk Assessment and Diagnosis. J. Clin. Med. 2025, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, N.; Glastras, S.J. The Emerging Role of Biomarkers in the Diagnosis of Gestational Diabetes Mellitus. J. Clin. Med. 2018, 7, 120. [Google Scholar] [CrossRef]
- Hall, P.M. Study of Glycosylated Proteins in Diabetes Mellitus; University of Surrey: Surrey, UK, 1985. [Google Scholar]
- Al-Jipouri, A.; Eritja, À.; Bozic, M. Unraveling the Multifaceted Roles of Extracellular Vesicles: Insights into Biology, Pharmacology, and Pharmaceutical Applications for Drug Delivery. Int. J. Mol. Sci. 2024, 25, 485. [Google Scholar] [CrossRef]
- Aye, I.L.M.H. Cholesterol and Oxysterol Transport in Human Placental Trophoblasts: Implications for Placental Cell Viability, Differentiation and Inflammation; University of Western Australia: Perth, Australia, 2011. [Google Scholar]
- Choubey, M.; Bora, P. Emerging Role of Adiponectin/AdipoRs Signaling in Choroidal Neovascularization, Age-Related Macular Degeneration, and Diabetic Retinopathy. Biomolecules 2023, 13, 982. [Google Scholar] [CrossRef]
- Mandò, C.; Castiglioni, S.; Novielli, C.; Anelli, G.M.; Serati, A.; Parisi, F.; Giovarelli, M. Placental Bioenergetics and Antioxidant Homeostasis in Maternal Obesity and Gestational Diabetes. Antioxidants 2024, 13, 858. [Google Scholar] [CrossRef]
- Bakkali, M.E.; Derdaki, M.; Quyou, A. Advanced Maternal Age, Gestational Diabetes, and Parity: A Moderated Mediation Model for Preeclampsia. Tanzania J. Health Res. 2024, 25, 524–542. [Google Scholar]
- Grzeszczak, K.; Łanocha-Arendarczyk, N.; Malinowski, W.; Ziętek, P.; Kosik-Bogacka, D. Oxidative Stress in Pregnancy. Biomolecules 2023, 13, 1768. [Google Scholar] [CrossRef]
- Jantape, T.; Kongwattanakul, K.; Arribas, S.M.; Rodríguez-Rodríguez, P.; Iampanichakul, M.; Settheetham-Ishida, W.; Phuthong, S. Maternal obesity alters placental and umbilical cord plasma oxidative stress, a cross-sectional study. Intern. J. Mol. Sci. 2024, 25, 10866. [Google Scholar] [CrossRef]
- Santos-Rosendo, C.; Bugatto, F.; González-Domínguez, A.; Lechuga-Sancho, A.M.; Mateos, R.M.; Visiedo, F. Placental adaptive changes to protect function and decrease oxidative damage in metabolically healthy maternal obesity. Antioxidants 2020, 9, 794. [Google Scholar] [CrossRef] [PubMed]
- Boyadzhieva, M.; Atanasova, I.; Zacharieva, S.; Kedikova, S. Adipocytokines during pregnancy and postpartum in women with gestational diabetes and healthy controls. J. Endocrinol. Investig. 2013, 36, 944–949. [Google Scholar]
- Powden, T.C.; Zarek, S.M.; Rafique, S.; Sjaarda, L.A.; Schisterman, E.F.; Silver, R.M.; Yeung, E.H.; Radin, R.; Hinkle, S.N.; Galai, N.; et al. Preconception leptin levels and pregnancy outcomes: A prospective cohort study. Obes. Sci. Pract. 2020, 6, 181. [Google Scholar] [CrossRef]
- Salem, M.M.; El-Sokary, A.A.; Hazzaa, S.M.; El-Gharib, M.N.E. Predictive value of first trimester measurement of adiponectin and 1,5 anhydroglucitol in diagnosis of gestational diabetes mellitus. J. Adv. Med. Med. Res. 2020, 32, 111. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Y.H.; Chen, Y.P.; Yuan, X.L.; Wang, J.; Zhu, H.; Lu, C.M. Maternal circulating concentrations of tumor necrosis factor-alpha, leptin, and adiponectin in gestational diabetes mellitus: A systematic review and meta-analysis. Sci. World J. 2014, 1, 926932. [Google Scholar] [CrossRef]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 1, 7489795. [Google Scholar] [CrossRef]
- Kelly, C.B.; Karanchi, H.; Yu, J.Y.; Leyva, M.J.; Jenkins, A.J.; Nankervis, A.J.; Hanssen, K.F.; Garg, S.K.; Scardo, J.A.; Hammad, S.M.; et al. Plasma AGE and Oxidation Products, Renal Function, and Preeclampsia in Pregnant Women with Type 1 Diabetes: A Prospective Observational Study. J. Diabetes Res. 2023, 1, 8537693. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Y.; Geng, L.; Xu, Y.; Zhao, M. Effect of different nutrients on blood glucose, inflammatory response and oxidative stress in gestational diabetes mellitus: A network meta-analysis. British J. Nutrit. 2024, 131, 1513. [Google Scholar] [CrossRef]
- López-Tinoco, C.; Roca, M.; García-Valero, A.; Murri, M.; Tinahones, F.J.; Segundo, C.; Aguilar-Diosdado, M. Oxidative stress and antioxidant status in patients with late-onset gestational diabetes mellitus. Acta Diabetol. 2013, 50, 201. [Google Scholar] [CrossRef]
- Panayotova, M.; Penkova, M. Measurement of oxidative stress-related markers in gastro-intestinal damages in Bulgarian pediatric patients. Bulg ChemComm 2024, 142. [Google Scholar] [CrossRef]
- Freire, V.A.F.; de Melo, A.D.; de Lima Santos, H.; Barros-Pinheiro, M. Evaluation of oxidative stress markers in subtypes of preeclampsia: A systematic review and meta-analysis. Placenta 2023, 132, 55. [Google Scholar] [CrossRef]
- Yang, M.; Peng, S.; Li, W.; Wan, Z.; Fan, L.; Du, Y. Relationships between plasma leptin levels, leptin G2548A, leptin receptor Gln223Arg polymorphisms and gestational diabetes mellitus in Chinese population. Sci. Rep. 2016, 6, 23948. [Google Scholar] [CrossRef]
- Xiao, W.Q.; He, J.R.; Shen, S.Y. Maternal circulating leptin profile during pregnancy and gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2020, 161, 108041. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef]
- Sande, A.K.; Dalen, I.; Torkildsen, E.A.; Sande, R.K.; Morken, N.H. Pregestational maternal risk factors for preterm and term preeclampsia: A population-based cohort study. AOGS 2023, 102, 1549. [Google Scholar] [CrossRef]
- Ibrahim, A.; Khoo, M.I.; Ismail, E.H.E.; Hussain, N.H.N.; Zin, A.A.M.; Noordin, L.; Lah, N.A.Z.N. Oxidative stress biomarkers in pregnancy: A systematic review. Reprod. Biol. Endocrinol. 2024, 22, 93. [Google Scholar] [CrossRef]
- Pancheva, R.; Dolinsek, J.; Panayotova, M.; Yankov, I.; Kofinova, D.; Nikolova, S.; Baycheva, M.; Georgieva, M. Bridging the Gap: Awareness, Knowledge, and Challenges of Living with Celiac Disease in Bulgaria. Nutrients 2025, 17, 1267. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Yang, B.; Zhang, W.; Wang, Y. Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism. Open Life Sci. 2024, 19, 20220928. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martinez, O.; De Leon-Oliva, D.; Boaru, D.L.; Garcia-Puente, L.M.; De León-Luis, J.A.; Bravo, C.; Diaz-Pedrero, R.; Lopez-Gonzalez, L.; Álvarez-Mon, M.; et al. Exploring the Role of Mediterranean and Westernized Diets and Their Main Nutrients in the Modulation of Oxidative Stress in the Placenta: A Narrative Review. Antioxidants 2023, 12, 1918. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Fragoso, M.B.T.; Bueno, N.B.; Goulart, M.O.F.; de Oliveira, A.C.M. Oxidative stress markers in preeclamptic placentas: A systematic review with meta-analysis. Placenta 2020, 99, 89. [Google Scholar] [CrossRef]
- Sweeting, A.N.; Wong, J.; Appelblom, H.; Ross, G.P.; Kouru, H.; Williams, P.F.; Sairanen, M.; Hyett, J.A. A novel early pregnancy risk prediction model for gestational diabetes mellitus. Fetal Diagn. Ther. 2019, 45, 76. [Google Scholar] [CrossRef] [PubMed]
- Panayotova, M. Oxidative stress and inflammatory bowel disease in pediatrics. Trakia J. Sci. 2023, 375. [Google Scholar] [CrossRef]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Díaz-Velázquez, M.F.; Meixueiro-Calderón, C.; Valencia-Ortega, J. Role of Oxidative Stress and Inflammation in Gestational Diabetes Mellitus. Antioxidants 2023, 12, 1812. [Google Scholar] [CrossRef] [PubMed]
- Yañez, M.J.; Leiva, A. Human Placental Intracellular Cholesterol Transport: A Focus on Lysosomal and Mitochondrial Dysfunction and Oxidative Stress. Antioxidants 2022, 11, 500. [Google Scholar] [CrossRef]
- Espinoza, C.; Fuenzalida, B.; Leiva, A. Increased fetal cardiovascular disease risk: Potential synergy between gestational diabetes mellitus and maternal hypercholesterolemia. Curr. Vasc. Pharmacol. 2021, 19, 601. [Google Scholar] [CrossRef]
- Dahn, R. Deciphering the Molecular Mechanisms of Endothelial Cell Dysfunction in Preeclampsia: The Impact of TNF-α and Interleukins Elevated in Preeclamptic Subjects on Endothelial Monolayer Disruption vs. Cell State Changes; The University of Wisconsin-Madison: Madison, WI, USA, 2023. [Google Scholar]
- Hao, W.; Chang, Z.; Dou, B.; Li, F.; Qiao, Z.; Zhang, L. The relationship between vitamin A and E levels and the severity of disease and oxidative stress injury in patients with preeclampsia. Am. J. Transl. Res. 2024, 16, 4601. [Google Scholar] [CrossRef]
- McElwain, C.J.; Tuboly, E.; McCarthy, F.P.; McCarthy, C.M. Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: Windows into future cardiometabolic health? Front. Endocrinol. 2020, 11, 655. [Google Scholar] [CrossRef]
- Puttaiah, A.; Kirthan, J.A.; Sadanandan, D.M.; Somannavar, M.S. Inflammatory markers and their association with preeclampsia among pregnant women: A systematic review and meta-analysis. Clin. Biochem. 2024, 129, 110778. [Google Scholar] [CrossRef]
- Shi, H.; Sui, Y.; Wang, X.; Luo, Y.; Ji, L. Hydroxyl radical production and oxidative damage induced by cadmium and naphthalene in liver of Carassius auratus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2005, 140, 115–121. [Google Scholar] [CrossRef]
- Yokoyama, K.; Hashiba, K.; Wakabayashi, H.; Hashimoto, K.; Satoh, K.; Kurihara, T.; Sakagami, H. Inhibition of LPS-stimulated NO production in mouse macrophage-like cells by tropolones. Anticancer Res. 2004, 24, 3917. [Google Scholar]
- Yoshioka, T.; Iwamoto, N.; Ito, K. An application of electron paramagnetic resonance to evaluate nitric oxide and its quenchers. J. Am. Soc. Nephrol. 1996, 7, 961. [Google Scholar] [CrossRef]
- Simenauer, A.M. HIV Tat Mediation of Redox Sensitive Transcription Factors in Pulmonary Vascular Cells; University of Colorado Denver: Denver, CO, USA; CU Anschutz: Aurora, CO, USA, 2020. [Google Scholar]
| Variables | Normal Pregnancies (Controls) (n = 27) | GDM (n = 30) | GDM + PE (n = 28) |
|---|---|---|---|
| Age (years) | 27.33 ± 9.28 | 31.21 ± 9.23 | 30.25 ± 8.14 |
| Gestational age at sampling (weeks) | 20–40 | 24–28 | 24–28 |
| BMI (kg/m²), mean ± SD | 25.23 ± 3.52 | 30.82 ± 4.26 | 31.52 ± 5.89 |
| BMI > 30 kg/m², n (%) | 7.4% | 59.2% | 58.1% |
| Blood sugar (mmol/L), mean ± SD | 4.97 ± 0.32 | 8.77 ± 0.75 | 9.52 ± 5.18 |
| HbA1C% (mmol/mol) | 5.09 ± 0.71 | 6.90 ± 1.07 | 7.78 ± 0.67 |
| Fasting glucose (mmol/L) | 4.72 ± 0.99 | 5.001 ± 0.56 | 4.98 ± 0.49 |
| 1 h plasma glucose after Oral glucose tolerance test OGTTb (mmol/L) 2 h plasma glucose after OGTT (mmol/L) | 5.62 ± 0.87 6.87 ± 0.87 | 10.06 ± 0.71 8.7 ± 0.85 | 10.80± 0.88 8.8 ± 0.70 |
| Insulin (uU/mL) | 15.33 ± 0.21 | 20.12 ± 0.34 | 25.10 ± 0.26 |
| Cholesterol (mmol/L) | 4.43 ± 0.76 | 5.47 ± 0.6 | 5.12 ± 1.38 |
| Triglycerides (mmol/L) | 1.52 ± 0.44 | 2.513± 0.17 | 2.43 ± 1.27 |
| HDL (mmol/L) | 1.01 ± 0.28 | 1.95 ± 0.13 | 1.25 ± 0.38 |
| LDL (mmol/L) | 2.32 ± 0.62 | 2.32 ± 0.15 | 2.86 ± 1.10 |
| sFlT-1/PIGF | 5.12 ± 3.54 | 38.07 ± 6.31 | 68.61 ± 12.43 |
| sFlT-1 (soluble thyrosin kinase) | 898 ± 1.25 | 4006 ± 154.01 | 4976 ± 115.32 |
| PIGF (placental growth factor) | 177 ± 14.13 | 103 ± 11.10 | 90 ± 11.96 |
| Blood pressure (mmHg) systolic diastolic | 120 80 | 120 80 | 140 90 |
| Proteinuria (mg/dL/day) | ≤150 | ≤150 | ≥300 |
| Estimated Birth weight (g) | 3000 ± 501 | 3800 ± 493 | 2750 ± 481 |
| SOD U/gHb | 121 ± 15.55 | 61.24 ± 18.15 | 50.14 ± 13.11 |
| CAT U/gHb | 48.59 ± 8.66 | 69.77 ± 7.58 | 72.69 ± 11.45 |
| GPx U/gHb | 289.87 ± 25.58 | 132.35 ± 15.61 | 94.28 ± 12.12 |
| Predictive Factor | Normal Pregnancies (Controls) (n = 27) | GDM (n = 30) | GDM + PE (n = 28) | p |
|---|---|---|---|---|
| Adiponectin (APN) pg/mL | 76.25 ± 18.66 | 38.33 ± 14.13 | 39.52 ± 8.01 | p = 0.004 vs. controls |
| Leptin pg/mL | 55.32 ± 19.17 | 78.99 ± 14.97 | 79.14 ± 20.01 | p= 0.001 |
| Human CRP pg/mL | 4.38 ± 0.21 | 7.47± 0.14 | 7.68 ± 0.26 | p = 0.005 |
| Advanced oxidation protein products (AOPP) ng/mL | 68.59 ± 16.09 | 89.56 ± 36.52 | 86.24 ± 16.98 | p = 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petkova-Parlapanska, K.; Kostadinova-Slavova, D.; Angelova, M.; Sadi J. Al-Dahwi, R.; Georgieva, E.; Goycheva, P.; Karamalakova, Y.; Nikolova, G. Oxidative Stress and Antioxidant Status in Pregnant Women with Gestational Diabetes Mellitus and Late-Onset Complication of Pre-Eclampsia. Int. J. Mol. Sci. 2025, 26, 3605. https://doi.org/10.3390/ijms26083605
Petkova-Parlapanska K, Kostadinova-Slavova D, Angelova M, Sadi J. Al-Dahwi R, Georgieva E, Goycheva P, Karamalakova Y, Nikolova G. Oxidative Stress and Antioxidant Status in Pregnant Women with Gestational Diabetes Mellitus and Late-Onset Complication of Pre-Eclampsia. International Journal of Molecular Sciences. 2025; 26(8):3605. https://doi.org/10.3390/ijms26083605
Chicago/Turabian StylePetkova-Parlapanska, Kamelia, Denitsa Kostadinova-Slavova, Mariya Angelova, Rafaah Sadi J. Al-Dahwi, Ekaterina Georgieva, Petya Goycheva, Yanka Karamalakova, and Galina Nikolova. 2025. "Oxidative Stress and Antioxidant Status in Pregnant Women with Gestational Diabetes Mellitus and Late-Onset Complication of Pre-Eclampsia" International Journal of Molecular Sciences 26, no. 8: 3605. https://doi.org/10.3390/ijms26083605
APA StylePetkova-Parlapanska, K., Kostadinova-Slavova, D., Angelova, M., Sadi J. Al-Dahwi, R., Georgieva, E., Goycheva, P., Karamalakova, Y., & Nikolova, G. (2025). Oxidative Stress and Antioxidant Status in Pregnant Women with Gestational Diabetes Mellitus and Late-Onset Complication of Pre-Eclampsia. International Journal of Molecular Sciences, 26(8), 3605. https://doi.org/10.3390/ijms26083605








