Tissue-Specific Differential Distribution of Cell Wall Epitopes in Sphagnum compactum and Marchantia polymorpha
Abstract
1. Introduction
2. Results
2.1. Spagnum compactum
2.2. Marchantia polymorpha
2.3. Comparison of Labeling Between S. compactum and M. polymorpha
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sørensen, I.; Domozych, D.; Willats, W.G. How Have Plant Cell Walls Evolved? Plant Physiol. 2010, 153, 366–372. [Google Scholar] [CrossRef]
- Sarkar, P.; Bosneaga, E.; Auer, M. Plant Cell Walls Throughout Evolution: Towards a Molecular Understanding of Their Design Principles. J. Exp. Bot. 2009, 60, 3615–3635. [Google Scholar] [CrossRef]
- Plancot, B.; Gügi, B.; Mollet, J.C.; Loutelier-Bourhis, C.; Giovind, S.R.; Lerouge, P.; Follet-Gueye, M.L.; Vicré, M.; Alfonso, C.; Nguema-Ona, E.; et al. Dessication tolerance in plants: Structural charactérization of the cell wall hemicellulosic polysaccharides in three Selaginella species. Carbohydr. Polym. 2019, 208, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Soriano, G.; Del-Castillo-Alonso, M.A.; Monforte, L.; Núñez-Olivera, E.; Martínez-Abaigar, J. Phenolic compounds from different bryophyte species and cell compartments respond specifically to ultraviolet radiation, but not particularly quickly. Plant Physiol. Biochem. 2019, 134, 137–144. [Google Scholar] [CrossRef]
- Niklas, K.J.; Cobb, E.D.; Matas, A.J. The evolution of hydrophobic cell wall biopolymers: From algae to angiosperms. J. Exp. Bot. 2017, 68, 5261–5269. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Rabanal, M.; Rebaque, D.; Largo-Gosens, A.; Encina, A.; Mélida, H. Cell Walls, a Comparative View of the Composition of Cell Surfaces of Plants, Algae and Microorganisms. J. Exp. Bot. 2024, erae512. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.J.; Szövényi, P.; Shaw, B. Bryophyte Diversity and Evolution: Windows into the Early Evolution of Land Plants. Am. J. Bot. 2011, 98, 352–369. [Google Scholar] [CrossRef]
- Ogwu, M.C. Ecological and Economic Significance of Bryophytes. In Current State and Future Impacts of Climate Change on Biodiversity; IGI Global: Hershey, PA, USA, 2020; pp. 54–78. [Google Scholar]
- Vitt, D.H.; House, M. Bryophytes as Key Indicators of Ecosystem Function and Structure of Northern Peatlands. Bryophyt. Divers. Evol. 2021, 43, 253–264. [Google Scholar] [CrossRef]
- Chen, K.H.; Nelson, J. A Scoping Review of Bryophyte Microbiota: Diverse Microbial Communities in Small Plant Packages. J. Exp. Bot. 2022, 73, 4496–4513. [Google Scholar] [CrossRef]
- Yadav, S.; Basu, S.; Srivastava, A.; Biswas, S.; Mondal, R.; Jha, V.K.; Mishra, Y. Bryophytes as Modern Model Plants: An Overview of Their Development, Contributions, and Future Prospects. J. Plant Growth Regul. 2023, 42, 6933–6950. [Google Scholar] [CrossRef]
- Flores-Sandoval, E.; Eklund, D.M.; Bowman, J.L. A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort Marchantia polymorpha. PLoS Genet. 2015, 11, e1005207. [Google Scholar] [CrossRef] [PubMed]
- Ligrone, R.; Duckett, J.G.; Renzaglia, K.S. Major Transitions in the Evolution of Early Land Plants: A Bryological Perspective. Ann. Bot. 2012, 109, 851–871. [Google Scholar] [CrossRef]
- Slate, M.L.; Antoninka, A.; Bailey, L.; Berdugo, M.B.; Callaghan, D.A.; Cárdenas, M.; Coe, K.K. Impact of Changing Climate on Bryophyte Contributions to Terrestrial Water, Carbon, and Nitrogen Cycles. New Phytol. 2024, 242, 2411–2429. [Google Scholar] [CrossRef]
- Pfeifer, L.; Mueller, K.-K.; Classen, B. The cell wall of hornworts and liverworts: Innovations in early land plant evolution? J. Exp. Bot. 2022, 73, 4454–4472. [Google Scholar] [CrossRef] [PubMed]
- Roig-Oliver, M.; Douthe, C.; Bota, J.; Flexas, J. Cell Wall Thickness and Composition Are Related to Photosynthesis in Antarctic Mosses. Physiol. Plant. 2021, 173, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Popper, Z.A.; Fry, S.C. Primary cell wall composition of bryophytes and charophytes. Ann. Bot. 2003, 91, 1–12. [Google Scholar] [CrossRef]
- Peña, M.J.; Darvill, A.G.; Eberhard, S.; York, W.S.; O’Neill, M.A. Moss and liverwort xyloglucans contain galacturonic acid and are structurally distinct from the xyloglucans synthesized by hornworts and vascular plants. Glycobiology 2008, 18, 891–904. [Google Scholar] [CrossRef]
- Medina, R.; Johnson, M.G.; Liu, Y.; Wickett, N.J.; Shaw, A.J.; Goffinet, B. Phylogenomic Delineation of Physcomitrium (Bryophyta: Funarineae) Based on Targeted Sequencing of Nuclear Exons and Their Flanking Regions Rejects the Retention of Physcomitrella, Physcomitridium, and Aphanorrhegma. J. Syst. Evol. 2019, 57, 404–417. [Google Scholar] [CrossRef]
- Shaw, A.J.; Schmutz, J.; Devos, N.; Shu, S.; Carrell, A.A.; Weston, D.J. The Sphagnum Genome Project: A New Model for Ecological and Evolutionary Genomics. Adv. Bot. Res. 2016, 78, 167–187. [Google Scholar]
- Shaw, J.A.; Devos, N.; Liu, Y.; Cox, C.J.; Goffinet, B.; Flatberg, K.I.; Shaw, B. Organellar Phylogenomics of an Emerging Model System: Sphagnum (Peatmoss). Ann. Bot. 2016, 118, 185–196. [Google Scholar] [CrossRef]
- Klavina, L.; Ramawat, K.; Mérillon, J.M. Polysaccharides from Lower Plants: Bryophytes. In Polysaccharides; Springer International Publishing: Cham, Switzerland, 2015; pp. 145–160. [Google Scholar]
- Hájek, T.; Beckett, R.P. Effect of water content components on desiccation and recovery in Sphagnum mosses. Ann. Bot. 2008, 101, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ballance, S.; Kristiansen, K.A.; Skogaker, N.T.; Tvedt, K.E.; Christensen, B.E. The Localisation of Pectin in Sphagnum Moss Leaves and Its Role in Preservation. Carbohydr. Polym. 2012, 87, 1326–1332. [Google Scholar] [CrossRef]
- Hymas, M.; Casademont-Reig, I.; Poigny, S.; Stavros, V.G. Characteristic Photoprotective Molecules from the Sphagnum World: A Solution-Phase Ultrafast Study of Sphagnic Acid. Molecules 2023, 28, 6153. [Google Scholar] [CrossRef] [PubMed]
- Bryan, L.; Shaw, R.; Schoonover, E.; Koehl, A.; DeVries-Zimmerman, S.; Philben, M. Sphagnan in Sphagnum-Dominated Peatlands: Bioavailability and Effects on Organic Matter Stabilization. Biogeochemistry 2024, 167, 665–680. [Google Scholar] [CrossRef]
- Tveit, A.T.; Kiss, A.; Winkel, M.; Horn, F.; Hájek, T.; Svenning, M.M.; Liebner, S. Environmental Patterns of Brown Moss- and Sphagnum-Associated Microbial Communities. Sci. Rep. 2020, 10, 22412. [Google Scholar] [CrossRef]
- Bowman, J.L.; Arteaga-Vazquez, M.; Berger, F.; Briginshaw, L.N.; Carella, P.; Aguilar-Cruz, A.; Zachgo, S. The Renaissance and Enlightenment of Marchantia as a Model System. Plant Cell 2022, 34, 3512–3542. [Google Scholar] [CrossRef]
- Shimamura, M. Marchantia polymorpha: Taxonomy, Phylogeny and Morphology of a Model System. Plant Cell Physiol. 2016, 57, 230–256. [Google Scholar] [CrossRef]
- Bowman, J.L. The Liverwort Marchantia polymorpha, a Model for All Ages. Curr. Top. Dev. Biol. 2022, 147, 1–32. [Google Scholar]
- Kolkas, H.; Burlat, V.; Jamet, E. Immunochemical identification of the main cell wall polysaccharides of the early land plant Marchantia polymorpha. Cells 2023, 12, 1833. [Google Scholar] [CrossRef]
- Kolkas, H.; Balliau, T.; Chourré, J.; Zivy, M.; Canut, H.; Jamet, E. The Cell Wall Proteome of Marchantia polymorpha Reveals Specificities Compared to Those of Flowering Plants. Front. Plant Sci. 2022, 12, 765846. [Google Scholar] [CrossRef]
- Apostolakos, P.; Galatis, B. Studies on the Development of the Air Pores and Air Chambers of Marchantia paleacea: III. Microtubule Organization in Preprophase-Prophase Initial Aperture Cells—Formation of Incomplete Preprophase Microtubule Bands. Protoplasma 1985, 128, 120–135. [Google Scholar] [CrossRef]
- Jibran, R.; Hill, S.J.; Lampugnani, E.R.; Hao, P.; Doblin, M.S.; Bacic, A.; Brummell, D.A. The Auronidin Flavonoid Pigments of the Liverwort Marchantia polymorpha Form Polymers That Modify Cell Wall Properties. Plant J. 2024, 120, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Popper, Z.A. Evolution and diversity of green plant cell walls. Curr. Opin. Plant Biol. 2008, 11, 286–292. [Google Scholar] [CrossRef]
- Kremer, C.; Pettolino, F.; Bacic, A.; Drinnan, A. Distribution of cell wall components in Sphagnum hyaline cells and in liverwort and hornwort elaters. Planta 2004, 219, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Carafa, A.; Duckett, J.G.; Knox, J.P.; Ligrone, R. Distribution of cell-wall xylans in bryophytes and tracheophytes: New insights into basal interrelationships of land plants. New Phytol. 2005, 168, 231–240. [Google Scholar] [CrossRef]
- Wallace, S.; Fleming, A.; Wellman, C.H.; Beerling, D.J. Evolutionary Development of the Plant and Spore Wall. AoB Plants 2011, plr027. [Google Scholar] [CrossRef]
- Henry, J.S.; Lopez, R.A.; Renzaglia, K.S. Differential Localization of Cell Wall Polymers across Generations in the Placenta of Marchantia polymorpha. J. Plant Res. 2020, 133, 911–924. [Google Scholar] [CrossRef]
- Renzaglia, K.; Duran, E.; Sagwan-Barkdoll, L.; Henry, J. Callose in Leptoid Cell Walls of the Moss Polytrichum and the Evolution of Callose Synthase across Bryophytes. Front. Plant Sci. 2024, 15, 1357324. [Google Scholar] [CrossRef]
- Ligrone, R.; Vaughn, K.C.; Renzaglia, K.S.; Knox, J.P.; Duckett, J.G. Diversity in the Distribution of Polysaccharide and Glycoprotein Epitopes in the Cell Walls of Bryophytes: New Evidence for the Multiple Evolution of Water-Conducting Cells. New Phytol. 2002, 156, 491–508. [Google Scholar] [CrossRef]
- Anderson, L.E.; Ammann, K. Cell Wall Ornamentation in the Hyaline Cells of Sphagnum. J. Hattori Bot. Lab. 1991, 69, 49–63. [Google Scholar]
- Kremer, C.L.; Drinnan, A.N. Secondary Walls in Hyaline Cells of Sphagnum. Aust. J. Bot. 2004, 52, 243–256. [Google Scholar] [CrossRef]
- Plank, N. The Nature of Cellulose in Sphagnum. Am. J. Bot. 1946, 33, 335–337. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Kim, S.J.; Renna, L.; Anozie, F.C.; Sharkey, T.D.; Brandizzi, F. Pectin methylesterification impacts the relationship between photosynthesis and plant growth. Plant Physiol. 2016, 171, 833–848. [Google Scholar]
- Daher, F.B.; Braybrook, S.A. How to Let Go: Pectin and Plant Cell Adhesion. Front. Plant Sci. 2015, 6, 523. [Google Scholar] [CrossRef]
- Fradera-Soler, M.; Grace, O.M.; Jørgensen, B.; Mravec, J. Elastic and collapsible: Current understanding of cell walls in succulent plants. J. Exp. Bot. 2022, 73, 2290–2307. [Google Scholar] [CrossRef] [PubMed]
- Ropitaux, M.; Bernard, S.; Follet-Gueye, M.L.; Vicré, M.; Boulogne, I.; Driouich, A. Xyloglucan and cellulose form molecular cross-bridges connecting root border cells in pea (Pisum sativum). Plant Physiol. Biochem. 2019, 139, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Schuette, S.; Wood, A.J.; Geisler, M.; Geisler-Lee, J.; Ligrone, R.; Renzaglia, K.S. Novel Localization of Callose in the Spores of Physcomitrella patens and Phylogenomics of the Callose Synthase Gene Family. Ann. Bot. 2009, 103, 749–756. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Verhoef, R.; Schols, H.A.; McCleary, B.V.; Knox, J.P. Developmental Complexity of Arabinan Polysaccharides and Their Processing in Plant Cell Walls. Plant J. 2009, 59, 413–425. [Google Scholar] [CrossRef]
- Ligrone, R.; Duckett, J.G. The leafy stems of Sphagnum (Bryophyta) contain highly differentiated polarized cells with axial arrays of endoplasmic microtubules. New Phytol. 1998, 140, 567–579. [Google Scholar] [CrossRef]
- Popper, Z.A.; Michel, G.; Hervé, C.; Domozych, D.S.; Willats, W.G.; Tuohy, M.G.; Stengel, D.B. Evolution and Diversity of Plant Cell Walls: From Algae to Flowering Plants. Annu. Rev. Plant Biol. 2011, 62, 567–590. [Google Scholar] [CrossRef]
- Melton, L.D.; Smith, B.G.; Ibrahim, R.; Schröder, R. Mannans in Primary and Secondary Plant Cell Walls. N. Z. J. For. Sci. 2009, 39, 153–160. [Google Scholar]
- Popper, Z.A.; Tuohy, M.G. Beyond the green: Understanding the evolutionary puzzle of plant and algal cell walls. Plant Physiol. 2010, 153, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Giannoutsou, E.; Apostolakos, P.; Galatis, B. Spatio-temporal Diversification of the Cell Wall Matrix Materials in the Developing Stomatal Complexes of Zea mays. Planta 2016, 244, 1125–1143. [Google Scholar] [CrossRef] [PubMed]
- Giannoutsou, E.; Sotiriou, P.; Nikolakopoulou, T.L.; Galatis, B.; Apostolakos, P. Callose and Homogalacturonan Epitope Distribution in Stomatal Complexes of Zea mays and Vigna sinensis. Protoplasma 2020, 257, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Apostolakos, P.; Livanos, P.; Giannoutsou, E.; Panteris, E.; Galatis, B. The Intracellular and Intercellular Cross-Talk during Subsidiary Cell Formation in Zea mays: Existing and Novel Components Orchestrating Cell Polarization and Asymmetric Division. Ann. Bot. 2018, 122, 679–696. [Google Scholar] [CrossRef]
- Kohchi, T.; Yamato, K.T.; Ishizaki, K.; Yamaoka, S.; Nishihama, R. Development and molecular genetics of Marchantia polymorpha. Annu. Rev. Plant Biol. 2021, 72, 677–702. [Google Scholar] [CrossRef]
- Rodríguez-Gacio, M.D.C.; Iglesias-Fernández, R.; Carbonero, P.; Matilla, Á.J. Softening-up mannan-rich cell walls. J. Exp. Bot. 2012, 63, 3976–3988. [Google Scholar] [CrossRef]
- Brett, C.T.; Baydoun, E.H.; Abdel-Massih, R.M. Pectin-xyloglucan linkages in type I primary cell walls of plants. Plant Biosyst. 2005, 139, 54–59. [Google Scholar] [CrossRef]
- Thomas, R.J. Wall Analyses of Lophocolea seta Cells (Bryophyta) Before and After Elongation. Plant Physiol. 1977, 59, 337–340. [Google Scholar] [CrossRef]
- Carroll, S.; Amsbury, S.; Durney, C.H.; Smith, R.S.; Morris, R.J.; Gray, J.E.; Fleming, A.J. Altering Arabinans Increases Arabidopsis Guard Cell Flexibility and Stomatal Opening. Curr. Biol. 2022, 32, 3170–3179. [Google Scholar] [CrossRef]
- Ellis, M.; Egelund, J.; Schultz, C.J.; Bacic, A. Arabinogalactan-Proteins: Key Regulators at the Cell Surface? Plant Physiol. 2010, 153, 403–419. [Google Scholar] [CrossRef]
- Casero, P.J.; Casimiro, I.; Knox, J.P. Occurrence of Cell Surface Arabinogalactan-Protein and Extensin Epitopes in Relation to Pericycle and Vascular Tissue Development in the Root Apex of Four Species. Planta 1998, 204, 252–259. [Google Scholar] [CrossRef]
- Cao, J.G.; Dai, X.L.; Zou, H.M.; Wang, Q.X. Formation and development of rhizoids of the liverwort Marchantia polymorpha. J. Torrey Bot. Soc. 2014, 141, 126–134. [Google Scholar] [CrossRef]
- Duckett, J.G.; Ligrone, R.; Renzaglia, K.S.; Pressel, S. Pegged and smooth rhizoids in complex thalloid liverworts (Marchantiopsida): Structure, function and evolution. Bot. J. Linn. Soc. 2014, 174, 68–92. [Google Scholar] [CrossRef]
- Lu, Y.T.; Loue-Manifel, J.; Bollier, N.; Gadient, P.; De Winter, F.; Carella, P.; Goodrich, J. Convergent evolution of water-conducting cells in Marchantia recruited the ZHOUPI gene promoting cell wall reinforcement and programmed cell death. Curr. Biol. 2024, 34, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Happ, K.; Classen, B. Arabinogalactan-proteins from the liverwort Marchantia polymorpha L., a member of a basal land plant lineage, are structurally different to those of angiosperms. Plants 2019, 8, 460. [Google Scholar] [CrossRef]
- Bartels, D.; Baumann, A.; Maeder, M.; Geske, T.; Heise, E.M.; von Schwartzenberg, K.; Classen, B. Evolution of plant cell wall: Arabinogalactan-proteins from three moss genera show structural differences compared to seed plants. Carbohydr. Polym. 2017, 163, 227–235. [Google Scholar] [CrossRef]
- Stech, M.; Câmara, P.E.; Medina, R.; Muñoz, J. Advances and challenges in bryophyte biology after 50 years of International Association of Bryologists. Bryophyt. Divers. Evol. 2021, 43, 6–9. [Google Scholar] [CrossRef]
- Harris, B.J.; Clark, J.W.; Schrempf, D.; Szöllősi, G.J.; Donoghue, P.C.; Hetherington, A.M.; Williams, T.A. Divergent Evolutionary Trajectories of Bryophytes and Tracheophytes from a Complex Common Ancestor of Land Plants. Nat. Ecol. Evol. 2022, 6, 1634–1643. [Google Scholar] [CrossRef]
- González, M.L.; Mallon, R.; Reinoso, J.; Rodríguez-Oubina, J. In vitro micropropagation and long-term conservation of the endangered moss Splachnum ampullaceum. Biol. Plant. 2006, 50, 339–345. [Google Scholar] [CrossRef]
- Nelson, J.M. Diversity and Effects of the Fungal Endophytes of the Liverwort Marchantia polymorpha. Ph.D. Dissertation, Duke University, Durham, NC, USA, 2017. [Google Scholar]
- Meidani, C.; Ntalli, N.G.; Giannoutsou, E.; Adamakis, I.D.S. Cell wall modifications in giant cells induced by the plant parasitic nematode Meloidogyne incognita in wild-type (Col-0) and the fra2 Arabidopsis thaliana katanin mutant. Int. J. Mol. Sci. 2019, 20, 5465. [Google Scholar] [CrossRef] [PubMed]
- Ursache, R.; Andersen, T.G.; Marhavý, P.; Geldner, N. A Protocol for Combining Fluorescent Proteins with Histological Stains for Diverse Cell Wall Components. Plant J. 2018, 93, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; McCully, M.E. A Critical Evaluation of the Specificity of Aniline Blue Induced Fluorescence. Protoplasma 1978, 95, 229–254. [Google Scholar] [CrossRef]
- Giannoutsou, E.; Galatis, B.; Apostolakos, P. De-esterified homogalacturonan enrichment of the cell wall region adjoining the preprophase cortical cytoplasmic zone in some protodermal cell types of three land plants. Int. J. Mol. Sci. 2019, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, P.; Giannoutsou, E.; Panteris, E.; Galatis, B.; Apostolakos, P. Local differentiation of cell wall matrix polysaccharides in sinuous pavement cells: Its possible involvement in the flexibility of cell shape. Plant Biol. 2018, 20, 223–237. [Google Scholar] [CrossRef]
- Pappas, D.; Giannoutsou, E.; Panteris, E.; Gkelis, S.; Adamakis, I.D.S. Microcystin-LR and cyanobacterial extracts alter the distribution of cell wall matrix components in rice root cells. Plant Physiol. Biochem. 2022, 191, 78–88. [Google Scholar] [CrossRef]
- Azariadis, A.; Vouligeas, F.; Salame, E.; Kouhen, M.; Rizou, M.; Blazakis, K.; Sotiriou, P.; Ezzat, L.; Mekkaoui, K.; Monzer, A.; et al. Response of Prolyl 4 Hydroxylases, Arabinogalactan Proteins and Homogalacturonans in Four Olive Cultivars under Long-Term Salinity Stress in Relation to Physiological and Morphological Changes. Cells 2023, 12, 1466. [Google Scholar] [CrossRef]
- Gkolemis, K.; Giannoutsou, E.; Adamakis, I.S.; Galatis, B.; Apostolakos, P. Cell wall anisotropy plays a key role in Zea mays stomatal complex movement: The possible role of the cell wall matrix. Plant Mol. Biol. 2023, 113, 331–351. [Google Scholar] [CrossRef]
- He, X.; He, K.S.; Hyvönen, J. Will Bryophytes Survive in a Warming World? Perspect. Plant Ecol. Evol. Syst. 2016, 19, 49–60. [Google Scholar] [CrossRef]
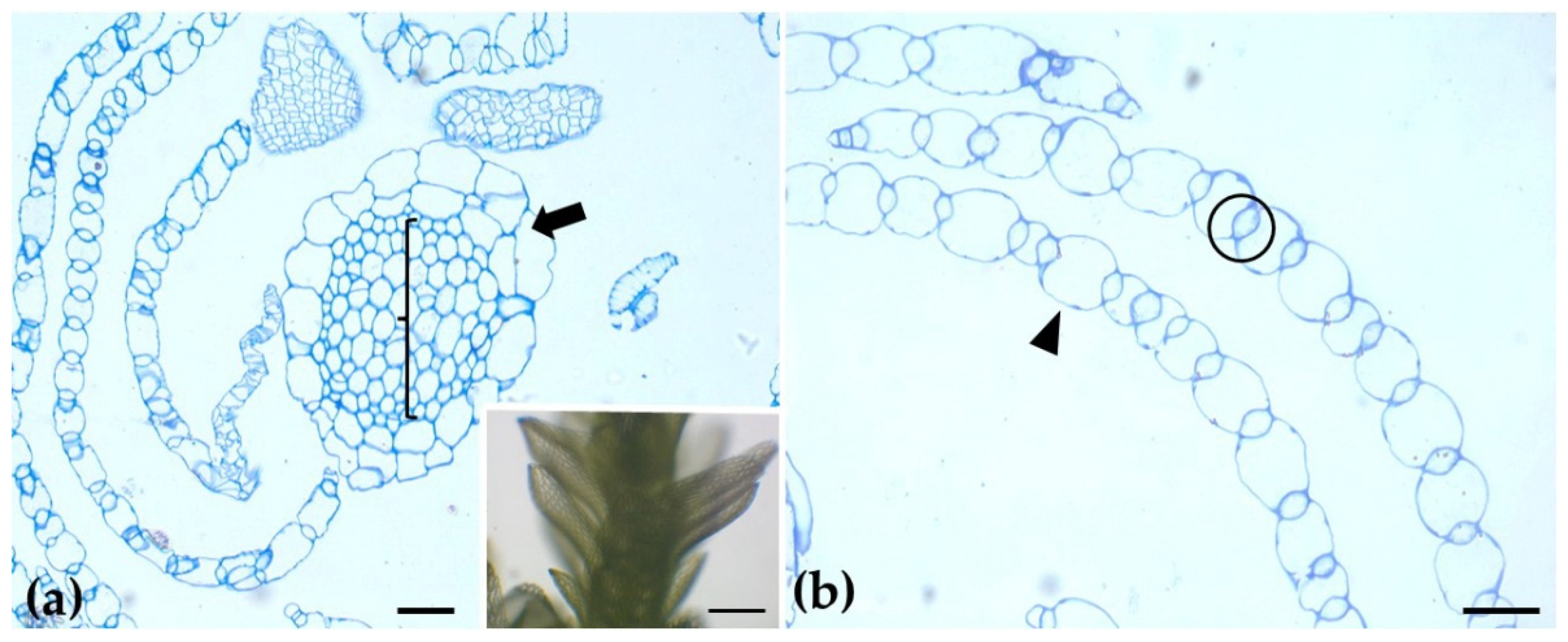


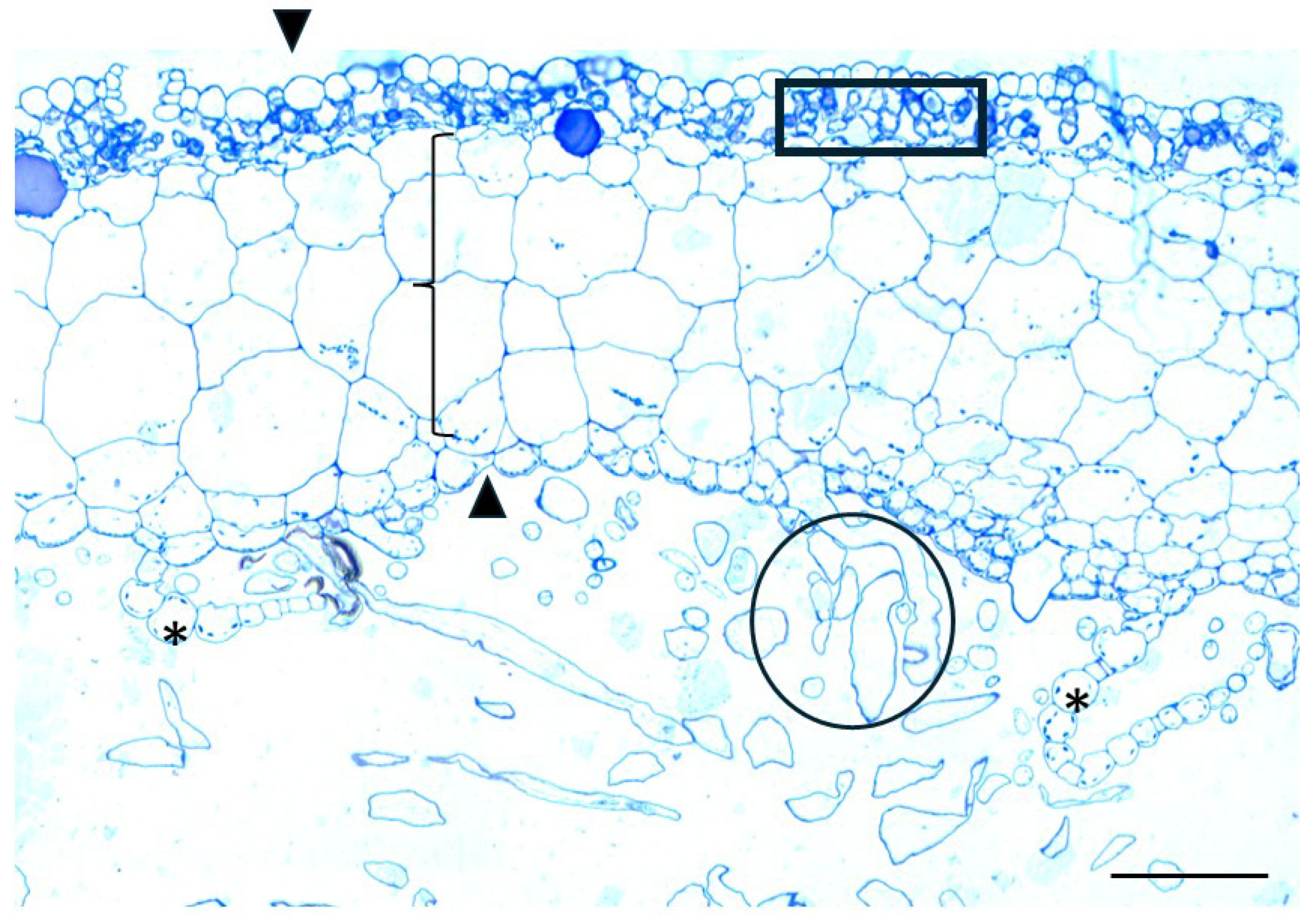

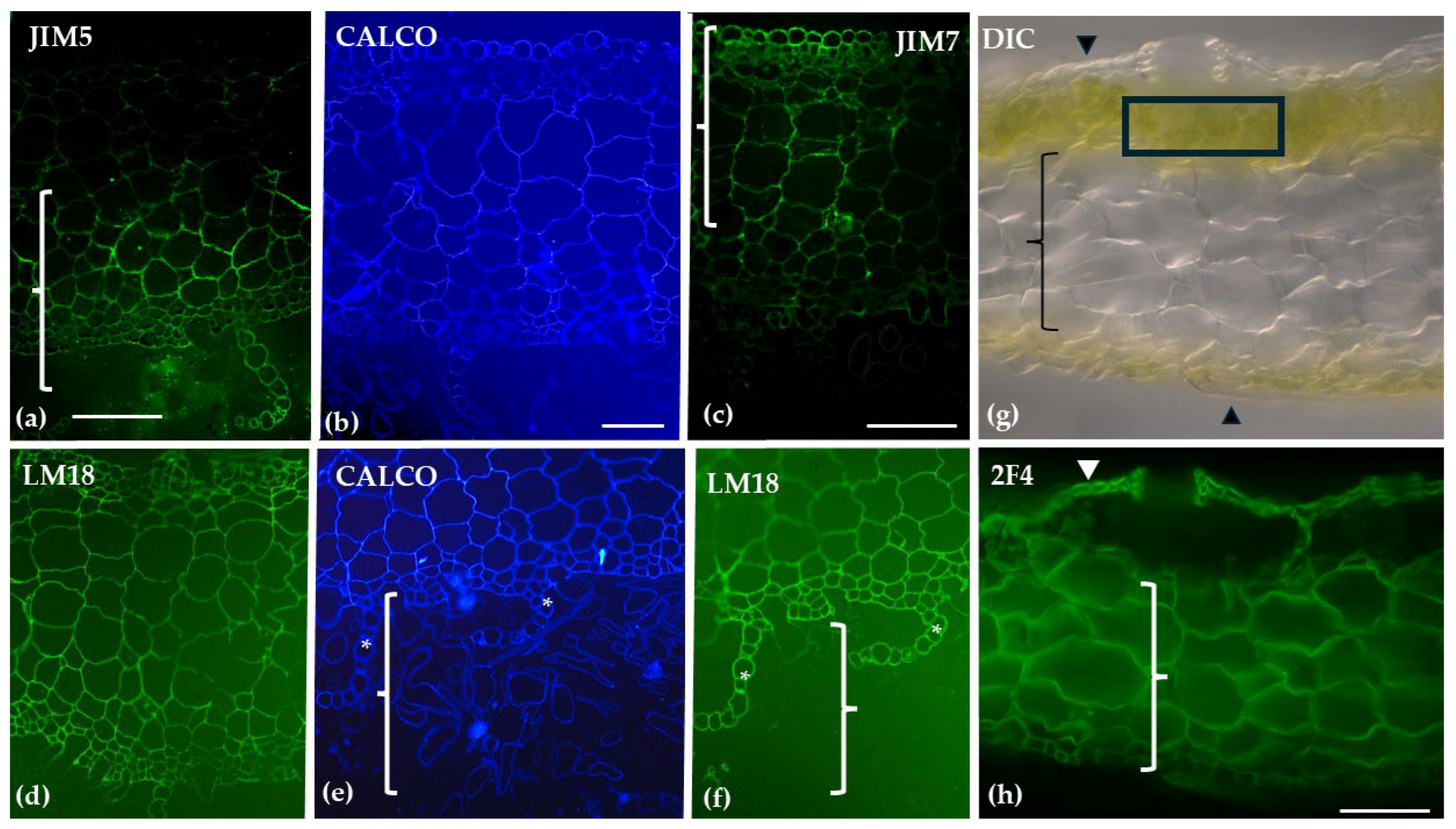

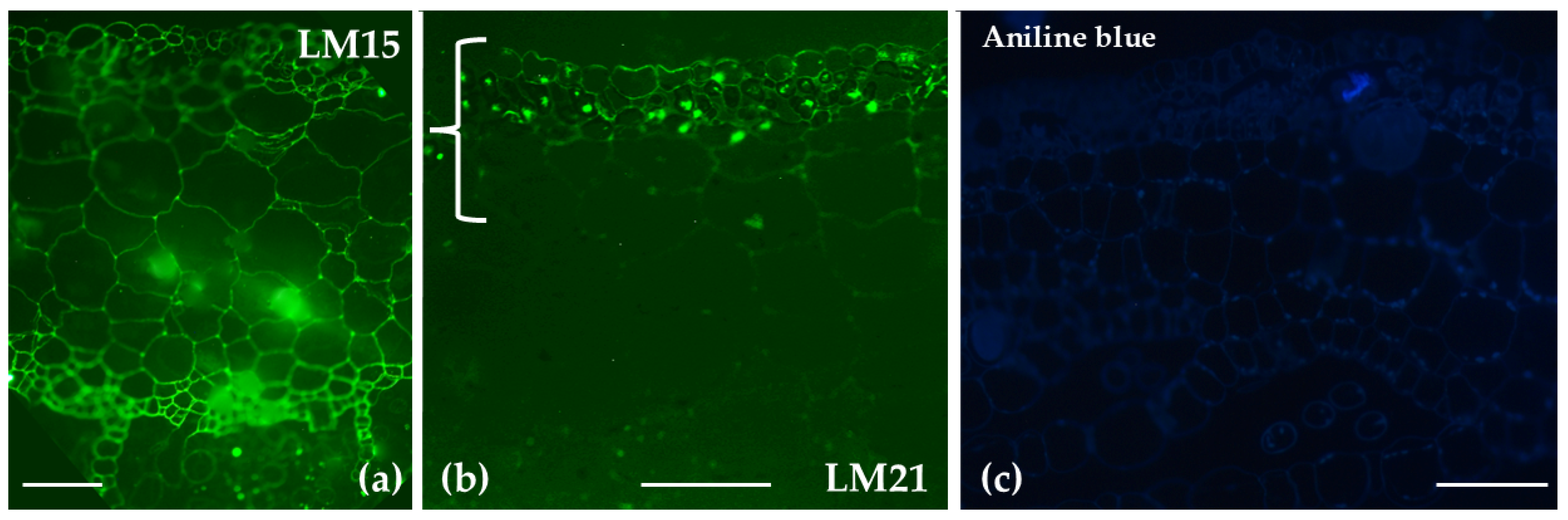
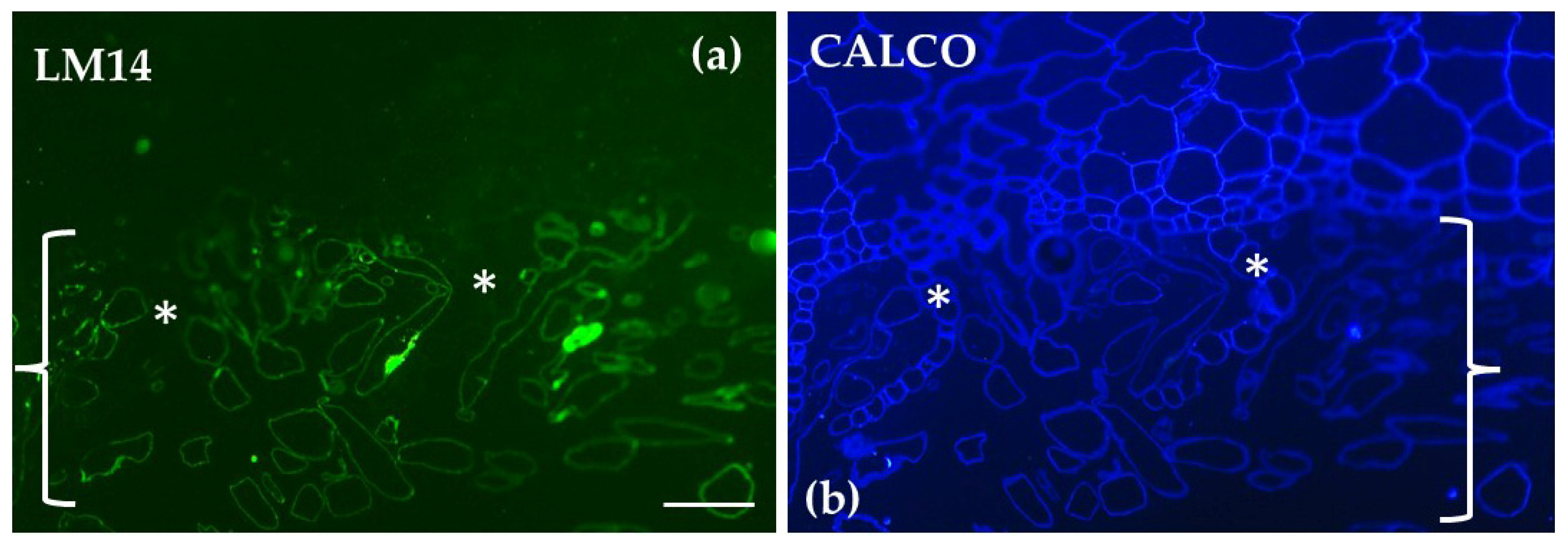

| Polysaccharide/ Protein | Antibody/ Label | Distribution in Sphagnum compactum | Distribution in Marchantia polymorpha |
|---|---|---|---|
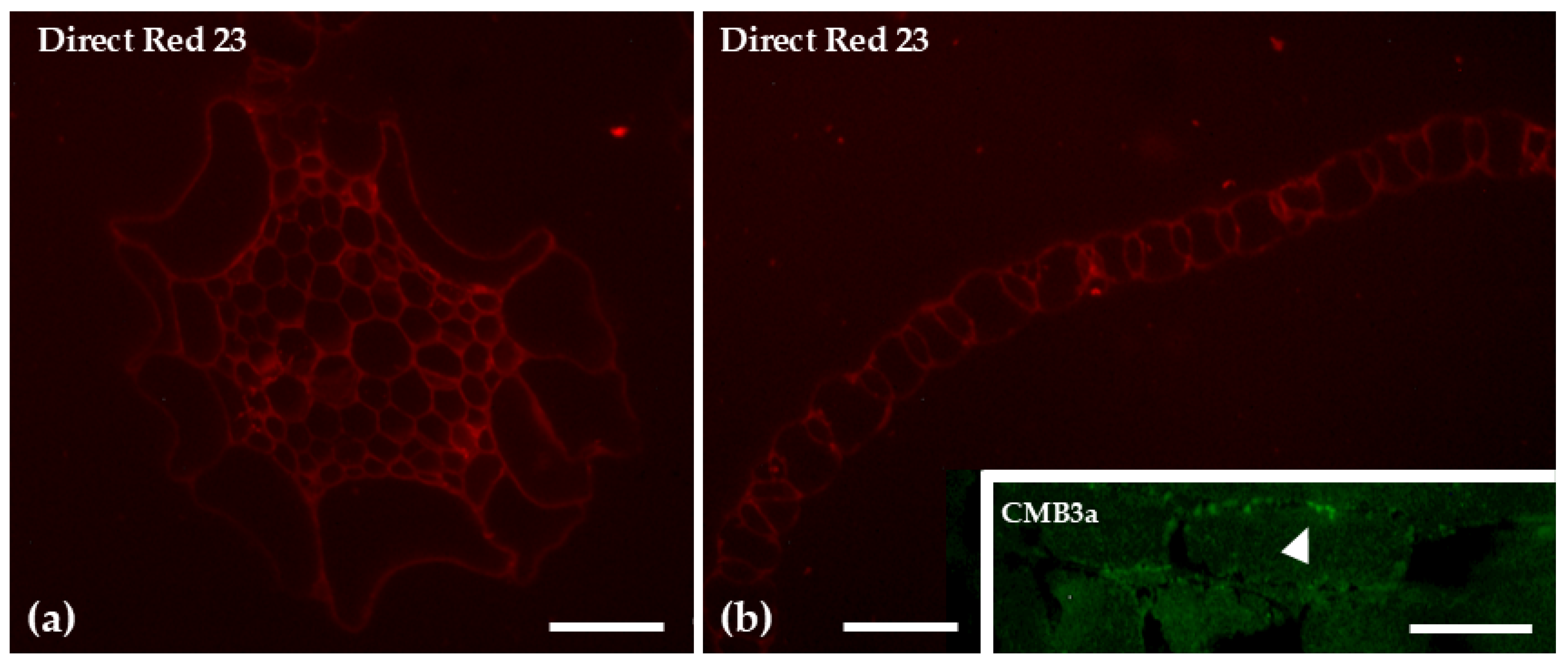
| Glucans | Direct Red 23 | Uniform and evenly distributed signal across the various tissues | Uniform and evenly distributed signal across the various tissues |
| CBM3a | Weak labeling in hyaline leaf cells | Weak labeling in chlorophyllous filaments and scales | |
| Aniline Blue | Staining was observed in the central cylinder of the stem, as well as at the cell junctions of the hyaline stem cells and the photosynthetic cells in the leaves | No prominent signal was detected | |
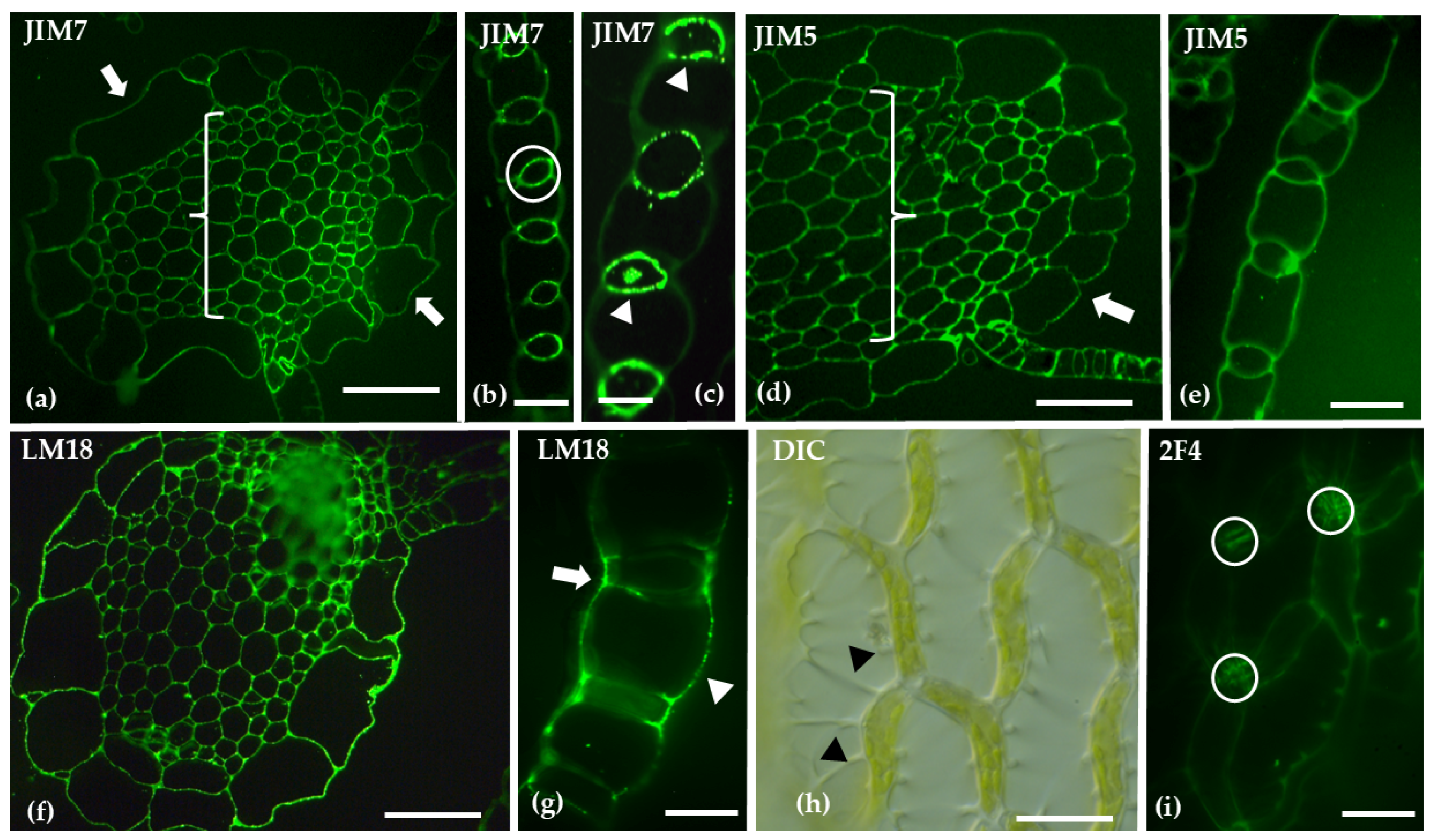
| Homogalacturonans (HGs) | JIM7 | Weak labeling in hyaline cell epidermis of stem; stronger in central cylinder | Polar distribution to upper epidermis and upper thallus parenchyma |
| LM18 | Widespread in stem; outer walls of hyaline and photosynthetic cells in branch leaves | Broader distribution across all histological regions, including scales; absent in the rhizoids | |
| JIM5 | Weak in hyaline cell epidermis of stem; stronger in central cylinder | Polar distribution to the lower epidermis/thallus parenchyma and the scales. | |
| 2F4 | Labeling was primarily concentrated on the transverse walls of chlorocyst cells in branch leaves | Signaling present in the upper epidermis and parenchymatic tissue, absent from the chlorophyllous filaments | |
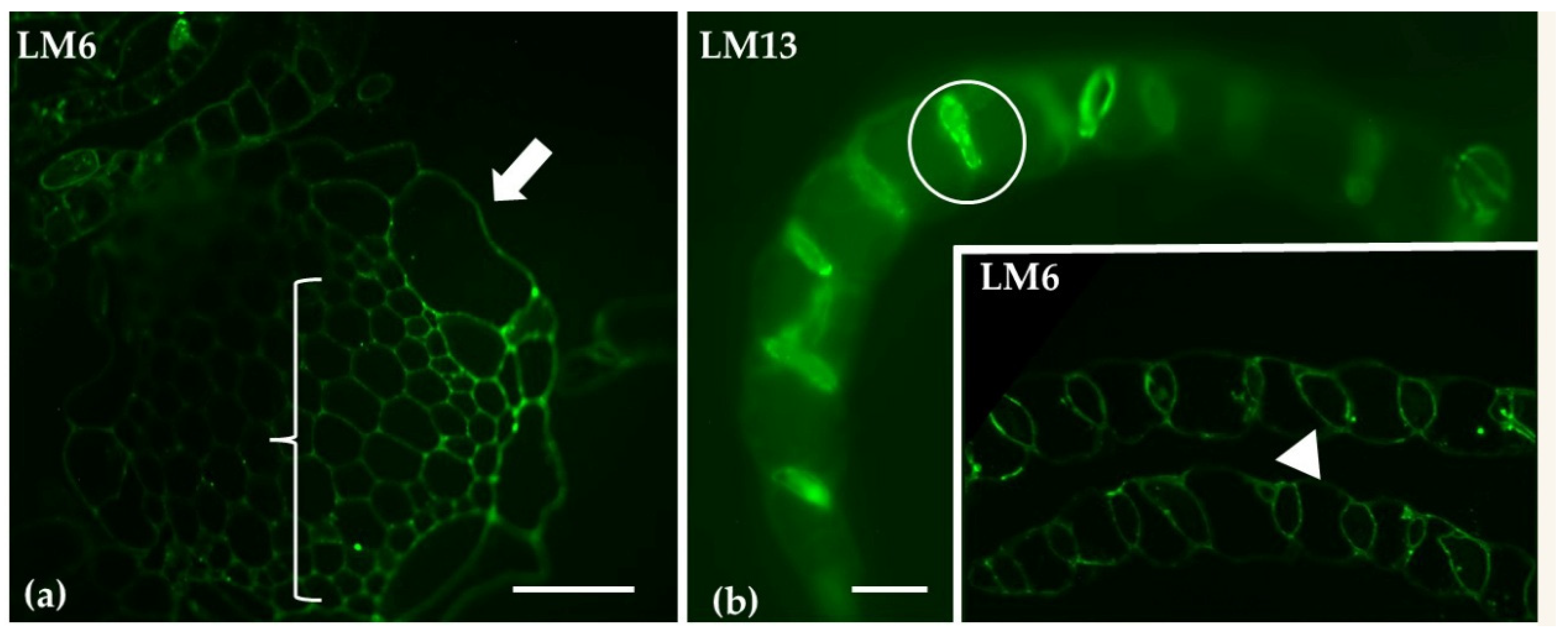
| Arabinans | LM6 | Reduced signal in stem; pronounced labeling in photosynthetic cells | Low signal in thallus parenchyma; labeling in idioblast cells |
| LM13 | Pronounced labeling in photosynthetic cells; reduced signal in stem | Low signaling throughout thallus parenchyma | |
| Xyloglucans | LM15 | Predominantly present at cell junctions in stem and branch leaves | Broad distribution across the thallus |
| Mannans | LM21 | Found in outer cell walls of hyaline and photosynthetic cells | Restricted to the upper epidermis, chlorophyllous filaments, and parenchyma closer to the upper epidermis |
| Arabinogalactan Proteins (AGPs) | LM14 | No signal in epidermis of hyaline cells; clear signal in central cylinder | Exclusively localized in the rhizoids; absent in other parts of the thallus |
| Antibody | Epitope Recognized |
|---|---|
| Crystalline cellulose | |
| CBM3a | Carbohydrate Binding Module family 3 |
| Hemicelluloses | |
| LM15 | XXLG and XLLG motifs of xyloglucan |
| LM21 | β-(1-4)-Manno-oligosaccharides from DP2 to DP5 |
| Pectins | |
| LM6 | (1→5)-α-L-arabinans |
| LM18 | Homogalacturonan (HG) domain of pectic polysaccharides (binds to both partially methyl-esterified and un-esterified HGs) |
| JΙΜ5 | HG domain of pectic polysaccharides (binds strongly to un-esterified HGs) |
| JIM7 | HG domain of pectic polysaccharides (requires methyl-esters for HG recognition, does not bind to un-esterified HGs) |
| 2F4 | Non-esterified or de-esterified HGAs that are cross-linked by calcium |
| Arabinogalactan Proteins (AGPs) | |
| LM14 | Arabinogalactan/AGP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamakis, I.-D.S.; Sotiriou, P.; Ntanou, N.; Nelson, J.M.; Giannoutsou, E. Tissue-Specific Differential Distribution of Cell Wall Epitopes in Sphagnum compactum and Marchantia polymorpha. Int. J. Mol. Sci. 2025, 26, 3602. https://doi.org/10.3390/ijms26083602
Adamakis I-DS, Sotiriou P, Ntanou N, Nelson JM, Giannoutsou E. Tissue-Specific Differential Distribution of Cell Wall Epitopes in Sphagnum compactum and Marchantia polymorpha. International Journal of Molecular Sciences. 2025; 26(8):3602. https://doi.org/10.3390/ijms26083602
Chicago/Turabian StyleAdamakis, Ioannis-Dimosthenis S., Penelope Sotiriou, Natalia Ntanou, Jessica M. Nelson, and Eleni Giannoutsou. 2025. "Tissue-Specific Differential Distribution of Cell Wall Epitopes in Sphagnum compactum and Marchantia polymorpha" International Journal of Molecular Sciences 26, no. 8: 3602. https://doi.org/10.3390/ijms26083602
APA StyleAdamakis, I.-D. S., Sotiriou, P., Ntanou, N., Nelson, J. M., & Giannoutsou, E. (2025). Tissue-Specific Differential Distribution of Cell Wall Epitopes in Sphagnum compactum and Marchantia polymorpha. International Journal of Molecular Sciences, 26(8), 3602. https://doi.org/10.3390/ijms26083602







