Abstract
Vitamin E, mainly encompassing tocopherols and tocotrienols, is an essential antioxidant synthesized in the photosynthetic tissues of plants and photosynthetic bacteria, as well as in certain algae, yet dietary intake often falls short of recommended levels. Although synthetic supplements are available, natural vitamin E demonstrates higher bioavailability, creating a need for biofortification strategies to enrich crops with this nutrient. Recent advances in molecular genetics have elucidated key components of the vitamin E biosynthesis pathway, uncovering complex regulatory mechanisms and expanding opportunities for genetic enhancement. This review integrates current advances in vitamin E biosynthesis, novel gene discovery, diverse biofortification strategies, and insights into transporter-mediated regulation to enhance tocopherol and tocotrienol levels in staple crops. By aligning these advances, this review provides a framework to drive innovative biofortification efforts, positioning vitamin E enrichment as a sustainable solution for improved human and animal health.
1. Introduction
Vitamin E, initially identified in 1922 by Evans and Bishop as a growth factor essential for mouse fertility [], has had its biosynthesis pathways and functional roles increasingly elucidated over time. It is primarily synthesized by photosynthetic organisms, such as plants and algae [,,]. Vitamin E contributes significantly to cellular processes, including scavenging reactive oxygen species (ROS), preventing lipid peroxidation, and protecting photosystems. It also aids in stabilizing biological membranes, facilitating signal transduction and glucose transport, and bolstering plant resilience to abiotic stressors [,,,,,,,,,]. Vitamin E is a crucial micronutrient that humans and animals must obtain exogenously due to their inability to synthesize it. Beyond its nutritional value, vitamin E demonstrates diverse biological activities, including antioxidant effects, anti-tumorigenic properties, cholesterol-lowering actions, neuroprotection, and prevention of cardiovascular and cerebrovascular diseases [,,,,,,,,,]. In animals, vitamin E is essential for growth and development, often requiring substantial supplementation in animal feeds to meet nutritional needs [,].
Vitamin E, mainly comprising tocopherols and tocotrienols, is widely distributed in nature. Tocopherols are primarily found in plant leaves, dicot seeds, and monocot embryos, while tocotrienols are predominantly present in the endosperm of most monocots []. Despite its broad availability, low dietary intake of vitamin E remains a significant concern, with many populations experiencing chronic deficiencies [,,]. Statistics up to 2024 show that 64% of the world’s population is deficient in vitamin E []. Observational studies suggest that a serum α-tocopherol concentration of over 30 μmol/L is associated with health benefits; however, a global survey indicated that only 21% of the population meets this threshold, highlighting widespread inadequacies in α-tocopherol levels [].
In China, the “Reference Intake of Dietary Nutrients for Chinese Residents (2023 Edition)” recommends a daily intake of 14 mg α-tocopherol equivalents (α-TE) for its population []. The animal feed industry is also a major consumer of vitamin E, with China reportedly incorporating 17,500 tons of synthetic vitamin E into animal feed annually, resulting in an expenditure of approximately RMB 2.6 billion [,]. This rising demand highlights the significant economic impact of vitamin E supplementation.
Natural vitamin E, primarily occurring as d-α-tocopherol, exhibits higher bioavailability and potency than its synthetic counterpart, which consists mostly of dl-α-tocopherol-acetate, a mixture of stereoisomers in which only half align with the natural form, leaving the remainder poorly recognized by the human body [,]. Given vitamin E’s indispensable role in human and animal health, as well as the economic impacts of synthetic supplementation, boosting the vitamin E content of plants (particularly staple crops) has become a critical goal. Doing so would not only address nutritional deficiencies but also decrease dependency on synthetic supplements, providing a sustainable solution to meet the vitamin E needs of both humans and livestock.
Advances in science and technology have clarified the biosynthesis pathway of vitamin E, paving the way for genetic engineering approaches to enhance its levels in crops. Nonetheless, the varied outcomes of these biofortification strategies reveal the complexity of regulatory mechanisms within plant systems. Recently identified regulatory factors, including newly discovered genes in the vitamin E biosynthesis pathway, can synergistically improve biofortification when integrated with previously known target genes. Studies show that overexpressing these novel elements—alongside established genes—can produce larger increases in vitamin E content, thereby enhancing the effectiveness of biofortification strategies in a range of crops []. This review summarizes current knowledge of the vitamin E biosynthesis pathway and examines various biofortification strategies aimed at increasing vitamin E content in crops. Additionally, it investigates potential regulatory factors affecting vitamin E synthesis and explores the transport mechanisms of vitamin E and its precursors, proposing innovative strategies to strengthen biofortification efforts. Considering the varied metabolic pathways for tocopherols and tocotrienols across different plant species, the prospects for vitamin E biofortification remain highly promising. Targeted approaches, leveraging newly discovered regulatory factors and tailored to each crop’s specific metabolism, can significantly boost vitamin E levels and overall nutritional value. By synthesizing these insights, this review aims to guide future research and application on adopting distinct biofortification strategies for different crops.
2. The Synthetic Pathway of Vitamin E
The biosynthetic pathway of vitamin E, an amphipathic molecule distinguished by an aromatic ring head and a phytyl side chain tail, is intricately regulated within plastids []. Vitamin E mainly includes two primary groups, tocopherols and tocotrienols, which differ in the saturation of the phytyl side chain (Figure 1). Each group is further divided into four isomeric forms—α, β, γ, and δ—based on the number and position of methyl groups on the aromatic ring, with α-tocopherol displaying the highest biological activity [,].
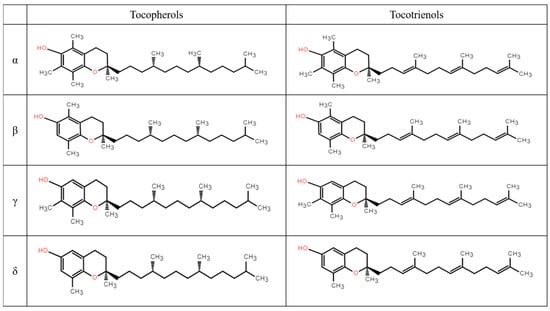
Figure 1.
Chemical structures of the α-, β- γ-, and δ- homologs of tocopherols and tocotrienols.
The core synthesis pathway of vitamin E begins with homogentisate (HGA) supplying the aromatic ring head, while geranylgeranyl-pyrophosphate (GGPP) and phytyl-pyrophosphate (PPP) contribute the phytyl side chains [,]. The enzyme homogentisate phytyltransferase (HPT/VTE2) catalyzes the condensation of HGA with PPP to form 2-methyl-6-phytylbenzoquinol (MPBQ), marking the commencement of tocopherol synthesis. Concurrently, homogentisate geranylgeranyltransferase (HGGT) facilitates the condensation of HGA with GGPP to form 2-methyl-6-geranylgeranylbenzoquinone (MGGBQ), initiating tocotrienol synthesis. Further enzymatic transformations convert MPBQ and MGGBQ into their respective derivatives, 2,3-dimethyl-5-phytylbenzoquinone (DMPBQ) and 2,3-dimethyl-5-geranylgeranylbenzoquinone (DMGGBQ), by MPBQ methyltransferase (MPBQ-MT, VTE3). Tocopherol cyclase (TC/VTE1) then catalyzes the conversion of these intermediates into δ-tocopherol, γ-tocopherol, δ-tocotrienol, and γ-tocotrienol. Subsequently, γ-tocopherol methyltransferase (γ-TMT/VTE4) methylates these compounds to produce β-tocopherol, α-tocopherol, β-tocotrienol, and α-tocotrienol (Figure 2).
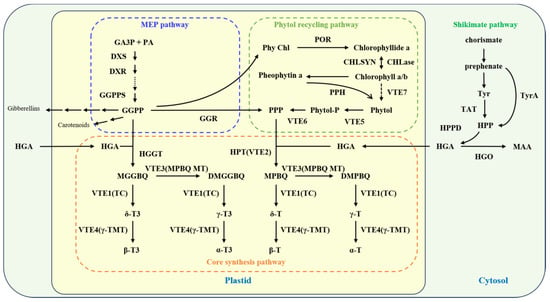
Figure 2.
Vitamin E synthesis pathway. Abbreviations: MGGBQ: 2-methyl-6-geranylgeranylbenzoquinone; DMGGBQ: 2,3-methyl-5-geranylgeranylbenzoquinone; δ-T3: δ-tocotrienol; γ-T3: γ-tocotrienol; β-T3: β-tocotrienol; α-T3: α-tocotrienol; MPBQ: 2-methyl-6-phytylbenzoquinone; DMPBQ: 2,3-Methyl-5-phytylquinone; δ-T: δ-tocopherol; γ-T: γ-tocopherol; β-T: β-tocopherol; α-T: α-tocopherol; VTE3(MPBQ MT): 2-methyl-6-phytoquinone methyltransferase; VTE1(TC): tocopherol cyclase; VTE4(γ-TMT): γ-tocopherol methyltransferase; HPT(VTE2): homogentisate phytyltransferase; HGGT: homogentisate geranylgeranyl transferase; HGA: homogentisate; PPP: phytyl pyrophosphate; GGPP: geranylgeranyl pyrophosphate; GGR: GGPP reductase; CHLSYN: chlorophyll synthase; GG Chl: geranylgeranyl chlorophyll; phy Chl: phytyl chlorophyll; VTE5: phytol kinase; VTE6: phytyl monophosphate kinase; VTE7: hydrolase with α/β esterase activity; phytyl-P: phytyl monophosphate; GA3P: glyceraldehyde 3-phosphate; PA: pyruvate; MEP pathway: methylerythritol phosphate pathway; HPP: 4-hydroxypyruvate; HPPD: 4-hydroxypyruvate dioxygenase; Tyr: L-tyrosine; TAT: tyrosine aminotransferase; TyrA: prephenate dehydrogenase; MAA: 4-maleyl-acetoacetate, 4-Maleylacetoacetic acid; DXS: deoxyxylulose-5-phosphate synthase; DXR: deoxyxylulose-5-phosphate reductoisomerase; GGPPS: geranylgeranyl pyrophosphate synthase; CHLase: chlorophyllase; PPH: pheophytin hydrolase; HGO: HGA dioxygenase.
Beyond the core pathway, the biosynthesis pathway of these precursors (HGA, PPP, GGPP) plays a crucial role in vitamin E synthesis. HGA is synthesized from 4-hydroxyphenylpyruvate (HPP) via HPP dioxygenase (HPPD) within the cytoplasmic shikimate pathway. In plants, HPP derives from tyrosine catabolism by tyrosine aminotransferase (TAT); in cyanobacteria, it is produced from chorismate/prephenate by chorismate/prephenate dehydrogenase (TyrA). The phytyl side chain precursors, GGPP and PPP, originate from the methylerythritol phosphate (MEP) pathway []. GGPP is synthesized by GGPP synthase (GGPPS) in plastids [], and PPP is formed by the reduction of GGPP through GGPP reductase (GGR) []. PPP can also derive from chlorophyll biosynthesis and degradation, involving chlorophyll synthase (CHLSYN) [,], chlorophyllase (CHLase) [], phytol kinase (VTE5), phytyl phosphate kinase (VTE6) [,,], and hydrolase (VTE7) [] (Figure 2). Because the biosynthesis of GGPP and PPP can vary widely among different plant species—both in terms of regulation and metabolic flux—biofortification strategies must be tailored to each species’ specific pathways. This targeted approach ensures that interventions effectively enhance vitamin E accumulation without disrupting other essential metabolic processes. In maize kernels, which have long been considered non-green tissues, research has identified two protochlorophyllide reductase (POR) genes associated with tocopherol content variations, confirming the roles of ZmPORB1 and ZmPORB2 in vitamin E synthesis [,,]. One of the main challenges in manipulating regulatory genes such as ZmPORB1 and ZmPORB2 is achieving tissue-specific expression without disrupting essential metabolic pathways or compromising overall plant growth. Additionally, maintaining metabolic balance—so that resource allocation to these pathways does not negatively affect related processes—is crucial for sustaining healthy crop development. Depending on the vitamin E synthesis pathway, biofortification strategies may involve overexpressing a single gene, multiple genes, or directing gene expression to specific tissues or organs to optimize vitamin E content. For example, seed-specific promoters can be employed to drive POR expression while combining these regulators with other vitamin E biosynthetic genes. However, such approaches require comprehensive validation to ensure stable expression, minimal side effects, and robust performance under field conditions.
3. Genetic and Regulatory Analysis of Vitamin E Synthesis
It is clear that the biofortification of tocopherols remains challenging, while the biofortification of tocotrienols and the conversion between vitamin E components has proven more effective. It is conceivable that there may be many unknowns in the anabolic pathway of tocopherol. In recent years, researchers have studied the genetic characteristics of vitamin E synthesis through QTL, linkage mapping (LM), and genome-wide association studies (GWASs) in order to provide new insights for the anabolic metabolism and biofortification of vitamin E.
Researchers first analyzed the vitamin E synthesis pathway in Arabidopsis, involving 36 enzymes encoded by 53 genes [,], fully elucidating the synthesis pathway of vitamin E synthesis and the synthesis pathway of its precursors. In recent years, in-depth research has been conducted on maize, and association analysis and linkage analysis have been conducted on an association population consisting of 513 maize materials. It was found that there are nine single nucleotide polymorphism (SNP) sites near the ZmTMT gene that affect the α-tocopherol content; resequencing analysis found that InDel 7 located in the 5′ untranslated region (5′UTR) of the ZmTMT gene and InDel 118 located in the promoter region significantly affect the α-tocopherol content. Based on this, molecular markers were developed, which facilitated the molecular marker-assisted breeding of maize with high α-tocopherol content and provided a reference for other studies []. Through a GWAS analysis of the American maize NAM (nested association mapping) population, it was found that HGGT1 and γ-TMT in the vitamin E synthesis pathway were significantly correlated with vitamin E content []. These findings indicate that natural variation in vitamin E content is primarily governed by the HGGT1 and γ-TMT genes, which explains the observed increase in tocotrienol levels through biofortification and the effective conversion between vitamin E components, while the impact of HPT overexpression remains moderate. This also suggests that vitamin E synthesis may be regulated by additional genes or genetic factors outside the core synthesis pathways. Genetic analysis in maize identified two SNP sites related to vitamin E synthesis within the intronic regions of a WRKY transcription factor gene and a PPR gene [], which are hypothesized to influence vitamin E synthesis, though detailed research remains lacking. Diepenbrock et al. identified a gene within QTL39 encoding a zinc finger domain transcription factor, an eQTL influencing α-tocotrienol content []. In a GWAS analysis of the relationship between tocopherol and fatty acids in soybean populations, the transcription factor GmZF351, which regulates fatty acid synthesis, was found to directly activate genes in the tocopherol synthesis pathway (Gmγ-TMT3, GmHPT, GmMPBQ-MT1, and GmHPPD1), increasing fatty acid and tocopherol contents in soybean seeds. This provides evidence for regulatory factors that control the expression of key enzyme genes in the vitamin E synthesis pathway []. These studies collectively suggest that the synthesis of plant vitamin E is subject to complex regulatory control. However, research on the regulation of vitamin E synthesis remains limited, highlighting a promising area for future investigation.
4. Biofortification of Vitamin E
Given the significance of vitamin E and the elucidation of its biosynthesis pathway, considerable efforts have been made to undertake biofortification targeting the precursors and the key enzyme genes within this pathway. Broadly, two principal strategies are employed. Firstly, vitamin E levels could be augmented by adding vitamin E precursors directly to cell cultures, providing a rapid method to assess biofortification effectiveness. Secondly, overexpressing key enzyme genes within the vitamin E biosynthetic pathway in plants could modulate precursor substances such as HGA, PPP, and GGPP. This approach can be combined with the enhanced expression of genes encoding rate-limiting enzymes like HPT and HGGT, which catalyze initial steps in vitamin E synthesis. Alternatively, upregulating γ-TMT expression could adjust the vitamin E composition by attenuating the proportion of less active components and converting them into more biologically active forms. A key objective of this approach is to increase the concentration of α-tocopherol, the most active form of vitamin E. This can be accomplished, which facilitates the conversion of lower-activity tocopherols into α-tocopherol. These two strategies can be applied independently or in combination to optimize the biofortification process, aiming to enhance the nutritional quality and health-promoting properties of crops through advanced genetic and biotechnological interventions. Given the significance of vitamin E and the elucidation of its biosynthesis pathway, considerable efforts have been made to undertake the biofortification targeting of the key enzyme genes within this pathway. Broadly, two principal strategies are employed. The first strategy involves augmenting overall vitamin E levels by modulating precursor substances such as HGA, PPP, and GGPP, coupled with enhancing the expression of genes encoding rate-limiting enzymes like HPT and HGGT, which catalyze the initial steps in vitamin E synthesis. The second strategy involves modifying the vitamin E composition by attenuating the proportion of less active components and converting them into more biologically active forms. A key objective of this approach is to increase the concentration of α-tocopherol, the most active form of vitamin E. This can be accomplished by upregulating the expression of γ-TMT, which facilitates the conversion of lower-activity tocopherols into α-tocopherol. These two strategies can be applied independently or in combination to optimize the biofortification process, aiming to enhance the nutritional quality and health-promoting properties of crops through advanced genetic and biotechnological interventions.
4.1. Addition of Vitamin E Precursors to Cell Cultures
Exogenous supplementation in plant cell cultures is a foundational approach to examining its effects on metabolite levels, particularly in vitamin E synthesis []. Studies reveal that the impact of added precursors varies with their type and concentration. In sunflower cell cultures, HGA supplementation at concentrations of as low as 100 mg/L and as high as 200 mg/L led to a 1.3-fold increase in α-tocopherol content, whereas the supplementation with phytol had no significant effect on tocopherol accumulation []. In safflower cultures, the tocopherol levels remained unchanged 3 days after the addition of HGA, but phytol addition led to a notable increase. After 14 days, both HGA and phytol supplementation led to significant enhancements in tocopherol content, showing a 3.3-fold and 18.4-fold increase, respectively []. In soybean suspension cultures, the tocopherol content tripled with the individual addition of HGA or phytol. When both were added together, the tocopherol content surged to six times the control level []. In some plant species, the efficacy of exogenous HGA and phytol supplementation is significantly influenced by species-specific differences in metabolic flux, subcellular compartmentalization, and regulatory control of key biosynthetic enzymes, causing variable responses to these precursors. Additionally, variations in enzyme expression, transport systems, and interactions with parallel pathways—such as chlorophyll biosynthesis, which also relies on phytol—can affect how efficiently supplemented HGA and phytol are converted into tocopherols. Consequently, future research should focus on identifying and alleviating metabolic bottlenecks, refining supplementation protocols (e.g., optimal dosages, delivery methods, timing), and exploring synergistic strategies (like combining supplementation with the overexpression of critical biosynthetic genes) to maximize vitamin E accumulation while sustaining normal plant growth.
Further, these cell cultures allow researchers to maintain precise control overgrowth conditions, nutrient supply, and precursor concentrations, making it easier to evaluate changes in vitamin E accumulation. Compared to in vivo approaches with whole plants, cell culture methods are faster and more amenable to large-scale screening; however, they do not fully replicate the complexity of intact organisms, where tissue-specific transport, developmental stages, and environmental factors can significantly influence vitamin E metabolism. Consequently, while in vitro experiments offer valuable insights and a foundation for optimizing precursor supplementation, subsequent validation in whole plants—ideally under field conditions—is essential to confirm real-world applicability.
4.2. Overexpression of Genes Involved in Vitamin E Biosynthetic Pathway in Plants
4.2.1. Boosting the Synthesis of HGA
The overexpression of HPPD, a crucial enzyme in the synthesis of HGA, has yielded varying results across different organisms in terms of vitamin E enhancement. In transgenic Arabidopsis, tobacco, and soybean, HPPD overexpression did not significantly increase the vitamin E content [,,,,]. In contrast, in cyanobacteria, HPPD overexpression resulted in a 7-fold increase in tocopherol content []. This disparity is likely due to differences in the HPP biosynthetic pathways between plants and bacteria. In bacteria, TyrA directly converts prephenate to HPP, whereas plants follow a more complex pathway, with tyrosine as an intermediate that feedback inhibits TyrA, tightly regulating HPP production and consequently limiting HGA and vitamin E synthesis []. Further research showed that the co-overexpression of HPPD and TyrA in Arabidopsis and tobacco significantly increased the vitamin E content, suggesting that enhancing HGA synthesis could boost vitamin E levels, particularly tocotrienols, in plants [,]. In transgenic Arabidopsis, canola, and soybean, this genetic modification resulted in notable increases in vitamin E content, highlighting the pivotal role of HGA in tocotrienol synthesis []. These findings suggest that while HGA availability is crucial for tocotrienol synthesis, its influence on tocopherol production may be less pronounced, reflecting a nuanced regulatory mechanism in vitamin E biosynthesis in plants. The simultaneous overexpression of HPPD and TyrA genes in transgenic Arabidopsis and soybean seeds led to substantial increases in HGA content—60-fold and 800-fold, respectively—resulting in the noticeable darkening of seed color []. However, this increase did not correlate with a proportional rise in vitamin E levels, suggesting that only a fraction of the newly synthesized HGA contributes to vitamin E synthesis, with possible alternative metabolic pathways for HGA utilization. In the soybean MO12 mutant, the mutation of HGA dioxygenase 1 (HGO1) caused a 30-fold increase in HGA but only a twofold increase in vitamin E content, indicating a regulatory mechanism that restricts HGA use for vitamin E synthesis []. In camelina, the simultaneous overexpression of HPPD and TyrA genes led to a 2.5-fold increase in vitamin E content, while the RNAi inhibition of HGO expression raised the vitamin E content by about 1.4 times. However, when HGO expression was inhibited in the transgenic lines overexpressing HPPD and TyrA, no further increase in vitamin E was observed []. HGO1’s role in converting HGA to MAA highlights its potential as a bottleneck in vitamin E biosynthesis. Notably, as in transgenic plants with elevated HGA, the rise in vitamin E levels in the MO12 mutant was primarily attributed to an increase in tocotrienols, without a significant change in tocopherol levels []. This reinforces the notion that HGA availability alone does not determine tocopherol levels. This observation raises a pertinent question regarding the role of other precursors like PPP or GGPP in vitamin E synthesis. Are these compounds more critical in determining the production of vitamin E, particularly tocopherols? Further investigation into the roles of PPP and GGPP may elucidate their relative importance to HGA in vitamin E biosynthesis and potentially reveal new biofortification targets to enhance tocopherol content in plants.
4.2.2. Genetic Enhancement of GGPP and PPP Contents
GGPP is a crucial precursor for several plant metabolites, including vitamin E, carotenoids, chlorophyll, gibberellins, and diterpenes, positioning GGPP synthesis as a key juncture in isoprenoid biosynthesis. Enhancing the expression of enzymes upstream in the GGPP synthesis pathway has been employed as a strategy to increase GGPP availability. The overexpression of deoxyxylulose-5-phosphate synthase (DXS), the initial rate-limiting enzyme in the MEP pathway, led to an approximate 2-fold increase in tocopherol content in Arabidopsis seedlings, primarily in the form of α-tocopherol []. This suggests that elevated DXS expression raises GGPP levels, subsequently increasing PPP and tocopherol production. However, this enhancement in isoprenoid content was not observed in mature plant leaves, indicating that DXS’s effect may be limited to younger tissues []. In contrast, overexpressing deoxyxylulose-5-phosphate reductoisomerase (DXR), the second key enzyme in the MEP pathway, did not result in a similar increase in tocopherol content, suggesting potential regulatory mechanisms or substrate limitations affecting pathway flux. Specifically, the concentration of D-5-deoxyxylulose phosphate, a substrate in the MEP pathway, may limit the effectiveness of DXR overexpression in boosting isoprenoid production []. To address the limitations in DXP production when overexpressing DXR, one potential strategy is co-expressing upstream genes like DXS (which synthesizes DXP) alongside DXR to ensure a balanced flux through the MEP pathway. Additionally, investigating other rate-limiting enzymes and regulatory elements in the MEP pathway (such as GcpE, LytB, or transcription factors that coordinate chloroplast development) could offer further avenues to enhance isoprenoid production—particularly in mature tissues, where physiological constraints often reduce metabolic plasticity. Similarly, increasing the expression of key enzymes in the PPP synthesis pathway has been used to improve the effectiveness of PPP. Studies in rice have shown that products of both GGR1 and GGR2 exhibit GGR activity, with double mutations in these genes significantly reducing tocopherol content, underscoring GGR’s critical role in tocopherol synthesis []. Additionally, in maize, the overexpression of ZmPORB increased the tocopherol content by 1.5-fold in leaves and 1.19-fold in grains []. In Arabidopsis, the vte7 mutant showed a 0.45-fold reduction in seed tocopherol content without a decrease in leaves, while in maize, the ZmVTE7 mutation reduced the total vitamin E content by 0.62-fold in kernels and 0.51-fold in leaves []. The overexpression of AtVTE7 and ZmVTE7 partially restored seed tocopherol levels and significantly increased the leaf tocopherol content by 3.6-fold and 6.9-fold in Atvte7 []. In Arabidopsis, the vte5 mutant showed a reduction in tocopherol to 0.2-fold in seeds and 0.35-fold in leaves, which was almost recovered by VTE5 overexpression. Additionally, VTE5 overexpression in wild-type plants slightly increased tocopherol levels []. Transgenic Arabidopsis seeds expressing the VTE6 gene showed a 1.15-fold increase in tocopherol content []. In green seeds, the simultaneous inhibition of CHLSYN and overexpression of HPT increased the tocopherol content in Arabidopsis seeds by over 2-fold []. These findings indicate that boosting PPP and GGPP levels has a more limited effect on overall vitamin E enhancement but notably influences the tocopherol content in green seeds. This suggests that other regulatory factors or mechanisms may influence tocopherol biosynthesis efficiency in plants. Collectively, these findings highlight the potential to amplify vitamin E accumulation by strategically combining these genes in plants.
4.2.3. Overexpression of Rate-Limiting Enzymes HPT and HGGT
The enzymes HPT and HGGT play crucial roles in vitamin E biosynthesis, rendering them key targets for genetic engineering aimed at enhancing the total vitamin E content. The overexpression of AtHPT in various plants has led to significant increases in α-tocopherol levels across different tissues, although the extent varies by plant species and tissue type. For example, the overexpression of AtHPT increased α-tocopherol levels in the leaves of Arabidopsis, tobacco, potato, lettuce, and rice by 4.2, 5.4, 4.6, 2.7, and 5.3 times, respectively [,,,,,,]. However, these studies also showed that tocopherol increases in other organs were less pronounced, particularly in soybean, rapeseed, and potato tubers, where levels increased by only 1.75, 1.2, and 2.2 times, respectively. This suggests that HPT overexpression may have a more substantial impact on tocopherol biosynthesis in vegetative tissues compared to reproductive tissues, such as seeds. Further experiments with HPT overexpression from different sources, such as apple MdHPT1 in tomato and lettuce in Arabidopsis, also led to substantial increases in α-tocopherol content in leaves by 3.6 and 18 times, respectively [,]. Combining the overexpression of HPT with other genes in the vitamin E pathway has shown additive effects, particularly in leaf tissues, though less so in seeds. For instance, the co-overexpression of AtHPT with AtVTE7 in Arabidopsis [] and with AtTC in rice [] led to substantial increases in leaf tocopherol levels by 7.4 and 5.3 times, respectively. These findings suggest that while HPT activity is crucial for tocopherol synthesis in leaves, it may not be the primary limiting factor in seed tocopherol biosynthesis. This indicates a nuanced regulation of tocopherol biosynthesis across different plant tissues and developmental stages, providing valuable insights for targeted biofortification strategies to enhance the vitamin E content. The overexpression of the barley HvHGGT has been shown to increase tocotrienol levels across various crops, approximately 1.1 to 1.15-fold in barley grains, 6-fold in maize kernels, 10~15-fold in Arabidopsis leaves, and 10-fold and 3-fold in soybean and upland cotton grains, all with notable increases in the tocotrienol content [,,,]. Moreover, overexpressing rice OsHGGT in tobacco resulted in a 3.4~4.7-fold increase in leaf vitamin E content []. This indicates that HGGT is pivotal in tocotrienol biosynthesis. However, in maize, while tocotrienol levels in kernels and extracted oil increased 18-fold, tocopherol content decreased by 18% [], suggesting a potential metabolic trade-off between tocopherol and tocotrienol biosynthesis when HGGT is overexpressed. These findings imply that while HGA, a precursor for both tocopherols and tocotrienols, is vital, its availability may limit synthesis under high demand, affecting the balance between tocopherol and tocotrienol accumulation. Thus, enhancing HGGT activity is a promising strategy for boosting tocotrienol levels, but the impact on overall vitamin E composition and potential trade-offs with tocopherol synthesis must be carefully considered in biofortification efforts. Overall, there have been no reports of adverse effects of the overexpression of HPT and HGGT on plants.
4.2.4. Increase the Content of Highly Active α-Tocopherol
The overexpression of γ-TMT boosts the conversion of less active vitamin E forms (γ- and δ-tocopherol/tocotrienol) into their more potent α- and β-counterparts, yet research has primarily centered on α-tocopherol because (1) α-tocopherol is preferentially recognized by the α-tocopherol transfer protein (α-TTP) in humans and animals, and (2) the δ- and β- forms typically occur at lower levels and exhibit lower bioactivity. Consequently, enhancing α-tocopherol emerges as a key focus in biofortification efforts. The overexpression of the γ-TMT gene has been shown to significantly increase the α-tocopherol content by 80-fold and shift the α-/γ-tocopherol ratio from 0.01 to 13 across various plant species, underscoring its potential in biofortification strategies to improve the vitamin E profile in crops, indicating the naturally low activity of γ-TMT in wild-type seeds []. Similar enhancements were observed in transgenic lettuce T2 lines expressing the Arabidopsis AtTMT gene, where the α-/γ-tocopherol ratio increased from 1.0 to 138.0 []. Further, the overexpression of AtTMT in mustard and tobacco resulted in 6-fold and 9.6-fold increases in α-tocopherol content, respectively [,]. In soybeans, the introduction of AtTMT, PfTMT (from Perilla frutescens), and BnTMT (from Brassica napus) increased the α-tocopherol content in seeds by 4, 10.4, and 11.1-fold, respectively, illustrating the efficacy of cross-species gene transfers in enhancing α-tocopherol levels [,,]. Similarly, the overexpression of MsTMT in Arabidopsis demonstrated an 11-fold increase in seed α-tocopherol content [,]. The co-overexpression of AtTMT and AtHPT in potato led to a 2.6 to 2.8-fold increase in α-tocopherol content in tubers and a 4.8 to 5.1-fold increase in leaves, suggesting a synergistic effect []. In maize, the overexpression of soybean GmTMT2a and maize ZmTMT converted over 90% of γ-tocopherol in kernels to α-tocopherol [,], significantly altering the vitamin E profile and also increasing the α-tocotrienol content []. Overexpressing the γ-TMT gene specifically enhances levels of α-tocopherol, the most biologically active form of vitamin E, without affecting the total tocopherol content. This targeted increase highlights γ-TMT’s role in improving plant nutritional quality by selectively enriching the α-form content while maintaining overall vitamin E quantity. Similarly, the overexpression of the MPBQ MT does not modify the total tocopherol content but alters the tocopherol composition. The concurrent overexpression of γ-TMT and MPBQ-MT shows a synergistic effect, as seen in transgenic soybean seeds where the α-tocopherol content increased 8-fold, with α-tocopherol comprising over 95% of total tocopherols []. This additive effect underscores the potential of combinatorial gene overexpression strategies to optimize vitamin E composition in crops. Additionally, the co-overexpression of HPT with γ-TMT not only boosts the total tocopherol content but also significantly increases the α-tocopherol proportion [,]. This suggests that the simultaneous upregulation of these genes effectively enhances both tocopherol quantity and quality, presenting a promising biofortification approach to increase the α-tocopherol content for improved nutritional outcomes. In summary, the overexpression of γ-TMT drives the robust conversion of γ-tocopherol to α-tocopherol in both vegetative and reproductive tissues, though the extent of this conversion can vary based on tissue-specific factors. Increasing α-tocopherol does not appear to diminish the levels or function of other antioxidant compounds, suggesting minimal competition among different antioxidant pathways. Moreover, a higher α-tocopherol content can enhance the plant’s resilience to biotic and abiotic stresses, potentially by boosting overall antioxidant capacity and reinforcing defense mechanisms. Current biofortification efforts for vitamin E primarily rely on conventional transformation techniques to overexpress key genes such as HPT and HGGT. While CRISPR/Cas9 gene editing holds great promise for precisely enhancing these pathways, its large-scale application is presently limited by the lack of identified negative regulatory factors in the vitamin E biosynthetic pathway. Once these factors are discovered, CRISPR-based approaches could be deployed to inactivate them, offering a more targeted and potentially scalable strategy for boosting the vitamin E content in staple crops.
Synthesizing the findings of various research reports, it becomes evident that the outcomes of biofortification using different strategies vary significantly (Table 1). Notably, the enhancement effects of HGGT and γ-TMT stand out. This variability suggests the presence of complex regulatory mechanisms within plants []. Consequently, these observations have encouraged researchers to pursue more detailed investigations into the genetic regulation of vitamin E biosynthesis.

Table 1.
Summary of vitamin E biofortification.
5. Perspectives
Vitamin E is predominantly synthesized within the plastids, yet certain genes associated with its synthesis pathway are also located in other cellular compartments, such as the cytoplasm. The synthesis process necessitates the translocation of some substrates or products across the plastid membrane to the endoplasmic reticulum or other organelles for functional roles []. Despite the importance of this process, there is a notable scarcity of reports on proteins or transporters in plants that bind or transport vitamin E []. To date, the SlTBP protein found in tomato represents the only identified plant protein involved in the intracellular transport of α-tocopherol. This plastidial protein also plays a role in regulating lipid transport within tomato and is expressed in source leaves, sink leaves, and fruits. Nevertheless, the precise mechanisms by which SlTBP contributes to vitamin E transport require further elucidation []. The transport of precursor substances crucial for vitamin E synthesis is a significant focus for future research. HGA originates from the breakdown of Tyr within the shikimate pathway, where Tyr is transformed into HPP by TAT, followed by the conversion of HPP into HGA by HPPD. Recent findings indicate that TAT1 is localized in the cytoplasm [], and HPPD has been identified in both the chloroplast and cytoplasm [,], suggesting that HGA synthesis may occur entirely or partially in the cytoplasm before being transported to the plastid for vitamin E synthesis via an as-yet unidentified transporter. Furthermore, the identification of HGO in soybean within the cytoplasm suggests that, at least in soybeans, a mechanism exists for transporting HGA from the cytosol to the plastid for vitamin E synthesis [].
PDP is derived from chlorophyll synthesis and degradation pathways, yet there is limited research on the relationship between CHLSYN, CHLase, and vitamin E synthesis. Additionally, no studies confirm a direct role of chlorophyll degradation in vitamin E synthesis. Thus, producing significant quantities of PDP through chlorophyll degradation to boost vitamin E production presents a key challenge for future genetic engineering in vitamin E biosynthesis.
In conclusion, future research on vitamin E biosynthesis should prioritize the following: (1) identifying new genes involved in vitamin E synthesis to deepen our understanding of the biosynthetic pathway; (2) integrating biofortification strategies with the genetic framework of vitamin E-related genes, with a focus on regulatory mechanisms; and (3) developing novel tools or methodologies to study the vitamin E transporters and precursor transport, which will advance our knowledge and facilitate further progress in vitamin E biofortification.
Author Contributions
Screening the literature, Y.L. (Yanjiao Li), D.Y. and Y.R.; writing—original draft preparation, Y.L. (Yanjiao Li) and L.Z.; writing—review and editing, L.W. and L.Z.; supervision, Y.L. (Yanzhong Luo), H.Z., Y.L. (Yuan Liu), L.W. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Hainan Province Academician Innovation Platform Research Project (YSPTZX202140), and the Innovation Program of the Chinese Academy of Agricultural Sciences.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Sussmann, R.A.; Angeli, C.B.; Peres, V.J.; Kimura, E.A.; Katzin, A.M. Intraerythrocytic stages of Plasmodium falciparum biosynthesize vitamin E. FEBS Lett. 2011, 585, 3985–3991. [Google Scholar] [CrossRef] [PubMed]
- Mène-Saffrané, L.; Pellaud, S. Current strategies for vitamin E biofortification of crops. Curr. Opin. Biotechnol. 2017, 44, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in Plants: Novel Concepts, New Perspectives, and Outstanding Issues. Antioxid. Redox Signal. 2020, 32, 463–485. [Google Scholar] [CrossRef]
- Che, P.; Zhao, Z.Y.; Glassman, K.; Dolde, D.; Hu, T.X.; Jones, T.J.; Gruis, D.F.; Obukosia, S.; Wambugu, F.; Albertsen, M.C. Elevated vitamin E content improves all-trans beta-carotene accumulation and stability in biofortified sorghum. Proc. Natl. Acad. Sci. USA 2016, 113, 11040–11045. [Google Scholar] [CrossRef]
- FRYER, M.J. The antioxidant effects of thylakoid Vitamin E (α-tocopherol). Plant Cell Environ. 1992, 15, 381–392. [Google Scholar] [CrossRef]
- Salimath, S.S.; Romsdahl, T.B.; Konda, A.R.; Zhang, W.; Cahoon, E.B.; Dowd, M.K.; Wedegaertner, T.C.; Hake, K.D.; Chapman, K.D. Production of tocotrienols in seeds of cotton (Gossypium hirsutum L.) enhances oxidative stability and offers nutraceutical potential. Plant Biotechnol. J. 2021, 19, 1268–1282. [Google Scholar] [CrossRef]
- Elstner, E.F. Oxygen radicals—biochemical basis for their efficacy. Klin. Wochenschr. 1991, 69, 949–956. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Matringe, M.; Ksas, B.; Rey, P.; Havaux, M. Tocotrienols, the unsaturated forms of vitamin E, can function as antioxidants and lipid protectors in tobacco leaves. Plant Physiol. 2008, 147, 764–778. [Google Scholar] [CrossRef]
- Muñoz, P.; Munné-Bosch, S. Vitamin E in Plants: Biosynthesis, Transport, and Function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Hussain, S.; Hussain, N.; Kakar, K.U.; Shah, J.M.; Zaidi, S.H.R.; Jan, M.; Zhang, K.; Khan, M.A.; Imtiaz, M. Tocopherol as plant protector: An overview of Tocopherol biosynthesis enzymes and their role as antioxidant and signaling molecules. Acta Physiol. Plant. 2022, 44, 1–11. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef]
- Lan, D.; Yao, C.; Li, X.; Liu, H.; Wang, D.; Wang, Y.; Qi, S. Tocopherol attenuates the oxidative stress of BMSCs by inhibiting ferroptosis through the PI3k/AKT/mTOR pathway. Front. Bioeng. Biotechnol. 2022, 10, 938520. [Google Scholar] [CrossRef]
- Eitsuka, T.; Nakagawa, K.; Miyazawa, T. Down-regulation of telomerase activity in DLD-1 human colorectal adenocarcinoma cells by tocotrienol. Biochem. Biophys. Res. Commun. 2006, 348, 170–175. [Google Scholar]
- He, L.; Mo, H.; Hadisusilo, S.; Qureshi, A.A.; Elson, C.E. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J. Nutr. 1997, 127, 668–674. [Google Scholar]
- Khanna, S.; Roy, S.; Ryu, H.; Bahadduri, P.; Swaan, P.W.; Ratan, R.R.; Sen, C.K. Molecular basis of vitamin E action: Tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J. Biol. Chem. 2003, 278, 43508–43515. [Google Scholar]
- Qureshi, A.A.; Peterson, D.M.; Hasler-Rapacz, J.O.; Rapacz, J. Novel tocotrienols of rice bran suppress cholesterogenesis in hereditary hypercholesterolemic swine. J. Nutr. 2001, 131, 223–230. [Google Scholar]
- Qureshi, A.A.; Sami, S.A.; Salser, W.A.; Khan, F.A. Synergistic effect of tocotrienol-rich fraction (TRF25) of rice bran and lovastatin on lipid parameters in hypercholesterolemic humans. J. Nutr. Biochem. 2001, 12, 318–329. [Google Scholar]
- Sen, C.K.; Khanna, S.; Roy, S.; Packer, L. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60c-Src kinase activation and death of HT4 neuronal cells. J. Biol. Chem. 2000, 275, 13049–13055. [Google Scholar]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Serbinova, E.; Khwaja, S.; Reznick, A.Z.; Packer, L. Thioctic acid protects against ischemia-reperfusion injury in the isolated perfused langendorff heart. Free Radic. Res. Commun. 1992, 17, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.M.; Bhuvanendran, S.; Nair, R.S.; Radhakrishnan, A.K. Exploring the anti-inflammatory activities, mechanism of action and prospective drug delivery systems of tocotrienol to target neurodegenerative diseases. F1000 Res. 2023, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- National Feed Work Office CFIA. China Feed Industry Yearbook 2006/2007; China Commercial Press: Beijing, China, 2008. [Google Scholar]
- National Feed Work Office CFIA. China Feed Industry Yearbook 2008; China Commercial Press: Beijing, China, 2009. [Google Scholar]
- Hunter, S.C.; Cahoon, E.B. Enhancing vitamin E in oilseeds: Unraveling tocopherol and tocotrienol biosynthesis. Lipids 2007, 42, 97–108. [Google Scholar] [CrossRef]
- Kim, Y.N.; Cho, Y.O. Vitamin E status of 20-to 59-year-old adults living in the Seoul metropolitan area of South Korea. Nutr. Res. Pract. 2015, 9, 192–198. [Google Scholar] [CrossRef]
- Polito, A.; Intorre, F.; Andriollo-Sanchez, M.; Azzini, E.; Raguzzini, A.; Meunier, N.; Ducros, V.; O’Connor, J.M.; Coudray, C.; Roussel, A.M.; et al. Estimation of intake and status of vitamin A, vitamin E and folate in older European adults: The ZENITH. Eur. J. Clin. Nutr. 2005, 59, S42–S47. [Google Scholar] [CrossRef]
- Chaudhary, R.; Chaturvedi, S.; Sharma, R.; Tiwari, S. Global Scenario of Vitamin Deficiency and Human Health. In Advances in Agri-Food Biotechnology; Sharma, T.R., Deshmukh, R., Sonah, H., Eds.; Springer Singapore: Singapore, 2020; pp. 199–220. [Google Scholar]
- Passarelli, S.; Free, C.M.; Shepon, A.; Beal, T.; Batis, C.; Golden, C.D. Global estimation of dietary micronutrient inadequacies: A modelling analysis. Lancet Glob. Health 2024, 12, e1590–e1599. [Google Scholar] [CrossRef]
- Peter, S.; Friedel, A.; Roos, F.F.; Wyss, A.; Eggersdorfer, M.; Hoffmann, K.; Weber, P. A Systematic Review of Global Alpha-Tocopherol Status as Assessed by Nutritional Intake Levels and Blood Serum Concentrations. J. Int. Vitaminol. Nutr. 2015, 85, 261–281. [Google Scholar] [CrossRef]
- Society, C.N. Reference Intake of Dietary Nutrients for Chinese Residents in 2023. J. Nutr. 2023, 45, 414. [Google Scholar] [CrossRef]
- Borel, P.; Preveraud, D.; Desmarchelier, C. Bioavailability of vitamin E in humans: An update. Nutr. Rev. 2013, 71, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Metabolism of natural forms of vitamin E and biological actions of vitamin E metabolites. Free. Radic. Biol. Med. 2022, 179, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Kim, S.; Magallanes-Lundback, M.; Bao, Y.; Deason, N.; Danilo, B.; Wu, D.; Li, X.; Wood, J.C.; Bornowski, N.; et al. Genome-wide association identifies a missing hydrolase for tocopherol synthesis in plants. Proc. Natl. Acad. Sci. USA 2022, 119, e2113488119. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhang, Q.; Wang, J.; Li, Y.; Wang, X.; Bao, Y. Vitamin E synthesis and response in plants. Front. Plant Sci. 2022, 13, 994058. [Google Scholar] [CrossRef]
- Herbers, K. Vitamin production in transgenic plants. J. Plant Physiol. 2003, 160, 821–829. [Google Scholar]
- Wang, H.; Xu, S.; Fan, Y.; Liu, N.; Zhan, W.; Liu, H.; Xiao, Y.; Li, K.; Pan, Q.; Li, W.; et al. Beyond pathways: Genetic dissection of tocopherol content in maize kernels by combining linkage and association analyses. Plant Biotechnol. J. 2018, 16, 1464–1475. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Ren, G.; Li, D.; Cahoon, R.E.; Chen, M.; Zhou, Y.; Yu, B.; Cahoon, E.B. Chlorophyll Synthase under Epigenetic Surveillance Is Critical for Vitamin E Synthesis, and Altered Expression Affects Tocopherol Levels in Arabidopsis. Plant Physiol. 2015, 168, 1503–1511. [Google Scholar] [CrossRef]
- Ruiz-Sola, M.Á.; Coman, D.; Beck, G.; Barja, M.V.; Colinas, M.; Graf, A.; Welsch, R.; Rütimann, P.; Bühlmann, P.; Bigler, L.; et al. Arabidopsis GERANYLGERANYL DIPHOSPHATE SYNTHASE 11 is a hub isozyme required for the production of most photosynthesis-related isoprenoids. New Phytol. 2016, 209, 252–264. [Google Scholar] [CrossRef]
- Tanaka, R.; Rothbart, M.; Oka, S.; Takabayashi, A.; Takahashi, K.; Shibata, M.; Myouga, F.; Motohashi, R.; Shinozaki, K.; Grimm, B.; et al. LIL3, a light-harvesting-like protein, plays an essential role in chlorophyll and tocopherol biosynthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 16721–16725. [Google Scholar] [CrossRef]
- Qin, P.; Chen, P.; Zhou, Y.; Zhang, W.; Zhang, Y.; Xu, J.; Gan, L.; Liu, Y.; Romer, J.; Dörmann, P.; et al. Vitamin E biofortification: Enhancement of seed tocopherol concentrations by altered chlorophyll metabolism. Front. Plant Sci. 2024, 15, 1344095. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, T.; Ren, G.; Hörtensteiner, S.; Zhou, Y.; Cahoon, E.B.; Zhang, C. Chlorophyll degradation: The tocopherol biosynthesis-related phytol hydrolase in Arabidopsis seeds is still missing. Plant Physiol. 2014, 166, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, E.C.; Ntourou, T.; Goulas, V.; Manganaris, G.A.; Kalaitzis, P.; Fotopoulos, V. Temporal analysis reveals a key role for VTE5 in vitamin E biosynthesis in olive fruit during on-tree development. Front. Plant Sci. 2015, 6, 871. [Google Scholar] [CrossRef]
- Almeida, J.; Azevedo, S.; Spicher, L.; Glauser, G.; vom Dorp, K.; Guyer, L.; del Valle Carranza, A.; Asis, R.; de Souza, A.P.; Buckeridge, M.; et al. Down-regulation of tomato PHYTOL KINASE strongly impairs tocopherol biosynthesis and affects prenyllipid metabolism in an organ-specific manner. J. Exp. Bot. 2016, 67, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Vom Dorp, K.; Hölzl, G.; Plohmann, C.; Eisenhut, M.; Abraham, M.; Weber, A.P.; Hanson, A.D.; Dörmann, P. Remobilization of phytol from chlorophyll degradation is essential for tocopherol synthesis and growth of Arabidopsis. Plant Cell 2015, 27, 2846–2859. [Google Scholar] [CrossRef]
- Diepenbrock, C.H.; Kandianis, C.B.; Lipka, A.E.; Magallanes-Lundback, M.; Vaillancourt, B.; Gongora-Castillo, E.; Wallace, J.G.; Cepela, J.; Mesberg, A.; Bradbury, P.J.; et al. Novel loci underlie natural variation in Vitamin E levels in maize grain. Plant Cell 2017, 29, 2374–2392. [Google Scholar] [CrossRef]
- Liu, N.; Du, Y.; Yan, S.; Chen, W.; Deng, M.; Xu, S.; Wang, H.; Zhan, W.; Huang, W.; Yin, Y.; et al. The light and hypoxia induced gene ZmPORB1 determines tocopherol content in the maize kernel. Sci. China Life Sci. 2024, 67, 435–448. [Google Scholar] [CrossRef]
- Zhan, W.; Liu, J.; Pan, Q.; Wang, H.; Yan, S.; Li, K.; Deng, M.; Li, W.; Liu, N.; Kong, Q.; et al. An allele of ZmPORB2 encoding a protochlorophyllide oxidoreductase promotes tocopherol accumulation in both leaves and kernels of maize. Plant J. Cell Mol. Biol. 2019, 100, 114–127. [Google Scholar] [CrossRef]
- Muthulakshmi, M.; Srinivasan, A.; Srivastava, S. Antioxidant Green Factories: Toward Sustainable Production of Vitamin E in Plant In Vitro Cultures. ACS Omega 2023, 8, 3586–3605. [Google Scholar] [CrossRef]
- Caretto, S.; Bray, S.; Fachechi, C.; Gala, R.; Zacheo, G.; Giovinazzo, G. Enhancement of vitamin E production in sunflower cell cultures. Plant Cell Rep. 2004, 23, 174–179. [Google Scholar] [CrossRef]
- Furuya, T.; Yoshikawa, T.; Kimura, T.; Kaneko, H. Production of tocopherols by cell culture of safflower. Phytochemistry 1987, 26, 2741–2747. [Google Scholar] [CrossRef]
- Karunanandaa, B.; Qi, Q.; Hao, M.; Baszis, S.R.; Jensen, P.K.; Wong, Y.-H.H.; Jiang, J.; Venkatramesh, M.; Gruys, K.J.; Moshiri, F.; et al. Metabolically engineered oilseed crops with enhanced seed tocopherol. Metab. Eng. 2005, 7, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Tsegaye, Y.; Shintani, D.K.; DellaPenna, D. Overexpression of the enzyme p-hydroxyphenolpyruvate dioxygenase in Arabidopsis and its relation to tocopherol biosynthesis. Plant Physiol. Biochem. 2002, 40, 913–920. [Google Scholar] [CrossRef]
- Zhang, C.; Cahoon, R.E.; Hunter, S.C.; Chen, M.; Han, J.; Cahoon, E.B. Genetic and biochemical basis for alternative routes of tocotrienol biosynthesis for enhanced vitamin E antioxidant production. Plant J. Cell Mol. Biol. 2013, 73, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Falk, J.; Andersen, G.; Kernebeck, B.; Krupinska, K. Constitutive overexpression of barley 4-hydroxyphenylpyruvate dioxygenase in tobacco results in elevation of the vitamin E content in seeds but not in leaves. FEBS Lett. 2003, 540, 35–40. [Google Scholar] [CrossRef]
- Rippert, P.; Scimemi, C.; Dubald, M.; Matringe, M. Engineering plant shikimate pathway for production of tocotrienol and improving herbicide resistance. Plant Physiol. 2004, 134, 92–100. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Stacey, M.G.; Cahoon, R.E.; Nguyen, H.T.; Cui, Y.; Sato, S.; Nguyen, C.T.; Phoka, N.; Clark, K.M.; Liang, Y.; Forrester, J.; et al. Identification of Homogentisate Dioxygenase as a Target for Vitamin E Biofortification in Oilseeds. Plant Physiol. 2016, 172, 1506–1518. [Google Scholar] [CrossRef]
- Konda, A.R.; Gelli, M.; Pedersen, C.; Cahoon, R.E.; Zhang, C.; Obata, T.; Cahoon, E.B. Vitamin E biofortification: Maximizing oilseed tocotrienol and total vitamin E tocochromanol production by use of metabolic bypass combinations. Metab. Eng. 2023, 79, 66–77. [Google Scholar] [CrossRef]
- Estévez, J.M.; Cantero, A.; Reindl, A.; Reichler, S.; León, P. 1-Deoxy-d-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J. Biol. Chem. 2001, 276, 22901–22909. [Google Scholar] [CrossRef]
- Wright, L.P.; Rohwer, J.M.; Ghirardo, A.; Hammerbacher, A.; Ortiz-Alcaide, M.; Raguschke, B.; Schnitzler, J.-P.; Gershenzon, J.; Phillips, M.A. Deoxyxylulose 5-phosphate synthase controls flux through the methylerythritol 4-phosphate pathway in Arabidopsis thaliana. Plant Physiol. 2014, 165, 1488–1504. [Google Scholar] [CrossRef]
- Carretero-Paulet, L.; Cairó, A.; Botella-Pavía, P.; Besumbes, O.; Campos, N.; Boronat, A.; Rodríguez-Concepción, M. Enhanced flux through the methylerythritol 4-phosphate pathway in Arabidopsis plants overexpressing deoxyxylulose 5-phosphate reductoisomerase. Plant Mol. Biol. 2006, 62, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Kimura, E.; Abe, T.; Murata, K.; Kimura, T.; Otoki, Y.; Yoshida, T.; Miyazawa, T.; Nakagawa, K. Identification of OsGGR2, a second geranylgeranyl reductase involved in α-tocopherol synthesis in rice. Sci. Rep. 2018, 8, 1870. [Google Scholar] [CrossRef] [PubMed]
- Valentin, H.E.; Lincoln, K.; Moshiri, F.; Jensen, P.K.; Qi, Q.; Venkatesh, T.V.; Karunanandaa, B.; Baszis, S.R.; Norris, S.R.; Savidge, B.; et al. The Arabidopsis vitamin E pathway gene5-1 mutant reveals a critical role for phytol kinase in seed tocopherol biosynthesis. Plant Cell 2006, 18, 212–224. [Google Scholar] [PubMed]
- Harish, M.C.; Dachinamoorthy, P.; Balamurugan, S.; Bala Murugan, S.; Sathishkumar, R. Overexpression of homogentisate phytyltransferase (HPT) and tocopherol cyclase (TC) enhances α-tocopherol content in transgenic tobacco. Biol. Plant. 2013, 57, 395–400. [Google Scholar] [CrossRef]
- Collakova, E.; DellaPenna, D. The role of homogentisate phytyltransferase and other tocopherol pathway enzymes in the regulation of tocopherol synthesis during abiotic stress. Plant Physiol. 2003, 133, 930–940. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.M.; Park, S.R.; Jung, J.; Moon, J.K.; Cheong, J.J.; Kim, M. Overexpression of Arabidopsis homogentisate phytyltransferase or tocopherol cyclase elevates vitamin E content by increasing gamma-tocopherol level in lettuce (Lactuca sativa L.). Mol. Cells 2007, 24, 301–306. [Google Scholar]
- Sundararajan, S.; Rajendran, V.; Sivakumar, H.P.; Nayeem, S.; Mani Chandra, H.; Sharma, A.; Ramalingam, S. Enhanced vitamin E content in an Indica rice cultivar harbouring two transgenes from Arabidopsis thaliana involved in tocopherol biosynthesis pathway. Plant Biotechnol. J. 2021, 19, 1083–1085. [Google Scholar] [CrossRef]
- Upadhyaya, D.C.; Bagri, D.S.; Upadhyaya, C.P.; Kumar, A.; Thiruvengadam, M.; Jain, S.K. Genetic engineering of potato (Solanum tuberosum L.) for enhanced α-tocopherols and abiotic stress tolerance. Physiol. Plant. 2021, 173, 116–128. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, L.; Zhang, L.; Wang, Y.; Cui, L.; Tang, Y.; Sun, X.; Tang, K. Molecular analysis of a homogentisate phytyltransferase gene from Lactuca sativa L. Mol. Biol. Rep. 2011, 38, 1813–1819. [Google Scholar] [CrossRef]
- Seo, Y.S.; Kim, S.J.; Harn, C.H.; Kim, W.T. Ectopic expression of apple fruit homogentisate phytyltransferase gene (MdHPT1) increases tocopherol in transgenic tomato (Solanum lycopersicum cv. Micro-Tom) leaves and fruits. Phytochemistry 2011, 72, 321–329. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.; Shi, B.; Chai, Y.; Han, N.; Zhu, M.; Bian, H. Overexpression of HvHGGT enhances tocotrienol levels and antioxidant activity in barley. J. Agric. Food Chem. 2017, 65, 5181–5187. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, E.B.; Hall, S.E.; Ripp, K.G.; Ganzke, T.S.; Hitz, W.D.; Coughlan, S.J. Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat. Biotechnol. 2003, 21, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Konda, A.R.; Nazarenus, T.J.; Nguyen, H.; Yang, J.; Gelli, M.; Swenson, S.; Shipp, J.M.; Schmidt, M.A.; Cahoon, R.E.; Ciftci, O.N.; et al. Metabolic engineering of soybean seeds for enhanced vitamin E tocochromanol content and effects on oil antioxidant properties in polyunsaturated fatty acid-rich germplasm. Metab. Eng. Commun. 2020, 57, 63–73. [Google Scholar] [CrossRef]
- Tanaka, H.; Yabuta, Y.; Tamoi, M.; Tanabe, N.; Shigeoka, S. Generation of transgenic tobacco plants with enhanced tocotrienol levels through the ectopic expression of rice homogentisate geranylgeranyl transferase. Plant Biotechnol. 2015, 32, 233–238. [Google Scholar] [CrossRef]
- Dolde, D.; Wang, T. Oxidation of Crude Corn Oil with and without Elevated Tocotrienols. J. Am. Oil Chem. Soc. 2011, 88, 1367–1372. [Google Scholar] [CrossRef]
- Shintani, D.; DellaPenna, D. Elevating the vitamin E content of plants through metabolic engineering. Science 1998, 282, 2098–2100. [Google Scholar] [CrossRef]
- Cho, E.A.; Lee, C.A.; Kim, Y.S.; Baek, S.H.; de los Reyes, B.G.; Yun, S.J. Expression of gamma-tocopherol methyltransferase transgene improves tocopherol composition in lettuce (Latuca sativa L.). Mol. Cells 2005, 19, 16–22. [Google Scholar] [CrossRef]
- Jin, S.; Daniell, H. Expression of γ-tocopherol methyltransferase in chloroplasts results in massive proliferation of the inner envelope membrane and decreases susceptibility to salt and metal-induced oxidative stresses by reducing reactive oxygen species. Plant Biotechnol. J. 2014, 12, 1274–1285. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Sarin, N.B. Antioxidant value addition in human diets: Genetic transformation of Brassica juncea with gamma-TMT gene for increased alpha-tocopherol content. Transgenic Res. 2007, 16, 109–113. [Google Scholar] [CrossRef]
- Chen, D.F.; Zhang, M.; Wang, Y.Q.; Chen, X.W. Expression of γ-tocopherol methyltransferase gene from Brassica napus increased α-tocopherol content in soybean seed. Biol. Plant. 2012, 56, 131–134. [Google Scholar] [CrossRef]
- Youngjin, K.; HongYul, S.; TaeIl, P.; SoHyeon, B.; WoonChul, S.; Hyunsoon, K.; Junggon, K.; YongEui, C.; SongJoong, Y. Enhanced biosynthesis of α-tocopherol in transgenic soybean by introducing γ-TMT gene. Plant Biotechnol. J. 2005, 7, 1–7. [Google Scholar]
- Tavva, V.S.; Kim, Y.H.; Kagan, I.A.; Dinkins, R.D.; Kim, K.H.; Collins, G.B. Increased alpha-tocopherol content in soybean seed overexpressing the Perilla frutescens gamma-tocopherol methyltransferase gene. Plant Cell Rep. 2007, 26, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Jia, H.; Feng, G.; Wang, Z.; Li, J.; Gao, H.; Wang, X. Overexpression of Medicago sativa TMT elevates the α-tocopherol content in Arabidopsis seeds, alfalfa leaves, and delays dark-induced leaf senescence. Plant Sci. 2016, 249, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qiu, D.; Gao, H.; Wen, H.; Wu, Y.; Pang, Y.; Wang, X.; Qin, Y. Over-expression of a γ-tocopherol methyltransferase gene in vitamin E pathway confers PEG-simulated drought tolerance in alfalfa. BMC Plant Biol. 2020, 20, 226. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Liu, B.; Zhang, L.; Zhang, W.; Chen, R.; Wang, L. Overexpression of the maize γ-tocopherol methyltransferase gene (ZmTMT) increases α-tocopherol content in transgenic Arabidopsis and maize seeds. Transgenic Res. 2020, 29, 95–104. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Liu, R.R.; Xu, G.; Zhang, P.; Li, Y.; Tang, K.X.; Liang, G.H.; Liu, Q.Q. Increased α-tocotrienol content in seeds of transgenic rice overexpressing Arabidopsis γ-tocopherol methyltransferase. Transgenic res 2013, 22, 89–99. [Google Scholar] [CrossRef]
- YAO, X.; Yang, W.; LUO, Y.; CHEN, R.; WANG, L.; ZHANG, L. Acquisition and Characteristic Analysis of Transgenic Maize with phyA2, ZmTMT, and Bar. Sci. Agric. Sin. 2020, 53, 4982–4991. [Google Scholar]
- Van Eenennaam, A.L.; Lincoln, K.; Durrett, T.P.; Valentin, H.E.; Shewmaker, C.K.; Thorne, G.M.; Jiang, J.; Baszis, S.R.; Levering, C.K.; Aasen, E.D.; et al. Engineering vitamin e content: From Arabidopsis mutant to soy oil. Plant Cell 2003, 15, 3007–3019. [Google Scholar] [CrossRef]
- Collakova, E.; DellaPenna, D. Homogentisate phytyltransferase activity is limiting for tocopherol biosynthesis in Arabidopsis. Plant Physiol. 2003, 131, 632–642. [Google Scholar] [CrossRef]
- Meena, V.K.; Chand, S.; Shekhawat, H.V.S.; Choudhary, K.; Sharma, J.K.; Sheera, A.; Lekha; Yadava, D.K. Advances in plant tocopherol biosynthesis: From pathway elucidation to crop biofortification strategies. Discover Plants 2025, 2, 9. [Google Scholar] [CrossRef]
- Lipka, A.E.; Gore, M.A.; Magallanes-Lundback, M.; Mesberg, A.; Lin, H.; Tiede, T.; Chen, C.; Buell, C.R.; Buckler, E.S.; Rocheford, T.; et al. Genome-wide association study and pathway-level analysis of tocochromanol levels in maize grain. G3 Genes Genomes Genet. 2013, 3, 1287–1299. [Google Scholar] [CrossRef]
- Deng, M.; Chen, H.; Zhang, W.; Cahoon, E.B.; Zhou, Y.; Zhang, C. Genetic improvement of tocotrienol content enhances the oxidative stability of canola oil. Front. Plant Sci. 2023, 14, 1247781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, Y.; Zhu, Y.; Zhang, L.; Zhang, W.; Chen, R.; Xu, M.; Fan, Y.; Wang, L. GmTMT2a from soybean elevates the α-tocopherol content in corn and Arabidopsis. Transgenic Res. 2013, 22, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- DellaPenna, D.; Mène-Saffrané, L. Vitamin E. In Biosynthesis of Vitamins in Plants Part B; Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2011; pp. 179–227. [Google Scholar]
- Li, Q.; Yang, X.; Xu, S.; Cai, Y.; Zhang, D.; Han, Y.; Li, L.; Zhang, Z.; Gao, S.; Li, J.; et al. Genome-wide association studies identified three independent polymorphisms associated with α-tocopherol content in maize kernels. Public Libr. Sci. One 2012, 7, e36807. [Google Scholar] [CrossRef]
- Chu, D.; Zhang, Z.; Hu, Y.; Fang, C.; Xu, X.; Yuan, J.; Zhang, J.; Tian, Z.; Wang, G. Genome-wide scan for oil quality reveals a coregulation mechanism of tocopherols and fatty acids in soybean seeds. Plant Commun. 2023, 4, 100598. [Google Scholar] [CrossRef]
- Maeda, H.; Sage, T.L.; Isaac, G.; Welti, R.; Dellapenna, D. Tocopherols modulate extraplastidic polyunsaturated fatty acid metabolism in Arabidopsis at low temperature. Plant Cell 2008, 20, 452–470. [Google Scholar] [CrossRef]
- Austin, J.R.; Frost, E.; Vidi, P.A.; Kessler, F.; Staehelin, L.A. Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 2006, 18, 1693–1703. [Google Scholar] [CrossRef]
- Bermúdez, L.; del Pozo, T.; Lira, B.S.; de Godoy, F.; Boos, I.; Romanó, C.; Previtali, V.; Almeida, J.; Bréhélin, C.; Asis, R.; et al. A Tomato Tocopherol Binding Protein Sheds Light on Intracellular α-tocopherol Metabolism in Plants. Plant Cell Physiol. 2018, 59, 2188–2203. [Google Scholar] [CrossRef]
- Wang, M.; Toda, K.; Maeda, H.A. Biochemical properties and subcellular localization of tyrosine aminotransferases in Arabidopsis thaliana. Phytochemistry 2016, 132, 16–25. [Google Scholar] [CrossRef]
- Garcia, I.; Rodgers, M.; Lenne, C.; Rolland, A.; Sailland, A.; Matringe, M. Subcellular localization and purification of a p-hydroxyphenylpyruvate dioxygenase from cultured carrot cells and characterization of the corresponding cDNA. Biochem. J. 1997, 325 Pt 3, 761–769. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).