Abstract
Magnesium ions regulate synaptic and nonsynaptic neuronal excitability from intracellular (Mg2+i) and extracellular (Mg2+o) domains, modulating voltage- and ligand-gated ion channels. K+ inward rectifier (Kir) channel inward rectification arises from Mg2+i blocking the pore and outward K+ current, while Mg2+o targets external sites. Mg2+i causes voltage-dependent Ca2+ voltage-gated (CaV) and Na+ voltage-gated (NaV) channel block while phosphorylation modulates channel activity. Mg2+o elicits direct voltage-dependent CaV channel block, and screens surface charge, and in NaV channels reduces conduction and may cause depolarization by quantum tunneling across closed channels. Mg2+i is an allosteric large conductance Ca2+-activated K+ (BK) channel activator, binding to low-affinity sites to alter Ca2+ and voltage sensitivity but reduces small conductance Ca2+-activated K+ (SK) channels’ outward K+ current and induces inward rectification. N-Methyl-D-aspartate receptor (NMDAR) channels are inhibited by Mg2+i binding within the pore, while Mg2+o stabilizes excitability through voltage-dependent block, Mg2+o forms Mg-ATP complex modifying purinergic P2X receptor (P2XR) channel affinity and gating and directly blocks the pore. Mg2+o reduces gamma-aminobutyric acid type A receptor (GABAAR) channel Cl− current amplitude and augments susceptibility to blockers. Mg2+o and Mg2+i block nicotinic acetylcholine receptor (nAChR) channels through voltage-dependent pore binding and surface charge screening, impeding current flow and altering gating.
Keywords:
magnesium; Kir channel; CaV channel; NaV channel; BK channel; SK channel; NMDAR; P2XR; GABAAR; nAChR 1. Introduction
Magnesium (Mg) is a mineral micronutrient bioessential for human health and wellbeing. In our cells and tissues, it serves many structural and metabolic functions, as well as important electrophysiological roles. Magnesium ion (Mg2+) is an electrolyte present in all our body fluids with a dominant intracellular distribution. In the brain, Mg2+ is ubiquitous, but more abundant in the cerebrospinal and interstitial fluid than in the blood, reflecting high neuronal demand for Mg2+. Within the nerve cells, Mg2+ distribution is compartmentalized, with specific localization in different cellular structures: the cytoplasm, cell nucleus, mitochondria, and endoplasmic reticulum (ER). Since mitochondria serve as the site of cellular respiration and major adenosine triphosphate (ATP) synthesis, they also store the highest concentration of Mg2+ in the cell. They have the capacity to actively accumulate Mg2+ and release it in response to various biological stimuli. In eukaryotic cells, mitochondrial Mg2+ dynamics and Mg2+ permeation across the inner mitochondrial membrane are facilitated by specific Mg2+-sensitive channels [1]. Several other transporters and exchangers have been identified on the cell membrane and organelles’ membranes responsible for maintaining cellular Mg2+ homeostasis [2]. Studies focusing on the effects of intracellular Mg2+ (Mg2+i) on cell energy metabolism report several mechanisms of modulation of oxidative phosphorylation by Mg2+ in the mitochondrial matrix. The impact of Mg2+i on cell respiration is described to be manifold, with a resulting enhancement of mitochondrial ATP synthesis [3].
Mg2+ ions distributed throughout the cell and across various compartments serve many distinct roles, critical for neuronal health and activity. Magnesium is essential for various nerve cell functions, including enzyme activation, protein synthesis, genome stabilization, cell cycle regulation, energy metabolism, regulation of biochemical pathways, signaling pathways, and modulation of membrane ion transport mechanisms. Little is known that other than intracellular Ca2+ ion (Ca2+i), Mg2+i can also be considered an intracellular messenger, as Mg2+i relays signals within the cell between different compartments. This, in fact, refers to the free, unbound form of Mg2+i, also known as the ionized Mg2+ fraction, physiologically present in low concentrations in the cytosol of the resting cell (0.5–1.2 mmol/L) [4]. Cytosolic Mg2+ fluctuations occur upon stimulation by various extra- or intracellular stimuli, whereby Mg2+i concentrations ([Mg2+]i) can change dynamically. Mg2+i can rapidly be mobilized from the cellular stores or enter from the extracellular fluid, and act as a signal transducer and cellular regulator to perform many roles. It can directly control the activity of various target cell molecules: proteins, enzymes, nucleotides, ATP, etc., usually by binding to their specific motifs [5].
The concentration of free, ionized Mg2+ in human brain tissue fluid measures approximately 0.5 mmol/L (as determined by 31P phosphorus magnetic resonance spectroscopy), which is similar to that in nerve cell cytosol, adequate to ATP levels and energy demands of central neurons. In the blood and all our body fluids, Mg2+ exists in two main forms: the bound form (comprising the protein-bound Mg2+ and Mg2+ complexed with different ligands, including ATP), and the free form (ionized Mg2+ or unbound Mg2+). These three Mg2+ fractions (three states in which Mg2+ can be found) exist in equilibrium, but with constant dynamic Mg2+ shifts between them. It is the ionized Mg2+ fraction (iMg2+) that is free to exert regulatory Mg2+ effects (metabolic, immune, and electrophysiological). Only the iMg2+ is available for biological actions, being unbound and exchangeable between the compartments of body fluids. Although frequent Mg2+ shifts occur, equilibrium is maintained in a manner that both intra- and extracellular concentrations of iMg2+ are physiologically stable and approximately equal. Assessing the Mg2+i and extracellular (outer) Mg2+ (Mg2+o) levels in the brain provides better insights into neuronal Mg2+ homeostasis and its involvement in cell signaling, electrophysiology, and bioenergetics [6]. From both the intra- and the extracellular side of the cell membrane, Mg2+ serves the role of a regulatory cation, known for its overall stabilizing effect on electrically excitable membranes. Its homeostasis helps maintain the physiological functions of central neurons in a complex manner and even more tightly than in other types of excitable cells. Many of its actions couple cell metabolism to its electrical activity [4].
Regular neural and muscular electrical excitability and conductivity are essential to enable proper vital body functions such as sensorineural alertness, consciousness, and other nervous system functions, heart action, blood circulation, and respiration. Other than the traditionally considered electrolytes (Na+, K+, Ca2+, and Cl−), Mg2+ is also required for their maintenance, although it is frequently neglected when testing for the routine blood serum ionogram. Clinical implications of imbalances of Mg2+ metabolism are numerous. Systemic Mg2+ homeostasis with stable concentrations of Mg2+ ([Mg2+]) in the whole body is required for human health. On the other hand, chronic Mg2+ deficiency is associated with chronic low-grade hyperexcitability [4]. Because of its many pathophysiological effects, such as Mg2+ may be a biomarker of poor outcomes and unwanted cardiovascular events, including death, angina, myocardial infarction, heart failure, arrhythmias, and stroke [7]. In the fields of neurology, neuropsychiatry, neurosurgery, and neurorehabilitation, it is implicated in a number of conditions, like epilepsy, anxiety, depression, migraine and tension headache, Alzheimer’s disease, Parkinson’s disease, stroke recovery, cerebral vasospasm, insomnia, restless leg syndrome, etc. [8,9]. In order to better understand the potency of Mg2+ deficiency to mediate neuropathophysiological mechanisms of these diseases, one must comprehend well the basic electrophysiological effects of Mg2+ on a molecular level. This review aims to present some of them, affecting major voltage-gated and ligand-gated ion channels of central neurons.
2. Intracellular and Extracellular Magnesium as Modulators of Ion Channel Function
Ion channels are integral membrane proteins that allow the selective passage of ions across cellular membranes. Their activity is tightly regulated by voltage changes, ligand binding, and intracellular signaling molecules. Magnesium, the most abundant divalent intracellular cation, plays a dual role in modulating ion channels depending on its localization. Magnesium ions can affect both voltage-gated and ligand-gated ion channels, from both the extracellular and intracellular sides of the membrane. Due to their double positive charge, Mg2+ ions in aqueous solutions and all body fluids carry a large surface charge that strongly attracts dipoles of water molecules. In this hydrated state, as Mg2+(aq), they have a large ionic radius, which does not prevent them from being attracted to charged binding sites in the pores of ion channels more specific for some other ions (such as Ca2+, K+, or Na+). However, their double hydration shell is not easy to shed, making it very difficult for Mg2+(aq) ions to pass through the narrow pores of ion channels in biological membranes. This makes Mg2+ a suitable modulator of activity in a number of different ion channels. Once Mg2+ ions bind within the channel pore, it becomes challenging for the competing cations to displace it, and channel permeation becomes blocked [10]. Over time, it has been shown that Mg2+ influences ion channel function through several mechanisms, such as electrostatic interactions with channel pores or gating domains, allosteric modulation of channel conformation, surface charge shift that alters membrane potential sensing, or competition with other cations (Ca2+, Na+) for binding or permeating the channel pore.
Magnesium acts as an important determinant in physiological and pathophysiological conditions, and the mechanisms of its action that underlie therapeutic potential are of great interest to basic scientists and practicing clinicians. However, we are still lacking complete knowledge regarding the precise mechanisms of Mg2+ effects on excitable membranes. Understanding structural interactions of Mg2+ with different ion channels, ramifications of these interactions, and their effects on channel functionality, is thereafter crucial for our comprehension of the mechanisms underlying alterations of functions of excitable membranes associated with magnesium imbalances, as well as the effects of potential therapeutic applications of magnesium in the conditions of magnesium deficiency. Therefore, we aim here to present an overview of research data on the interactions between Mg2+i and Mg2+o with some of the most important voltage-gated and ligand-gated ion channels shown to be affected by Mg2+ (Figure 1).
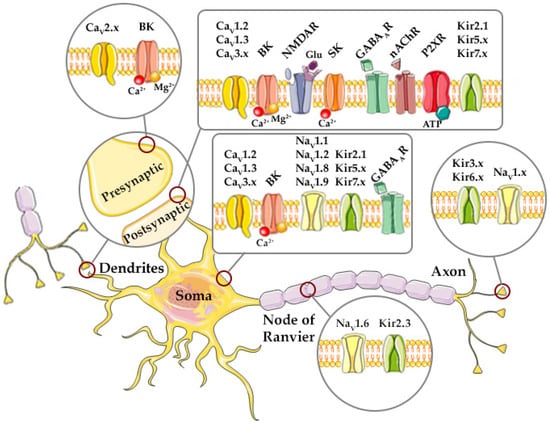
Figure 1.
Localization of major voltage-gated and ligand-gated ion channels in neurons shown to be regulated by Mg2+. Kir—inward rectifier K+ channel, CaV—voltage-gated Ca2+ channel, NaV—voltage-gated Na+ channel, BK—large conductance Ca2+-activated K+ channel, SK—small conductance Ca2+-activated K+ channel, P2XRs—purinergic P2X receptors, NMDAR—N-methyl-D-aspartate Receptor, Glu—glutamate, GABAAR—type A Gamma-Amino Butyric Acid Receptor, nAChR—nicotinic ACh receptor. Adapted from Servier Medical Art (https://smart.servier.com).
2.1. Voltage-Gated Ion Channels Regulated by Mg2+
Voltage-gated channels (VGCs) are transmembrane proteins that mediate ion flux across the cell membrane in response to changes in membrane potential. They play a pivotal role in electrical signaling processes in the body–neurotransmission, muscle contraction, and hormone secretion. The canonical action of these channels arises from an interplay of their components and functions, (a) channel voltage sensing is mediated by special voltage-sensing domains (VSD), (b) conformational changes of channel pores shift between pore opening and pore closing, (c) channels actively transition from open state to closed state, while channel inactivation results from a state of a channel already closed but which cannot be reopened temporarily, and finally (d) ion selectivity of some VGCs comes from specific ion-selectivity filter regions within the channel pore. Magnesium ions, normally present in the extracellular and intracellular fluids (both cytosolic and subcellular compartments), can affect the function of VGCs. Perturbations in the [Mg2+], even within their physiological range, can augment changes in channel activity. These effects are more prominently seen with [Mg2+]i changes and with larger variations in Mg2+o concentration ([Mg2+]o) changes. Here we present the findings concerning Mg2+ effects on several voltage-gated ion channels, inward rectifier K+ channels, voltage-gated Ca2+ channels, voltage-gated Na+ channels, and large conductance Ca2+-activated K+ channels, primarily in nerve cells, but also in some other cell types (microglia, astrocytes, cardiomyocytes, vascular smooth muscle cells, and endothelial cells).
2.1.1. Mg2+ Effects on Inward Rectifier Potassium (Kir) Channels
Kir channels allow influx of K+ ions into the cells and conduct a more inward K+ current during hyperpolarizing membrane potentials (negative to the K+ equilibrium potential), while restricting outward K+ current amid membrane depolarization [11,12,13]. These channels are tetramers, and each subunit contains two transmembrane (TM) helices (the outer, TM1 helix and the inner, TM2 helix), linked by the pore-forming region (H5) that carries the ion-selectivity filter and large N-terminal (amino, NH2−) and C-terminal (carboxy, COOH-) regions that constitute the cytoplasmic domain (CTD). The Kir channel structure is missing a VSD [14].
Functional studies suggest that K+ ions interact with certain charged residues in the Kir channel CTD, while traversing the extended channel pore composed of the CTD pore and TM pore [15]. The selectivity filter at the extracellular region of the channel pore can also serve as a gating element [16]. Though not being voltage-gated, Kir channels are of interest due to a highly voltage-dependent block of outward K+ currents, elicited by Mg2+i [11,12,17,18,19,20]. This group of K+-selective ion channels is critical for maintaining the resting membrane potential, regulating neuronal and myocyte excitability, and facilitating K+ homeostasis in the nervous system. For example, Kir1.1 channels act as K+ transporters, regulating their excretion and electrolyte balance as they are expressed in renal tubules [14], but their function in the hippocampus and cortex still remains unknown [21]. Kir2.1 channels are expressed diffusely in the whole brain, on neuron somata and dendrites, endothelium, heart muscle, vascular smooth muscles and skeletal muscles, Kir2.2 channels throughout the forebrain and strongly in the cerebellum, but also in the myocardium and skeletal muscles, Kir2.3 channels mainly in the forebrain, olfactory bulb, cardiomyocytes and skeletal myocytes and in the microvilli of Schwann cells at nodes on Ranvier, while Kir2.4 are found in the cranial nerve motor nuclei in the midbrain, pons, and medulla. Since being expressed in excitable tissues, the Kir2.x family helps maintain resting membrane potential and contributes to cardiac excitability. Kir3.1, Kir3.2, and Kir3.3 channel types are expressed throughout the brain, some such as Kir3.2 in homomeric form or as Kir3.1/3.2, Kir 3.2/3.3 heteromers, and mediate inhibitory neurotransmission. Kir4.1 and Kir5.1 channels are expressed on astrocytes in the brain either in Kir4.1 homomeric or Kir4.1/5.1 heteromeric form, respectively, and they mediate K+ buffering, which is important for the control of neuronal function in the central nervous system (CNS), but also in the spinal cord, retina, and stria vascularis in the inner ear. Kir6.x channels are expressed in all muscle cell types, the brain, and the pancreas, and act as metabolic sensors, while Kir7.1, found in the retina, contributes to ion transport and visual function [14,21].
Effects of Mg2+i on Kir Channels
Inward rectification of Kir channels is considered to result from an endogenous effect of Mg2+ binding to the channel from the cytoplasmatic, intracellular side, as registered in isolated murine cardiomyocytes [11,14,22], murine erythroleukaemia (MEL) cells expressing Kir1 channels [23], or modeled in silico [15]. At resting membrane potential level, electrostatic forces repel Mg2+ from the channel pore, allowing for inward K+ conduction, while membrane depolarization allows Mg2+i to flow into the Kir channel pore, physically blocking it and inhibiting the efflux of K+ ions, thus stabilizing membrane potential in neurons and glial cells [15,21]. This competitive blockage of Kir channels by Mg2+i at depolarizing membrane potentials is crucial for the magnitude of the outward K+ current and K+ balance within the cell. Additionally, this block allows astrocytes to uptake an excess of extracellular K+ ions to prevent hyperexcitability during high neuronal activity [14]. Mg2+i produces a rapid block of outward K+ current in bovine vascular endothelial cells, but there seems to be an internal additional voltage-dependent gating mechanism free of Mg2+ influence, leading to closure of Kir channels [24]. Mg2+ (0.1 mmol/L) on the cytoplasmatic side of the membrane diminishes the outward K+ currents through open Kir channels, which become flickery, while there is no effect of Mg2+i on the inward currents [12,18,19]. Electrophysiological experiments on murine ventricular cardiomyocytes show that progressive increase of [Mg2+]i modifies the outward K+ current, which evolves from an open channel current to zero-current level, as [Mg2+]i reaches 1 mmol/L [12,18]. Mg2+ ions have a diameter close to that of K+ ions, making it able to block the channels that permeate K+ and possess K+-binding sites [21].
Over time, it was established that Kir channel subunits possess more than one binding site for Mg2+. Mg2+i can interact with negatively charged residues of the Kir channel pore lining in the cytoplasmatic and transmembrane portion of the channel in the process of rectification [15]. Based on the sensitivity to Mg2+i block, Kir channels are recognized as either strong or weak inward rectifiers [17]. Electrophysiological experiments on Xenopus oocytes expressing various Kir channel mutants identified one of the crucial sites within the channel pore for Mg2+ to bind. This site is in the vicinity of a negatively charged residue, aspartate (Asp) side chain in position 171, as it is shown that substitution of Kir1.1 channel’s polar uncharged asparagine (Asn) with negatively charged Asp in this position (N171D) elicits a 20-fold stronger affinity of the channel to Mg2+ and blocks outward K+ currents [17]. Additionally, findings from the same model, expressing mutant Kir channels, reveal that a single substitution of Asp within the channel’s transmembrane M2 domain at position 172 with Asn (D172N) transforms a strong inward rectifier Kir2.1 channel into a channel with weak rectifier-like properties. Reversed substitution of Asn in position 171 with Asp (N172D) converts a weak rectifier Kir into a channel with a strong rectifier nature [25]. These findings are corroborated on MEL cells by showing that a mutation induced to a Kir2.1 channel, altering negatively charged Asp in position 172 to uncharged glutamine (Gln) (D172Q), reduces the channel’s affinity to Mg2+i more than five-fold [26]. Electrophysiological experiments on oocytes expressing mutant Kir channels suggest another site that is involved in channel rectification. Changing the negatively charged glutamate (Glu) in position 224 of the CTD C-terminus with non-polar glycine (Gly) or polar uncharged Gln or serine (Ser) (E224G/Q/S, respectively) drastically reduces rectification of the mutant channel. Furthermore, it is possible that the Asp residue at position 172 interacts with the Glu residue at position 224 to form a binding pocket for Mg2+ [27]. Multiscale modeling was utilized to investigate the path that Mg2+i crosses from the cytoplasm through the Kir2.1 channel pore, reaching the conclusion that these ions extensively interact with a negatively charged Glu residue in position 299 at the center of the intracellular domain, in order to block K+ outward current [28].
Membrane patches excised from murine cardiomyocytes [11], oocytes expressing Kir channels [29] or vascular endothelial cells [24] used to record single Kir channel currents exhibit rapid rundown of Kir activity in an internal solution containing physiological [Mg2+]i (~1 mmol/L), but restore channel rectification capability, while channel activity in Mg2+-free solution, although preserved, leads to a loss of inward rectification. Although it was initially thought that changes in intracellular K+ concentration ([K+]i) have little to no effect on the blocking action of Mg2+i on Kir outward currents [19], it is now known that a decrease in [K+]i augments the blocking affinity of Mg2+i on Kir1.1 expressed on Xenopus oocytes [30]. Additionally, as extracellular K+ concentration ([K+]o) increases, the Mg2+i blocking affinity for different families of Kir channels decreases, and a stronger depolarization is required to achieve the same amount of Kir blockage by Mg2+i [17,30].
Effects of Mg2+o on Kir Channels
Mg2+o can also modulate the activity of Kir channels. Electrophysiological recordings of membrane patches of vascular endothelial cells [24], oocytes expressing Kir2.1 [31], Kir2.2 [16] or Kir1.1 [20] channels exposed to increasing concentrations of Mg2+o demonstrate a decay of Kir channel activity, a reduction in the outward K+ current amplitude, and a rise in the extent of Kir channel inactivation. Removal of extracellular bivalent cations, such as Mg2+, reduces the extent of Kir channel voltage-dependent inhibition [16,20,24]. Presence of Mg2+o elicits longer periods of Kir channel closure intermittently separated by bursts of channel activity reflecting Mg2+ entry, association with the channel pore binding site, followed by dissociation from the site and exit from the channel [24]. Inward rectifier K+ channels (Kir2.1) expressed on Xenopus oocytes exhibit greater sensitivity to Mg2+o-induced voltage-dependent block than Kir2.2 and Kir2.3 channels. One of the mechanisms underlying this effect might be the interaction of Mg2+o with a negatively charged Glu residue at position 125 located in the extracellular loop between TM1 and pore domain (PD) of Kir2.1 channel. Substitution of Glu with Asp (E125D) preserves the sensitivity to Mg2+o-induced block of the mutant channel, proving that the negatively charged amino acid residue at position 125 is potentially a binding site for Mg2+o. Moreover, mutant Kir2.1 channel with polar uncharged Gln instead of Glu (E125Q) reduces channel sensitivity to Mg2+o, whereas substitution of Gln (Q126E) in mutant Kir2.2 and positively charged histidine (His) (H116E) in mutant Kir2.3 channels with Glu increases their sensitivity to Mg2+o block [32]. However, an increase in [K+]o reduces the Mg2+o-induced blocking effect on Kir channel activity, possibly by competing for the same binding site in the channel pore in murine cardiomyocytes [11,19], endothelial cells [24] and oocytes expressing Kir2.1 [31,32], Kir2.2 [16] or Kir1.1 [20] channels. Specific residues in the outer region of the channel could constitute a functional K+ sensor that alters its activity to changes in [K+]o. Negatively charged amino acid residues of the outer mouth of the Kir channel and the pore’s selectivity filter attract K+ ions, increasing their concentration in this region. Electrostatic repulsion between cations potentially repels Mg2+ from binding to the channel, thus reducing Mg2+o-induced voltage-dependent block of inward K+ current [16].
Finally, concerning other types of voltage-gated K+ (KV) channels, there are findings that KV1-KV3 are susceptible to increasing [Mg2+]i as it blocks outward K+ currents in a voltage-dependent manner [33,34]. Membrane depolarization can enhance Mg2+i blocking action of KV1.5 and KV2.1 channels to the degree that channels exhibit inward rectification in the presence of Mg2+i at positive membrane voltages, reminiscent of Kir channels [33]. In addition to the direct channel pore block, Mg2+i modulates the channel’s voltage sensor activity by screening negative cytosolic surface charges and shifts activation and inactivation to more negative membrane potentials [33,35]. Contrary to its direct channel blocking action, Mg2+i decreases KCNQ (K+ voltage-gated channel subfamily Q, KV7) channel currents by binding phosphatidylinositol 4,5-bisphosphate (PIP2), a membrane lipid instrumental for KCNQ channel activation, thereby leaving only a limited fraction of free PIP2 available to interact with the channel [36]. Conversely, voltage-dependent activation of EAG (ether-à-go-go, KV10) and hERG (human ether-à-go-go related gene, KV11) channels is modulated by Mg2+o, slowing the channel’s gating kinetics and transition to an open state as it screens negatively charged amino acid residues in the channel’s voltage-sensing region [37,38,39,40].
2.1.2. Mg2+ Effects on Voltage-Gated Calcium (CaV) Channels
In response to membrane depolarization, CaV channels mediate Ca2+ influx that regulates neurotransmitter release, excitability, and intracellular signaling in neurons; cardiac action potential (AP); muscle contractions; hormone secretion; and gene transcription. These channels consist of a pore-forming CaVα1 subunit and auxiliary subunits that attune gating activity–an intracellular CaVβ-subunit (CaVβ1–CaVβ4) and a complex extracellular CaVα2δ-subunit (CaVα2δ1–CaVα2δ4) [41,42]. The CaVα1 subunit is composed of four homologous repeats (I–IV), each containing six TM helices (S1-S6), where S5 and S6 form channel PD, a loop linking S5 and S6 (p-loop) carries the Ca2+ selectivity filter region, while S1–S4 helices constitute channel VSD [43]. CaV channels are classified into three subfamilies. Firstly, CaV1.1–CaV1.4 channels are defined as L-type for their large, long-lasting currents or as dihydropyridine receptors (DHPRs) due to their sensitivity to dihydropyridine (DHP). CaV1.1 channels are expressed in skeletal muscles and CaV1.4 channels in the retina, while CaV1.2 and CaV1.3 channels are present in cardiac and vascular smooth muscle cells, sinoatrial node (SAN), neuron soma and dendrites in the brain, intestinal/bladder smooth muscle, presynaptic side in cochlear ribbon synapses, and adrenal chromaffin cells [41,42,44]. CaV2.1 channels are called P-type, as they were first described and are abundant in cerebellar Purkinje neurons, and Q-type, which were initially identified in cerebellar granule cells, and help regulate cerebellar signaling [45]. CaV2.2 channels or N-type (neural type) are involved in neurotransmission in autonomic synapses and sensory synapses conveying nociceptive signals. CaV2.3 channels, known as R-type as they are not only resistant to DHP but mediate Ca2+ currents upon P/Q- and N-type channels block, are found in hippocampal and cortical neurons. The CaV2 channel subfamily is dominantly expressed in presynaptic nerve terminals and mediates coupling of presynaptic membrane depolarization and neurotransmitter release. Furthermore, some CaV2 channels (CaV2.1 and CaV2.2) in neuron somata and dendritic spines [46,47] can associate with Ca2+-activated K+ channels (presented in detail later) to shape AP repolarization and control of neuron firing frequency, by altering K+ conductance, while cooperation of these channels in presynaptic membrane microdomains regulates neurotransmission [48,49]. Lastly, CaV3.1–CaV3.3 channels, known as T-type for their tiny and transient currents, mediate SAN pace-making activity [43,50], but also neurotransmitter release in the retina, olfactory bulbs, hippocampal, and DRG neurons [42]. Orchestrating rebound excitation, CaV3 channels shape oscillatory neuron activity in the cerebellum and thalamocortical circuits as well as in sensory processing [51]. High-voltage-activated (HVA) Ca2+ channels (CaV1 and CaV2) contain CaVα1-, CaVβ-, and CaVα2δ-subunits, and their activation is achieved at more depolarized membrane potentials, while low-voltage-activated (LVA) Ca2+ channels (CaV3) are built solely of CaVα1-subunits, and they can be activated by depolarizations slightly above the resting membrane potential [41,43]. Channels interact directly or indirectly with various proteins that regulate their function, modulation, and localization within the presynaptic terminal [41]. Long-lasting Ca2+ currents critical for excitation and neuroendocrine regulation are mediated by CaV1.2 channels, while CaV2.1 channels play an essential role in neurotransmitter release. CaV channels possess self-regulatory mechanisms, allowing them to change ion permeation in response to prolonged depolarization and activity or high Ca2+i concentration ([Ca2+]i), namely through voltage-dependent inactivation (VDI) and Ca2+-dependent inactivation (CDI). These actions are a result of the coordinated binding of CaVα1 C-terminal cytoplasmatic isoleucine-glutamine (IQ) domains with a soluble Ca2+ sensor, calmodulin (CaM) already complexed with Ca2+ to induce CDI, or an interaction between the S1–S2 interdomain loop (α1-interacting domain, AID) region with auxiliary CaVβ-subunits to elicit VDI [43,50,52,53]. Another pivotal region for CDI is the Ca2+i-binding CaVα1-subunit’s C-terminus EF-hand motif, a site that is in the vicinity of the IQ domain and is proposed to also mediate in the CaV channel inactivation upon Ca2+-CaM binding [53].
Effects of Mg2+i on CaV Channel
The concentration of Mg2+ can vary in pathophysiological conditions in the brain or skeletal muscle, increase in transient cardiac ischemia, or decrease in heart failure. Increase of [Mg2+]i, in some cases even within physiological concentration range, alters the function of CaV channels, leading to their inhibition. The underlying mechanism of inhibition is multifaceted and can be interpreted as a concomitant effect of Mg2+i on the direct influence on the channel’s ion permeation and Mg2+i-induced modulation of the channel’s gating kinetics.
Extensive electrophysiological studies on isolated murine ventricular cardiomyocytes report on a progressive reduction in peak currents through CaV1.2 channels during application of [Mg2+]i that reaches the higher end of the physiological range and/or supraphysiological values [54,55,56]. Additionally, cardiomyocytes exhibit progressive shortening of AP duration with increasing [Mg2+]i used. These effects could not be abrogated by increasing levels of Ca2+o ([Ca2+]o) [54]. Variations of [Mg2+]i in isolated murine artery myocytes show a dichotomous response, an initial increase in Ca2+-and Ba2+-carrying inward currents through CaV1.2 channels in extremely low [Mg2+]i conditions, presumably due to instant reduction in Mg2+, followed by a progressive attenuation in current amplitude as [Mg2+]i increases [57]. Concentration-dependent inhibitory action of Mg2+i is also shown in human embryonic kidney (HEK) TsA-201 cells expressing rabbit wild-type CaV1.2 channels at fixed membrane potentials. Namely, increasing [Mg2+]i reduces CaV channel-mediated inward currents, an effect reversed by Mg2+i depletion [55,58]. Moreover, the inhibitory action of increasing [Mg2+]i on CaV channel permeation in isolated amphibian cardiac myocytes is also present, albeit more modest than in mammalian myocytes [59,60]. Concentration surpassing the physiological range; however, they elicit a pronounced voltage-dependent blocking effect on CaV channels, augmenting the rate and extent of channel inactivation. Membrane hyperpolarization undermines the Mg2+i-induced CaV block, promoting influx of Ca2+ during the channel activation phase, while depolarizing potentials allow Mg2+i to enter the cytoplasmatic side of the channel pore, hindering Ca2+ currents [61]. Voltage dependence of CaV channel activation and inactivation can be altered at extremely high and low [Mg2+]i, as shown in rabbit CaV1.2-expressing HEK-TsA-201 cells. High [Mg2+]i leads to a negative shift in the voltage dependence of both channel activation and inactivation, while very low [Mg2+]i causes a positive shift in the voltage–conductance relationship of CaV1.2 channels [58]. It is also proposed that the reduction in inward currents mediated by CaV channels can also partially be due to higher [Mg2+]i screening the negative charges on the intracellular membrane surface, thus modulating channel activity [58,61].
Mg2+i can modulate CaV1.2 channel activity by interacting with the putative Ca2+-binding motif of 12 amino acid residues (EF-hand region) on the C-terminal domain in CaVα1-subunit. Multiple studies on Mg2+ binding affinity to CaV channel pore demonstrate that negatively charged amino acid residues of the proximal C-terminal EF-hand motif are the possible binding sites in the Mg2+i-induced blocking cascade, probably competing with Ca2+-CaM complex binding and leading to a partial CaM displacement. Mutant rabbit CaV channels expressed on HEK-TsA-201 cells, where negatively charged Asp is substituted with either a nonpolar alanine (Ala), polar uncharged Asn or Ser, or positively charged lysine (Lys) (D1546A/N/S/K) in position 1546 of the EF-hand motif in CaVα1-subunit, show decreased affinity for Mg2+i. Conversely, exchanging positively charged Lys for negatively charged Asp in position 1453 or 1539 (K1453D, or K1539D) of the same region, significantly increases channel pore affinity to Mg2+i. In comparison to cells expressing wild-type rabbit CaV1.2, channel conductance decline is seen in lower [Mg2+]i in cells carrying mutant K1453D or K1539D CaV EF-hand motifs, while D1546A/N/S/K CaV mutants require a several-fold higher level of cytosolic Mg2+ to elicit the inhibitory action [58].
The interaction between the CaVα1-subunit’s distal C-terminal domain (DCT) and proximal C-terminal domain (PCT) can be autoinhibitory, as it reduces coupling of charge movement to channel opening, thus leading to prominent augmentation of voltage-dependent inactivation of CaV1.2 channels. Mg2+i can also modulate the noncovalent bond between DCT and PCT and the voltage-dependent inactivation of CaV1.2-mediated currents. The binding of Mg2+i to the PCT EF-hand motif is necessary for the DCT to exert the autoinhibitory effect [62]. Electrophysiological examination of isolated murine ventricular cardiomyocytes and HEK-TsA-201 cells expressing mutant variants of the channel’s CaVα1-subunits shows that an increase in [Mg2+]i not only reduces peak amplitudes of inward CaV1.2-mediated currents but also increases the rate and the duration of voltage-dependent inactivation of inward currents. Presence of mutant D1546A/N/S/K EF-hand motifs in CaVα1-subunits reduces VDI and Mg2+i-dependent inhibitory effects on CaV1.2 conductance, since only higher [Mg2+]i can elicit the steady state inactivation of the mutant channel compared to the wild-type one. The effect that the EF-hand motif mutation exerts on the rate and extent of VDI arises from the lack of Mg2+i binding affinity for the mutated region of the channel, thus failing to enhance VDI frequency and degree [62]. Elevated [Mg2+]i in amphibian cardiac myocytes also increases the rate and the extent of CaV channel VDI [61].
Earlier experiments on amphibian cardiomyocytes shed light on another mechanism through which Mg2+i can modulate CaV channels’ gating kinetics. Increased Mg2+i levels markedly reduce inward Ca2+ currents through CaV channels when they are phosphorylated [59,60]. On the other hand, some postulate that inhibitory effects on CaV conductance result from modulation of channel gating properties through phosphorylation of channel residues elicited by Mg2+i [63]. Having in mind that Mg2+i can interact with protein kinases and phosphatases to modify their activity [58], the effect that Mg2+i exerts on CaV channels seems dual, as Mg2+i possibly interacts directly with CaV channels, which, in a more phosphorylated state, are more susceptible to Mg2+i inhibitory effect. As with amphibian myocytes, electrophysiological recordings from murine ventricular cardiomyocytes show that rising [Mg2+]i causes a prominent reduction in Ca2+ currents through CaV1.2 channels conditioned to maximal phosphorylation. Changes from the CaV1.2 channel’s maximal phosphorylation state attenuate the inhibitory Mg2+i effect on inward Ca2+ currents, thus giving rise to the possibility that [Mg2+]i can antagonize the effects of phosphorylation on channel gating kinetics [64]. Dialysis of murine ventricular cardiomyocytes with K252a, a protein-kinase inhibitor, diminishes inward Ca2+ current density and any inhibitory effect of Mg2+i on CaV1.2 channel permeation, while phosphorylation of channels mediated by dialyzed cyclic adenosine monophosphate (cAMP) requires higher [Mg2+]i to reduce CaV channel conductance [63]. Inhibition of CaV1.2 channel conduction by high [Mg2+]i in isolated murine cardiomyocytes is potentiated by phosphorylation of the channel by protein kinase A (PKA), but application of phosphatase 2A (PP2A) dampens Mg2+i-induced inhibition as CaV channels become dephosphorylated [55]. Many potential phosphorylation sites are found in CaVα1-and CaVβ-subunits of CaV1.2 channels, and the most important ones for PKA-mediated phosphorylation are Ser residues at position 1928 (S1928) in α1C-subunit and positions 478 and 479 (S478 and S479, respectively), of β2A-subunits [65]. Recordings from HEK-TsA-201 cells expressing mutated rabbit CaV1.2 channels corroborate the significance of the channel’s phosphorylation state and sensitivity to Mg2+i-induced decrease in channel conduction. Namely, inward Ca2+ currents through mutant CaV1.2 channels lacking PKA phosphorylation sites are minutely affected by increasing [Mg2+]i, which is comparable to that of PP2A application in cells carrying wild-type channels. Conversely, truncation of the channel’s α1-subunit to increase channel pore open probability leads to a strong inhibition of inward currents through mutated channels when [Mg2+]i is high, comparable to blocking effects seen in dephosphorylated channels under the influence of dihydropyridine agonist BayK8644, which promotes channel pore opening [55,56]. Interestingly, increasing [Ca2+]i also has the capacity to dampen Mg2+i-induced inhibitory effects regardless of the channel phosphorylation status, indicating that, other than counteracting the CaV channel phosphorylation effects and gating kinetics, Mg2+i probably has a direct blocking effect on channel ion permeation [64].
While existing studies clarify the intricate mechanisms behind Mg2+i regulation of CaV1 channels, comparable direct channel-specific processes for other CaV channel subtypes, to the best of our knowledge, have not been reported.
Effects of Mg2+o on CaV Channels
While Mg2+i is considered to have an intricate effect on CaV channel conductance, aimed towards complex channel blocking and modulating channel activity, Mg2+o is considered to have the ability to directly block CaV channels. Early electrophysiological works on murine ventricular cardiomyocytes revealed that increasing [Mg2+]o transformed long-lasting single channel currents into bursts of brief openings, due to CaV channels’ discrete and rapid blocking and unblocking transitions. Channel blocking rate accelerates with increasing [Mg2+]o, and hyperpolarizing membrane potentials expedite channel opening and closing transitions as well. This gave rise to the idea that Mg2+o can lodge within the CaV channel pore and block the channel [66].
Mg2+o-induced blocking action is voltage-dependent. Hyperpolarization of membrane potential increases the degree of Mg2+o-induced steady state block of CaV channels [66]. Mg2+o can block CaV channels quite rapidly in isolated amphibian ventricular myocytes, but has little effect on channel inactivation kinetics [61]. Voltage-clamp experiments on isolated snail neurons and Na+ currents through CaV channels led to a suggestion that these channels have two functional regions–an external region where divalent cations bind with high-affinity to determine channel permeability to currents carried by monovalent cations, and the ion-selective filter that determines channel selectivity for different divalent cations [67].
Experiments on chick dorsal root ganglion (DRG) neurons exhibit a voltage-dependent block of CaV channels (L-and N-type) elicited by Mg2+o as inward Ca2+ and more prominently Na+ currents are reduced, respectively, to increasing [Mg2+]o. Inhibitory effect on inward currents is dominant at hyperpolarizing membrane potentials, and as the membrane becomes more positive, the blocking action declines. Upon cessation of depolarization and returning to negative membrane potentials, CaV channels are readily blocked again [68]. Given the potential clinical and experimental importance of Mg2+o in the hippocampus, the cellular and molecular bases for non-physiological excitability of hippocampal neurons induced by Mg2+ deficiency are of great practical interest. Electrophysiological findings of Mg2+o-induced CaV channel block are confirmed by visual observations of fluorescent probes in isolated murine hippocampal CA1 pyramidal neurons. Increase in Ca2+i-induced fluorescence in response to low [Mg2+]o, corresponds to increased inward Ca2+ currents through CaV channels upon removal of the Mg2+ block. Findings that intracellular fluorescence dissipates when [Mg2+]o increases or when CaV1.2 channel blockers are used further prove the blocking action of Mg2+o surrounding the neurons. Interestingly, Mg2+i levels do not significantly change in response to varying [Mg2+]o. It is proposed that Mg2+o modulates CaV channels and inward currents traversing the channel pore [69].
Equivalent concentration-dependent effects of Mg2+o are observed in recordings of rat pheochromocytoma (PC12) cells, possessing characteristics of peripheral sympathetic nerves. Increasing [Mg2+]o elicits progressive attenuation of inward currents through N-and L-type CaV channels [70]. Patch clamp recordings of isolated murine mesenteric artery myocytes show that increasing concentrations of Mg2+o elicit progressive reduction in Ca2+-and Ba2+-carried inward currents through CaV1.2 channels, with Ba2+ currents being more affected [57].
Apart from the blocking action on CaV channel conductance, high [Mg2+o] elicits surface charge screening near the mouth of the channel pore and a voltage shift in its gating kinetics [61,68]. As Mg2+o binds to the negatively charged Glu or Asp residues in the extracellular loop of the pore-forming α1-subunit, this reduces the effective voltage sensed by the VSD and shifts the voltage dependence of activation toward more positive potentials. This effect can decrease the amplitude of the Ca2+ current through CaV channels, in addition to elevating the voltage for channel activation [44,57]. Positive divalent cations can accumulate and “screen” or neutralize the negative charge of the cell surface.
2.1.3. Mg2+ Effects on Voltage-Gated Sodium (NaV) Channels
The NaV channels are critical for initiating and propagating APs in neurons, playing a pivotal role in neuronal excitability and signal transmission. These channels are composed of an NaVα-subunit with four homologous domains (I–IV), each with six TM segments (S1–S6), where S4 segment, being rich in positively charged amino acids, acts as a VSD, while S5–S6 segments with their connecting pore loop (p-loop) build the ion-conducting pore with an inactivation gate made out of regions that link III and IV domain. Auxiliary NaVβ-subunits (NaVβ1–NaVβ4) compete with each other (NaVβ1 v. NaVβ3 or NaVβ2 v. NaVβ4) for association with the NaVα-subunit. Although not obligatory for NaV channel function, they attune the activity of the NaVα-subunit to modulate channel kinetics, voltage sensitivity, and sensitivity to other ligands [50,71]. NaV1.1, 1.2, 1.3, and 1.6 channels are primarily expressed in the central nervous system (CNS), NaV1.7, 1.8, and 1.9 channels in the peripheral nervous system (PNS) at nociceptors, while NaV1.4 and NaV1.5 channels are the primary NaV channels in the skeletal muscles and the heart, respectively [50,72]. The channels open in response to membrane depolarization, allowing the influx of Na+ ions.
Effects of Mg2+i on NaV Channels
Electrophysiological experiments performed on murine cerebellar granule cells show that Mg2+i can elicit a voltage-dependent block of NaV channels by binding to the channel pore selectivity filter. During membrane depolarization, Mg2+i ions enter the NaV channel pore several times, reducing inward Na+ currents by half at depolarizing potential. Increase in [Mg2+]i augments the blocking effect of Mg2+i on NaV channels, while Mg2+i-induced inhibition of Na+ currents is reversed by an increase in Na+i concentration ([Na+]i) or Na+o concentration ([Na+]o), unveiling a competitive nature of this blocking effect. It is estimated that the presence of Mg2+ in the channel pore interferes with Na+ flux in both directions. Increase in [Mg2+]i from 0 to 30 mmol/L elicits negative V1/2 shifts in the range of −25 mV to −29 mV, although the voltage dependence of channel activation and inactivation does not change with [Mg2+]i increase [73]. Experimental recordings from Xenopus oocytes expressing rat brain type II Na+ channels [74,75], mutant K226Q NaV channels (Lys 226 substituted with Gln), type III Na+ channels, and neuron-like Na+ channels in chromaffin cells [75] demonstrate that Mg2+i markedly reduces Na+ currents through tested channels in a voltage- and concentration-dependent manner. Blocking effects become more prominent at more positive membrane potentials and with increasing [Mg2+]i in the patching pipette [75]. Examining the effects of Mg2+i on mutant cZ-2 Na+ channels known to exhibit slow and incomplete inactivation after opening demonstrates that increasing [Mg2+]i elicits a reduction in Na+ single-channel current amplitude. Since Mg2+i ions act as fast blockers, closing the Na+ channel pore completely for short time periods, they generate a flickery blocking action. Conversely, the blocking effect of Mg2+i on rat brain type II Na+ channels is augmented with an increase in [Na+]i. As Mg2+i is coupled to ATP–ADP hydrolysis, this balances the energy in the cell, so that a higher energy consumption leads to an increase in [Mg2+]i that modifies NaV channels to decrease in Na+ conductance and attenuate AP firing [75].
Mg2+i can modulate NaV channel activity through channel phosphorylation. Mg2+ is crucial for the function of PKA, an enzyme responsible for phosphorylation of NaV1.1 and NaV1.2 channels’ Ser residues in the NaVα-subunit’s intracellular loop, leading to the reduction in Na+ channel activity and conductance. High [Mg2+]i (10 mmol/L) can conversely lead to a decrease in PKA activity [72,76,77].
It is highly debatable whether high [Mg2+]i (30 mmol/L) elicits a negative shift in the NaV channel’s voltage dependence due to cytoplasmatic membrane surface potential change [73], since there is a relatively low half maximal inhibitory [Mg2+]i (4 mmol/L) compared to high blocking [Mg2+]o (35 mmol/L) that reduces inward Na+ channel conductance [74].
Effects of Mg2+o on NaV Channels
Electrophysiological examination of Mg2+o effects on murine hippocampal neurons presents a concentration-dependent increase in AP thresholds, resulting in decreased excitability. Higher [Mg2+]o (10 mmol/L) also reduces peak AP amplitude. As this action is comparable to the effect of tetrodotoxin (TTX), a highly selective antagonist of NaV channels, it is assumed that an increase in [Mg2+]o leads to a decreased availability and activation of NaV channels. Surface charge on the cell membrane created by sialic acid, phosphates, charged lipids, charged amino acids, and additional hydrophilic channel protein residues elicits local electrical fields near the channel voltage sensor [78]. However, some of the effects of Mg2+o on AP threshold and neuronal excitability are considered to result from surface charge screening, a process where extracellular cations bind to negative charges on the membrane, reduce surface potential, and produce local hyperpolarizing conditions for VSDs of Na+ channels. This charge screening effect is recognized as a depolarizing shift in Na+ voltage-dependent channel activation and inactivation [79]. Similar findings are seen in acutely isolated hippocampal CA1 neurons, as Mg2+o elicits a concentration-dependent reduction in NaV channel conductance. Moreover, exposing the cells to a fixed [Mg2+]o at different holding membrane potentials elicits a progressive reduction in Na+ current amplitude, indicating the voltage-dependence of Mg2+o-induced channel block [80]. NaV channel activity in murine hippocampal slices is susceptible to a concentration-dependent block by Mg2+o, as a decrease in NaV-mediated Na+ current amplitude is evident in high [Mg2+]o. On the other hand, exposing neurons to Mg2+o-free solution facilitates channel activation and leads to an increase in Na+ current amplitude [81]. In addition, studies on isolated murine saphenous nerves confirm that Mg2+o acts in a similar manner to TTX, via a concentration-dependent decrease in Na+ currents through NaV1.6 channels in Aβ fibers [82]. Altering the gating properties of the NaV channel leads to AP threshold shift, spontaneous synaptic activity, ectopic neuronal charges, and epileptic activity in in vitro and in vivo models.
Depolarization of the resting membrane potential by Mg2+o followed by decreased excitability of neurons seems contradictory. To explain this effect, a relatively novel hypothesis suggests that Mg2+o may induce subthreshold depolarization by quantum tunneling through closed NaV1.2 channels. Namely, it was calculated that at a concentration of 5.5 mmol/L Mg2+o passes through the channel and its intracellular hydrophobic gate, by acquiring the kinetic energy from neuronal membrane voltage and the thermal body energy, thus eliciting minor membrane depolarization by 5 mV. Unlike Mg2+o, Mg2+i has a lower tunneling probability, since they have lower kinetic energy. The degree of neuronal membrane depolarization by Mg2+o is determined by the tunneling probability, Na+ channel density in the membrane, and [Mg2+]o [83].
2.1.4. Mg2+ Effects on Large Conductance Ca2+-Activated Potassium (BK) Channels
BK channels have a large single-channel conductance. Activated by membrane voltage depolarization, micromolar [Ca2+]i, millimolar [Mg2+]i, or other ligands, BK channels mediate substantial K+ efflux and elicit repolarization of the membrane and/or closure of CaV channels to reduce Ca2+ influx. Assuming the role of a negative feedback to membrane depolarization and elevated [Ca2+]i, these channels regulate membrane excitability, intracellular ion homeostasis, and Ca2+ signaling, as they are often colocalized with CaV channels, N-methyl-D-aspartate receptors (NMDARs), and ryanodine receptors (RyRs) [84,85]. BK channels are expressed in skeletal and smooth muscles, neuron soma, axons, and presynaptic dendrite terminals, cochlear inner hair cells, and chromaffin cells, thus playing an important role in muscle contraction, neurotransmission, control of neuronal excitability (control of interspike interval and spike frequency adaptation), tuning hair cell firing, and hormone secretion. Native BK channels comprise α-subunits or a combination of α- and β-subunits (β1, β2/3, β4) or γ-subunits (γ1–γ4) in homotetrameric form [86,87]. Channels assembled from α-and β1-subunits are found in smooth muscle cells; ones made up of α-and β2/3-subunits exist in the brain, heart, and kidneys [86], while channels containing α-and β4-subunits are most abundant in the brain and spinal cord [87]. On the other hand, channels composed of α- and γ3-subunits are selectively expressed in the brain, while those built from α-and γ1-subunits are expressed in smooth muscle cells of cerebral arteries [87]. Each of the four α-subunits (Slo1 proteins) contains seven TM segments (S0–S6), including an additional S0 transmembrane segment that carries the N-terminus to the extracellular side and interacts with β-subunits, the pore–gate domain (PGD, S5–S6) with the C-linker (between S6 C-terminus and S7 N-terminus), which acts as an activation gate, and the voltage-sensing domain (VSD, S1–S4), whose positively charged Asp residues in the S4 helix represent a primary voltage sensor moving outward in response to membrane depolarization [88,89]. Furthermore, a large CTD or tail domain with four additional hydrophobic segments (S7–S10) is divided into two parts, homologous to a regulator of K+ conductance (RCK domains)–RCK1 (S7–S8) and RCK2 (S9–S10) [90] and serves as a primary ligand sensor [85]. RCK domains carry high-affinity Ca2+-binding sites–one on the RCK1 domain and one on the RCK2 domain (Ca2+ bowl), while the interface between the VSD and the cytoplasmatic domain and the RCK1 domain can be a target for Mg2+ binding [88]. BK channels owe their diversity to alternative splicing of Slo1 mRNA and accessory β subunits, which modify pharmacology, voltage dependence, and channel kinetics [86,88]. The VSD senses voltage, and the cytoplasmatic domain senses intracellular ligands, and they both allosterically control K+ efflux through PGD in response to various stimuli, linking cellular metabolism and membrane excitability [84]. As Mg2+i is one of the ligands that modulates the activity of BK channels, we will mostly focus on its effects.
Effects of Mg2+i on BK Channels
Early experiments on planar lipid bilayers with incorporated murine muscle transverse tubule membrane [91,92] or parotid acinar cell membrane [93] fragments or on cultured hippocampal neurons [94] show that Mg2+i acts like an allosteric BK channel stimulator, increasing the channel open probability [91,92,93], the affinity of the channel for Ca2+i [91,92] and channel activation [93] in a concentration-dependent manner. Mg2+i is suggested to bind to channel’s modulatory sites on its cytoplasmic side distinct from Ca2+-binding sites, as either physiological [Mg2+]i (1 μmol/L to 1 mmol/L) or high [Mg2+]i (10 mmol/L) at constant [Ca2+]i increase in the cooperativity of channel Ca2+ activation, with a two-fold increase in the Hill coefficient for Ca2+i-induced channel activation curve [93]. Interestingly, in the absence of Ca2+i, Mg2+i fails to activate the channel [91,93,94], even at extremely high [Mg2+]i of 50 mmol/L [92]. Increasing [Mg2+]i when Ca2+i is significantly reduced recovers channel open probability and single-channel current amplitude, with Mg2+i achieving almost the same efficacy as Ca2+i [94]. These findings indicate that Mg2+i is a potential internal modulator of BK channels in Ca2+i-dependent regulation of neuronal excitability.
Electrophysiological studies on cultured murine skeletal muscle cells [95], cerebrovascular smooth muscle cells [96], rabbit pulmonary artery [97], portal vein and coronary artery [98] smooth muscle cells, phospholipid bilayers containing rabbit colonocyte membrane fragments [99] or Xenopus oocytes expressing wild-type [100,101,102,103,104] or mutant mouse Slo1 (mSlo1) BK channels [105] show that Mg2+i can decrease channel outward K+ current amplitude and conductance in a concentration-dependent and voltage-dependent manner, acting as a fast blocker. The magnitude of Mg2+i-induced blocking action can be attenuated by [K+]i increase [95,99]. Interestingly, certain millimolar [Mg2+]i can, however, enhance channel open probability independently of membrane potential [96,98,100,101]. Patch-clamp recordings of macroscopic currents on oocytes expressing mSlo1 channel corroborate earlier findings, but also demonstrate that, at positive membrane voltage, Mg2+i reduces outward current amplitude regardless of [Ca2+]i, while negative membrane potential relieves channels from Mg2+i block [100]. Furthermore, Mg2+i can block mutant channels lacking CTD Ca2+-binding sites, but it does not affect channel activation. It is also proposed that channel sites involved in Mg2+-dependent channel activation are distinct from the ones mediating the previously described Mg2+ block [100].
Mg2+i competes with Ca2+i for BK channel low-affinity metal-binding sites (Mg2+-or Mg2+/Ca2+-binding sites). This competition is more evident in the presence of millimolar [Ca2+]i, which, being higher than physiological levels by three orders of magnitude, activates channels not only by occupying their high-affinity Ca2+-binding sites, but also by competitive binding to low-affinity metal-binding ones [100]. Moreover, low-affinity binding sites exhibit greater affinity for millimolar [Ca2+]i than for millimolar [Mg2+]i, indicating that, at high concentrations, Ca2+i and Mg2+i can use these binding sites to modulate channel-gating kinetics [101]. In contrast, apart from binding to the Mg2+-binding site, which activates the channel, Mg2+i can bind to the high-affinity Ca2+-binding site, without any effect, competitively inhibiting Ca2+-dependent activation [100,102]. As [Ca2+]i increases, Mg2+i binding to high-affinity Ca2+-binding sites declines, but when [Mg2+]i reaches supraphysiological levels, this inhibition is preserved [100,102]. Further electrophysiological examination on Xenopus oocytes expressing mSlo1 channels demonstrates that millimolar [Mg2+]i added to saturation micromolar [Ca2+]i (~110 μmol/L) enhances Ca2+i-triggered channel activation by interacting with low-affinity Mg2+-binding site [101]. However, it is debatable whether Mg2+i can activate BK channels by binding to the high-affinity Ca2+-binding site and substituting Ca2+i, and if this activation can occur in Ca2+i-free conditions [91,92,93,94,100,101]. Since Mg2+i causes a shift in channel gating as a result of channel closure retardation, rather than fast channel opening, Mg2+i effects are considered to be dependent on the channel open conformation [100,101]. Activation of BK channels by Ca2+i is probably potentiated by Mg2+i through channel allosteric modulation, as Mg2+i binding to low-affinity sites allosterically regulates channel open conformation, independently of Ca2+i binding to high-affinity binding sites and voltage sensor movement to membrane depolarization [101]. The binding of Ca2+i to the high-affinity binding sites, along with membrane depolarization, opens the channel, while Mg2+i bound to the low-affinity binding site potentiates channel activation.
In order to modulate BK channel activity, Mg2+i needs to simultaneously bind to several amino acid residues within the channel, prompting interdomain and intersubunit interactions. Studies on oocytes expressing mutant mSlo1 channel constructs reveal that the position 397 Gln oxygen-containing carbonyl group (Q397) coordinates Mg2+i ability to bind to Glu residues in 374 (E374) and 399 (E399) positions in the RCK1 domain [106,107]. Exchanging Gln with cysteine (Cys) in position 397 (Q397C) reduces the sensitivity of this mutant channel to Mg2+i both at zero and saturation [Ca2+]i, but it does not abolish it [106,107] and has no effect on the mutant channel conductance-voltage (G–V) relation [108]. Other mutant mSlo1 channel variants with Gln being replaced by arginine (Arg), Lys or tryptophan (Trp) (Q397R/K/W, respectively), exhibit decreased in Mg2+i sensitivity, but substitution with Glu (Q397E) or Asp (Q397D) enhances Mg2+i sensing, possibly due to the electrostatic attraction of negatively charged amino acid residues to bound Mg2+ [107,109] and shifts the channel G–V relation to more positive voltages [108]. Since Q397 is close to Mg2+i-binding sites E374 and E399, its mutation affects the channel’s Mg2+i sensitivity and binding affinity due to conformational changes in the binding sites or by an electrostatic interaction with already bound Mg2+ [107]. Interestingly, adding positive charge to this site, by substitution with Lys (Q397K) or Arg (Q397R) activates the mSlo1 mutant channel in absence of Mg2+i and shifts the G–V relation to more negative voltage, suggesting that the positively charged residue mimics Mg2+i bound to nearby sites (E374 and E399) and can electrostatically interact with positively charged Arg (R213) of the channel VSD [108]. Mutant Q397K or Q397R channels enhance the voltage sensor activation and mobility only when the channels are in the open state, similarly to wild-type channels upon Mg2+i binding [108]. Subsequent work on oocytes expressing several mutant channel variants reveals two additional Mg2+i-binding sites–a negatively charged, oxygen-bearing Asp residue in position 99 (D99) and a polar, uncharged Asn residue in position 172 (N172) in the α-subunit VSD, in addition to E374 and E399 in the RCK1 domain. Exchanging Asp in position 99 with nonpolar Ala (D99A) in the VSD makes the mutant channel not only resistant to Mg2+i but can abolish Mg2+i binding to E374 and E399 sites in the RCK1 domain. Furthermore, mutant channels where Asp is substituted by amino acids lacking oxygen in their side chain, such as Cys, Trp, Arg, or Lys (D99C/W/R/K, respectively), lose Mg2+ sensing as well. Conversely, Mg2+i can still exert its activating effect on a channel with preserved side chain oxygen, namely when Asp is interchanged with Gln, Asn, or Glu (D99Q/N/E, respectively) [109]. BK channels with a mutation of the other binding site, namely Asn replacement with Ala in position 172 (N172A), show a substantial decline of channel responsiveness to Mg2+i, whereas substitution with Arg (N172R) or Lys (N172K) abolishes Mg2+ sensitivity of the channel. Having a negatively charged Glu or Asp residue instead of Asn (N172D or N172E, respectively) enhances the mutant’s binding site interaction with Mg2+i [109]. Double mutant channels bearing Cys instead of Asp in position 99 and Gln in position 397 (D99C:Q397C) show that the VSD and RCK1 domains are close enough that a disulfide bond forms between their cysteine residues. This proves that the spatial proximity between D99 in VSD and E374 and E399 in the RCK1 domain fits the dimensions of a Mg2+-binding site [109]. Furthermore, double mutant channels comprising N172D substitution with either D99A, E374A, or E399N exhibit considerably stronger sensitivity to Mg2+i than those carrying single mutations, indicating that the Asp carboxylate group oxygen in position 172 rescues the channel’s ability to bind Mg2+i [109]. Experimental findings propose that Mg2+i binds to a complex site that consists of Asp residues at position 99 (D99) and Asn residues in position 172 (N127) in the VSD of one α-subunit and Glu residues at positions 374 (E374) and 399 (E399) in the RCK1 domain of a neighboring α-subunit, mediating an intersubunit interaction between the voltage-sensing and ligand-sensing domains [109]. This allows for the activation of the channel by electrostatic interaction between bound Mg2+i and the positively charged Arg (R213) residue of the VSD in the S4 segment [109]. Mutant mSlo1 channels with only preserved low-affinity Mg2+-binding site open to an increasing membrane voltage after a markedly shorter latency period in the presence of Mg2+i and a decrease in the interval duration of the channel closed state. Furthermore, Mg2+i prolongs bursts of channel openings with a higher channel opening rate, while the mean open interval duration increases in 2-fold compared to zero [Mg2+]i conditions. Mg2+i can bind to closed channels, while increasing the probability of channel opening, but is more efficient in binding to open channels, decreasing the probability of channel closing and closing rates [105].
Movement of the voltage sensor in response to membrane voltage change [108,110] generates a transient gating current (Ig) [108]. Whereas having no effect on the IgON, caused by voltage sensor movement from the resting to the activated state, when most channels are closed, Mg2+i slows down the return of the voltage sensor from the activated to the resting state, when most channels are open, and decreases the amplitude of the generated IgOFF [108]. Mutant channels where Asp is exchanged with Ala in position 99 (D99A) or position Glu is replaced with Asn in position 399 (E399N) become resilient to Mg2+i effect on IgOFF [109]. The transmembrane S4 segment is not only the primary voltage sensor that leads to the opening of the channel, but also the C-linker activation gate [110] but also mediates Mg2+i-dependent channel activation cascade. Channels carrying mutations of the VSD in the S4 segment, namely position 213 Arg substitution with polar uncharged Gln (R213Q) [110] or Cys (R213C) [108] have altered voltage dependence and lose sensitivity to Mg2+i and Mg2+i-dependent activation [108,110]. Time course of wild-type channel deactivation in the presence of Mg2+i is slower than in zero [Mg2+]i, but the R213Q mutant becomes unaffected by changes in [Mg2+]i [110]. It is shown that Mg2+i bound to E374 and E399 in the cytoplasmatic RCK1 domain can come close to the VSD in the S4 segment and electrostatically interact with the VSD positively charged R213 site, which is important for voltage sensing. The created electrical field at R213 and the repulsion of positive charges promote activation of the voltage sensor and favor the channel open state. It is proposed that VSD senses not only membrane voltage but also the bound Mg2+i. Therefore, the R213 residue acts both as a membrane voltage sensor and Mg2+i binding sensor, so that VSD can control channel activation [108]. Excitatory Mg2+i effect on BK channels requires activation of the channel voltage sensor. Substitution of Arg in position 210 with Cys leads to a constitutively active voltage sensor in the mutant channel, which can be opened by Mg2+i regardless of the membrane voltage. In addition, the channel’s open probability increases with increasing [Mg2+]i when they are activated [104,105]. However, it is possible that high [Mg2+]i (10–100 mmol/L) elicits a direct effect on channel opening independently of voltage sensor activation, similarly to that of Ca2+i, through an additional low-affinity binding site [104]. Mg2+i strengthens the coupling of voltage sensor activation and channel opening by interacting with multiple sites on the voltage sensor [104].
BK channel reactivity to Mg2+i is also affected by α-subunit mutations in the C-terminal half of the S4 segment and the N-terminal part of the S4–S5 linker. Specifically, mutant channels where Gln in position 216 or 222 is replaced with Arg (Q216R or Q222R, respectively), Glu in position 219 substituted with Gln (E219Q), leucine (Leu) in position 224 exchanged with Arg (L224Q) or channels bearing double mutation at positions 219 and 222 (E219R/Q222R) have a suppressed sensitivity to Mg2+i, albeit some of the positions in wild type channels do not directly participate in Mg2+i binding. These findings suggest that interactions between amino acid residues in the C-terminal part of S4 and the N-terminal part of S4–S5 linker with adjacent voltage-sensing moieties are crucial for Mg2+i-dependent channel activation [110]. Moreover, the interaction between the RCK1 domain and the voltage sensor conveys the energy from Mg2+i binding to open the channel gate [110]. Electrophysiological experiments on oocytes expressing wild-type mSlo1 channels propose that a ring composed of eight negatively charged Glu residues in positions 321 and 324 at the cytosolic channel entrance might be a possible target for Mg2+i blocking action. Moreover, mutant channels without the ring structure, stemming from Glu substitution with polar uncharged Asn (E321N:E324N), exhibit a significant resistance to Mg2+i-induced blocking action [103]. Substantial [K+]i increase equally mitigates Mg2+i block of wild type and mutant channels, suggesting that, when present, the ring of negative charge cannot facilitate blocking action in high [K+]i as it loses electrostatic attraction for Mg2+i and that there might be an additional site of Mg2+i action as the block is not abolished [103]. Namely, not only is Mg2+i attracted to the inner opening of the BK channel, making it easier to block K+ currents, but the screening of the negative charge by Mg2+i decreases in the polar attraction of the channel opening for cytosolic K+ and the outward current [103].
Effects of Mg2+o on BK Channels
Studies examining the effects of [Mg2+]o change on BK channel activity are scarce, as most studies investigate Mg2+i. Earlier electrophysiological experiments on murine muscle transverse tubule membranes incorporated into planar lipid bilayers indicate that high [Mg2+]o exerts no effect on BK channel activation or channel affinity to Ca2+ [91,92]. Subsequent studies on colonocyte membrane fragments incorporated into phospholipid bilayers; however, show that Mg2+o decreases in the channel outward current amplitude and channel conductance altogether in a concentration-dependent manner, suggesting that this might be a result of electrostatic screening of negative charges located at the channel’s extracellular pore opening [99,111].
2.1.5. Mg2+ Effects on Small Conductance Ca2+-Activated Potassium (SK) Channels
The SK channels are responsible for the medium membrane afterhyperpolarization (AHP) following APs, thus modulating intrinsic neuronal excitability and controlling spike firing rates and AP frequency, regulating dendritic excitability [112]. These channels are built as homotetramers of α subunits composed of six TM segments (S1–S6), with the PGD (S5–S6) surrounded by S1–S4 TM segments. Unlike BK channels, SK channels are voltage-insensitive and activate in response to low [Ca2+]i interacting with their CaM-binding domain (CaMBD). Each subunit binds one CaM through an interaction between the CaM C-lobe with the CaMBD independently of Ca2+i and between the CaM N-lobe and S4–S5 linker. In response to Ca2+i, the S4–S5 linker elicits a set of conformational changes, causing the S6 TM segment to move and open the channel pore for K+ efflux. SK channels are primarily expressed in the CNS, PNS, and the cardiovascular system [89]. Activation of SK channels in substantia nigra, ventral tegmental area, and cerebellar dopaminergic (DA) neurons to counteract CaV channel-or NMDAR-mediated Ca2+ influx and membrane excitation regulates muscle coordination and movement [113,114]. SK1 channels are expressed in the neocortex and co-expressed with SK2 channels in the hippocampus, thalamus, cerebellum, and brain stem, while SK3 channels are expressed in the midbrain and the cerebellum [89]. They are usually activated by Ca2+ influx through CaV channels during AP. SK channels couple their activity with postsynaptic modulators of [Ca2+]i, like NMDARs, in dendritic spines of neurons in the hippocampus and amygdala. They are involved in the regulation of the excitatory postsynaptic potential and can influence NMDAR activation through voltage-dependent Mg2+ block, affecting synaptic plasticity [115]. Inhibition of dendritic SK channels leads to long-term potentiation (LTP) and has an important role in memory and learning [116,117]. In cardiac atria and ventricles, SK channels act as an important negative feedback mechanism to [Ca2+]i increase through CaV1 channel activation or Ca2+ release from cellular storage, and lead to repolarization of cardiomyocytes and AP completion [118]. Interestingly, the evidence of direct Mg2+i effect on the function and gating kinetics of SK channels is scarce, while there are still no studies showing the effects of Mg2+o.
Patch-clamp recordings of Xenopus oocytes expressing cloned rat SK2 (rSK2) channels [119,120] or freshly isolated murine aorta endothelial cells [121] show that increasing [Mg2+]i within the physiological range reduces outward K+ channel currents [119,120,121]. Supraphysiological [Mg2+]i (up to 10 mmol/L) blocks the channel completely. Depolarizing shifts in membrane voltage enhance Mg2+i blocking action as if Mg2+i are pushed into the channel. Furthermore, increasing [Mg2+]i leads to inward rectification of the channel current-voltage (I–V) relation in a voltage-dependent manner [119,121]. Conversely, SK channel block elicited by high [Mg2+]i is attenuated with increasing [Ca2+]i, while a decrease of [K+]o potentiates the blocking effect and reduces its voltage-dependency [119]. Explaining the mechanisms of SK channel behavior in these conditions leads to the conclusion that they act similarly to Kir channels, so they can be referred to as “Ca2+-activated inward rectifier K+ channels” [119]. Electrophysiological studies on oocytes expressing mutant rSK2 channels were used to examine possible metal-binding sites in the channel pore-forming region that can mediate the Mg2+-induced block [120]. Mutant rSK2 channels where Ala substitutes Trp in position 379 (T379A), 398 (T398A), or Cys in position 386 (C386A) exhibit a comparable degree of channel block by divalent cations to that of wild-type channels. Conversely, replacement of Ser in position 359 with Ala (S359A) decreases the mutant channel susceptibility to any blocking action, proving that this is the intended site for direct interaction with Mg2+, among other divalent cations, and that it determines channel ionic selectivity. Mg2+i binding affinity to the S359A mutant rSK2 channel increases at lower positive membrane voltages [120]. Knowing that the activity of these channels is coupled with Ca2+i balance modulated by CaV channel, NMDAR, and nAChR activity, it is plausible that Mg2+i and Mg2+o can also indirectly affect SK channel gating kinetics by altering [Ca2+]i.
2.2. Ligand-Gated Ion Channels Regulated by Mg2+
Ligand-gated ion channels are oligomeric protein structures that convert a chemical signal into an ionic current flux through the central pore of the channel. They are involved in crucial signal transduction in the CNS and PNS. The canonical activation pathway of these channels starts with chemical messenger activation, i.e., ligand binding, conformational change, and restructuring of the channel’s integral protein components, opening of the channel pore, and ion flux across the membrane that alters the cell’s membrane potential and ends with a cellular response. The channel pore is permeable to cations such as Na+, K+, and Ca2+ or anions such as Cl−, with certain selectivity determined by ion size and charge. Here we present the findings concerning Mg2+ effects on several ligand-gated ion channels of ionotropic receptors, cationic channels of the N-methyl-D-aspartate (NMDA) glutamatergic receptor and purinergic P2X receptors, Cl− ion channel of type A receptor for gamma-Amino Butyric Acid (GABAAR), and cationic channel of the nicotinic cholinergic receptors (nAChR). There are a few studies demonstrating the effects Mg2+ exerts on GABAAR and nAChR function. We present their findings in two short overviews.
2.2.1. Mg2+ Effects on N-Methyl-D-aspartate Receptors (NMDARs)
The NMDARs belong to a family of ionotropic glutamate receptors (iGluRs) known for their essential contribution to excitatory synaptic plasticity and long-term signaling in the CNS. These are heterotetrametric structures composed of two GluN1 subunits and two of four types (A–D) of GluN2 subunits or two types (A–B) of GluN3 subunits. Each receptor subunit contains an extracellular amino-terminal domain (ATD), an extracellular agonist-or ligand-binding domain (LBD), three TM regions (TM1, TM3, and TM4), a re-entrant p-loop (M2 region) with a pore-lining segment and a selectivity filter, and an intracellular C-terminal domain [122,123]. Mg2+ plays a pivotal role in modulating NMDAR activity. Activation of the NMDAR is unique as it requires two signaling factors–Gly binding to a site on the GluN1 subunit and the agonist (NMDA, Glu, Asp) binding to a site on the GluN2 subunit in coordination with depolarization-dependent removal of Mg2+ from its binding site on the GluN2 subunit. Most excitatory synapses function as a result of AMPA (α-amino3-hydroxy-5-methyl-4-isoxazole propionic acid) and NMDA receptor interplay, and the resulting excitatory postsynaptic currents (EPSC) reflect this heterogeneity. Upon membrane voltage change stimulus, AMPA receptor (AMPAR) currents rise and subside fast, as they determine the onset and maximal amplitude of the EPSC, while NMDAR currents rise and decline more slowly, setting the decay of the EPSC and strongly influencing total positive charge entering the cell [124].
Effects of Mg2+i on NMDARs
Mg2+i can block NMDAR channels, and this blocking effect has yet to be explained for its physiological function. Electrophysiological examination of inside-out patches from oocytes expressing NMDAR GluN1 and GluN2A subunits shows that increasing [Mg2+]i in Ca2+o-free solution at positive membrane potentials reduces outward currents through the NMDAR channel. However, at more negative membrane potentials, blocking of inwardly directed currents by Mg2+i is stronger [125]. Mg2+i produces only slight inhibition of inward currents in CA1 neurons at negative potentials but has substantial blocking activity on outward currents at positive membrane potentials. The blocking effect of Mg2+i is more pronounced in conditions lacking Mg2+o [126]. Experiments on outside-out patches of neuronal membranes show that physiological [Mg2+]i can rapidly block the NMDA-activated channel, as it reduces the channel’s current amplitude at positive membrane potentials, without any flickering. This effect, which is augmented with increasing [Mg2+]i, is reversible and not detectable at negative membrane potentials [127,128,129]. The absence of the flickering activity during electrophysiological recording of cultured murine cortical neurons was hypothesized to be due to Mg2+i association and dissociation rates that are so high that the discrete open and blocked states cannot be discerned with signal filtering. The blocking rate constants increase with rising [Mg2+]i and membrane depolarization [129].
In contrast to Mg2+o eliciting channel blocking action by binding to Asn residues of GluN2 subunit, the blocking action of Mg2+i is primarily the result of an interaction with Asn residues in the channel’s GluN1 subunit. Mutant NMDAR expressed on oocytes where GluN1 subunit’s Asn is position 598 is exchanged with nonpolar Gly (N598G) or polar uncharged Gln (N598Q) or Ser (N598S) attenuates the Mg2+i-elicited channel block [125,130,131], while substitution with Asp (N598D) enhances the blocking action due to the negatively charged Asp residue. Asn residues of both GluN subunits form a narrow constriction of the channel pore and represent binding sites for Mg2+i, although GluN1 is the dominant binding site [125,131].
In addition to the binding site for Mg2+o and blockage deep in the pore (~0.64 through the electric field of the membrane from the extracellular side) [127,132], NMDAR channel possesses a divalent cation binding site near the external mouth of the pore (~0.2 through the electric field), to which Mg2+ binds slowly [133]. The difference in the channel’s blocking activity by Mg2+i and Mg2+o [127,130], as well as the markedly faster unblocking rate constants of Mg2+i at physiological voltage [128], suggest that Mg2+i and Mg2+o bind at different sites within the channel pore.
On the other hand, it is proposed that Mg2+ can unblock the deep binding site of the NMDAR channel either by moving back to the extracellular solution or by permeating the channel to the intracellular compartment [134]. Assuming that there are multiple binding sites in a narrow NMDAR channel pore, any other ions binding to more external sites than the one Mg2+ already binds to will trap Mg2+ in the pore (“lock-in effect”), enhancing the channel block [132,134,135]. Furthermore, some other cations from extra- and intracellular fluid, such as Ca2+o [133], Na+i or Na+o [132,134,136] or Cs+i [136], when occupying more external permeant binding sites of the channel pore, prevent Mg2+o from binding to their deep blocking site. Mg2+ can still occupy the blocking site in the NMDAR channel pore while the receptor agonist unbinds from its site, which is considered to be a trapping-block kinetic scheme. What is more interesting is that the closing rate constant of the blocked channel is several times faster than that of an unblocked channel, leading to an asymmetrical trapping-block kinetic scheme. This model suggests a link between Mg2+ presence in the NMDAR channel pore and its allosteric influence on the permeation gate closure [137].
Effects of Mg2+o on NMDARs
Mg2+o are well-known voltage-dependent blockers of NMDARs. Earlier works on murine neuron cultures [138,139] or neurons in murine hypothalamic slices [140] show that NMDAR response to Glu is potentiated when [Mg2+]o is reduced to subphysiological levels (below 1 mmol/L). It was then proposed that the voltage-dependence of NMDAR conductance is a result of a voltage-dependent Mg2+o block, as Mg2+ enters the receptor-channel, sensing the membrane electric field. Moreover, Mg2+o-free solution allows Glu, Asp, or NMDA to elicit stable inward currents, almost linearly with increasing membrane potential [138,139,140], but addition of Mg2+o strikingly reduces inward currents, while causing little change to outward currents [138,139]. Glutamate or NMDA in Mg2+o-free conditions is capable of opening the NMDAR cationic channel independently of the membrane potential level, showing reduced voltage sensitivity of the channel conductance in the absence of Mg2+o [138,139,140]. Elevated [Mg2+]o in isolated murine hippocampal neurons can potentiate outward currents through NMDAR channels in a concentration-dependent manner [141]. Under Mg2+o-free conditions, NMDA-induced nerve cell membrane depolarization in murine hippocampal slices [142], cerebral cortex slices [143,144] or spinal cord culture [139] is accompanied by a decrease in membrane input resistance. Conversely, [Mg2+]o increase elicits a persistent rise in the input resistance due to a voltage-dependent Mg2+ block of NMDA-evoked current [139,142,143,144]. NMDAR response to NMDA seen in neurons of murine brain slices or cultures at physiological [Mg2+]o (~1 mmol/L) exhibits a negative slope in the I–V relation, i.e., a conduction decrease over the membrane potential range of −70 to −30 mV, an effect that is absent in Mg2+o-free solution or at more positive membrane potentials and depolarization [139,140,141,145,146]. Hyperpolarization of the cell membrane potentiates the blocking effect of Mg2+o [138], but that effect subsides at positive membrane potentials (+20 mV) [145].
Morphological studies of neurons in culture report on the toxic effects of NMDA or Glu in Mg2+o-free conditions, suggesting that the relief from Mg2+o-induced block of NMDA-activated channels drives a more persistent stimulation of the cell membrane and influx of Ca2+ ions [147,148,149]. When membrane patches of neurons in brain slices or culture are held at negative membrane potential in the presence of Mg2+o, each stimulated channel opens in a form of grouped bursts, a flickering activity whose duration decreases as [Mg2+]o increases [135,138,139,143,150,151]. These bursts are a result of short current flow interruptions during periods when the channel is in the open configuration [151], implying a possibility that Mg2+o enters and blocks the open channel very briefly at a time [150]. In addition, Mg2+o reduces the frequency of the Glu-induced NMDAR channel open state [138]. The NMDAR channel sensitivity to Mg2+o block is suggested to result from Asn residues in the pore-forming TM2 segment acting as potential binding sites for Mg2+o. Electrophysiological experiments on mutant channels expressed on Xenopus oocytes demonstrate that the replacement of Asn with Gln in position 598 (N598Q) of the GluN1 subunit’s TM2 segment slightly reduces the Mg2+o block and decreases channel pore permeability for Ca2+ [152,153]. The same change in homologous position 595 (N595Q) of the GluN2 subunit (known as the Asn, N-site or Gln/Asn/Arg, Q/N/R-site) strongly diminishes Mg2+o blocking action and promotes Mg2+ ion permeability [152]. In the narrow constriction of the NMDAR channel, two adjacent GluN2 subunits align so that their Asn residues at positions N and N + 1, respectively, form a critical blocking site for Mg2+o [125]. However, GluN2 subunit’s differences in susceptibility to Mg2+o block cannot be explained by a single structural determinant such as Asn at the N-site. Namely, chimeric GluN2 subunits in channels expressed on Xenopus oocytes, constructed by replacing portions of the least sensitive GluN2C subunit with elements from the most susceptible GluN2B subunit, emphasize three determinants–the TM1 segment, the linker between TM2 and TM3 segments, and the TM4 segment as being important for Mg2+-dependent block in addition to the Q/N/R-site [154]. Large rapid membrane depolarizations consequently affect NMDAR channels (comprising GluN1 and GluN2A or GluN2B), by alleviating Mg2+o-imposed block with a complex time dependence [137,155]. The resulting release of Mg2+o block and elicited current has a relatively fast component due to the rapid Mg2+ unbinding kinetics (time constant 1 ms or less), and a slow component from an inherent voltage-dependent channel gating which increases in its open probability (time constant 10–15 ms) [137,156]. It is suggested that the specific slow Mg2+o unblock and the voltage-dependent gating properties of NMDAR GluN2 subunits are attributed to the Ser residue at the 632 position (S632) in the TM3 region of GluN2 subunits, as substitution of Ser with Leu at this site (S632L) reduces the subunit’s affinity to Mg2+ [156,157]. Both Mg2+o and Gly are necessary for the physiological function of NMDARs. Interestingly, Mg2+o allosterically increases NMDAR affinity for Gly, and it reduces the desensitization of the receptor by interacting with the Gly binding site [141].
In more recent studies on murine CA1 neurons, it is suspected that the blocking action of Mg2+o is possibly not solely regulated by changing membrane potential, and that a relative proportion of Na+ influx and efflux is an important factor. Changing [Na+]i and [Na+]o to create an inward or outward Na+ ion flux alters the efficacy of the Mg2+ block. Presence of Mg2+o elicits a prominent current inhibition during Na+ inward flux but has little blocking effect when Na+ outward flux occurs. The flow and tendency of movement of permeant ions such as Na+ can enhance the Mg2+o-induced block of the NMDAR channel, rather than interfering with it [126].
2.2.2. Mg2+ Effects on Purinergic P2X Receptors (P2XRs)
Purinergic P2 receptors are membrane proteins activated by extracellular nucleotides, which are widely expressed in many tissues and mediate cell communication. P2X receptors are ligand-gated channels conducting cation currents, while P2Y are G-protein coupled receptors. P2X (seven subtypes, P2X1-P2X7) mediate rapid cellular responses by opening their ligand-gated nonspecific cation channels in the cell membrane in response to ATP binding. These are unique, trimeric ATP-gated ion channels distinct from other transmitter-gated channels, forming pores that nonselectively allow small positive ions of Na+, K+, and Ca2+ to flow through. Most of the established Mg2+ actions on P2XRs arise from outside the cell, through changes in ATP speciation and potential allosteric modulation, while direct, defined intracellular mechanisms are less well characterized.
Effects of Mg2+i on P2XRs
P2XRs possess intracellular N- and C-termini and cytoplasmic domains essential for receptor function and downstream signaling. Their role is currently emphasized in channel gating rather than specific modulation by Mg2+i. Given the nonselective cation permeability of P2XR channels, interaction with Mg2+i could, in principle, influence their permeation or rectification. However, current data do not assign a direct effect of Mg2+i on P2X receptors, as no defined specific, reproducible Mg2+i binding sites or block phenomena have been revealed as canonical modulators across P2XR subtypes [158]. Interpretations of Mg2+ effects rather center on extracellular ATP/Mg-ATP dynamics [159].
Effects of Mg2+o on P2XRs
Available structural and pharmacological data primarily detail extracellular P2X control by Mg2+o. Namely, Mg2+o profoundly shapes P2XR activation by altering the chemical form of ATP and, in some cases, by interacting with allosteric sites on the ectodomain. Under physiological conditions, ATP is predominantly complexed with Mg2+, as it neutralizes the negative charges on its phosphate groups and stabilizes the molecule conformation into a compact Mg–ATP complex. P2X subtype-specific activation is associated with ATP speciation in such a way that the active nucleotide form differs by receptor subtype: P2X2 can be robustly activated by free ATP (ATP4−), whereas Mg-ATP2− promotes opening with very low efficacy at P2X2Rs, revealing a striking dependence of gating on the ATP speciation. Other subtypes also exhibit distinct profiles, establishing subtype-specific control by Mg2+o via the free ATP/Mg-ATP balance [160].
Human P2X2R subtype shows Mg2+o-dependent reduction in activation (while ligand binding remains relatively intact), P2X3R exhibits decreased activation at high Mg2+o levels with increased binding, while P2X2/3 heteromers display a hybrid effect, as determined using fluorescent ATP derivatives. These data indicate that Mg2+o causes P2XR binding–gating dissociation, as it can reduce gating efficacy without proportionally diminishing orthosteric binding, consistent with Mg-ATP favoring binding yet being a weaker gating agonist in certain subtypes. Additionally, evidence from ligand-binding and activation studies supports putative allosteric Mg2+o interactions, whereby Mg2+o can act beyond orthosteric Mg-ATP formation, potentially engaging an extracellular allosteric site to modulate P2XR function. This has been proposed to explain Mg2+o -dependent shifts in activation independent of binding loss, with subtype variability in magnitude and direction of the effect [161]. There is now an emerging recognition from subtype-specific experiments that Mg2+o is a functionally relevant P2XR modulator [162].
2.2.3. Mg2+ Effects on Gamma-Amino Butyric Acid A Type (GABAA) Receptor
GABA is one of the most important inhibitory neurotransmitters in the CNS, and most of its actions are mediated by GABAARs. These receptors consist of subunits that belong to seven classes, and each class has one or several members–α (1–6), β (1–4), γ (1–4), δ1, ε1, π1, or ϑ1. Being usually pentameric, GABAARs are a result of combining two α, two β, and one γ, δ, ε, π, or ϑ subunit (2:2:1 ratio) [163].
Electrophysiological experiments on Xenopus oocytes, expressing recombinant GABAARs (α1β2γ2S, α2β2γ2S, α1β2, and α2β2), show a biphasic change in GABA-induced inward Cl− currents in the presence of Mg2+o. An increase in inward current elicited by GABA binding to its receptor is evident in low [Mg2+]o (0.01 mmol/L), leading to a peak increase when the extracellular solution contains physiological [Mg2+]o of 1 mmol/L. However, high [Mg2+]o (10 mmol/L) reduces the GABA-activated current in most receptors [164]. Autoradiographic analysis of murine brain sections points to a decrease in the binding of a GABAAR Cl− channel blocker (t-butylbicyclophosphoro[S35]thionate, [S35]TBPS) across various anatomical structures in the presence of physiological [Mg2+]o (1 mmol/L), an effect that achieves reversed by high [Mg2+]o reaching supraphysiological level of 10 mmol/L. Furthermore, Mg2+o potentiates GABA-induced inhibition of Cl− channel blocker binding but also decreases the binding action of Cl− channel blocker in the presence of a GABAAR competitive antagonist (gabazine, SR-95531). It is suggested that Mg2+o exerts action on GABAARs independently of GABA presence [164]. Effects of Mg2+o on GABAARs are potentially mediated by two receptor binding site types–a high-affinity potentiating site and a low-affinity inhibitory site.
2.2.4. Mg2+ Effects on Nicotinic Acetylcholine Receptors (nAChRs)
The nAChRs are concentrated at the vertebrate neuromuscular junction, at synapses on autonomic ganglion cells and central neurons. nAChR is a pentameric structure, where each of the five subunits is arranged around the central pore. They can be homomeric, where subunits are identical, or heteromeric, where different subunits complex together. Neuronal nAChRs are heteromeric α4β2 pentamers formed in a 2:3 or 3:2 subunit ratio, while muscle nAChRs are most commonly composed of two α1- and one of β1-, δ-, and γ-or ε-subunits. Each subunit has a long extracellular N-terminal domain acting as an ACh- or agonist-binding site (extracellular domain, ECD), four TM regions (TM1–4), an intracellular loop that connects TM3 and TM4, and a short extracellular C-terminal domain. The TM2 regions of all five subunits form the lining of the receptor-associated ion channel pore [165].
The mechanism of nAChR activation by ACh has been examined. It proposes that two agonist acetylcholine oxyanions (ACh+) react with the nAChR recognition site and exchange for one Mg2+ ion. This exchange with Mg2+ occurs at two closely positioned negatively charged groups within the nAChR recognition site. The resting state of the membrane potential allows for electrostatic attraction between these negatively charged groups and Mg2+. However, when Mg2+ is replaced with two acetylcholine (ACh) molecules, they form two mutually repelling ACh+ receptor dipoles that cause the receptor groups to be forced apart, opening the receptor pore [166].
Mg2+o alters the time course of synaptic currents through nAChR channel, as excitatory miniature end-plate currents elicited by ACh binding to this receptor, exhibit slower decay when [Mg2+]o is higher [167]. Since receptor’s channel kinetics and channel gating determine current dynamics, two mechanisms have been proposed–cations such as Mg2+o interact with binding sites within the channel pore, and they alter the surface potential near the nAChR, indirectly influencing channel gating properties [168,169].
Patch-clamp recordings of sympathetic ganglion neurons and PC12 cells show that Mg2+i can block the channel pore, and that this blocking action is potentiated by membrane depolarization, i.e., it is voltage-dependent. However, extremely positive membrane potentials elicit permeation of the channel by Mg2+ and an outward current [170,171]. Electrophysiological examination of single-channel currents from cell-attached and outside-out patches of PC12 cells [169,171,172], guinea pig outer hair cells [173] or Xenopus oocytes expressing nAChRs [174] demonstrate that channel’s outward conductance is more noticeably reduced in the presence of high [Mg2+]i, an effect that is reversed when the intracellular solution dialyzing the cells is depleted of Mg2+i [169,171,172,173,174]. It is suggested that Mg2+i blocks the channel pore and changes the intrinsic channel gating of nAChR channel, and that both of these processes are voltage-dependent [169]. High [Mg2+]o decreases in ACh-evoked single-channel inward conductance, while conductance increases in Mg2+o-free solutions [171,172,173].
On the other hand, the effects of Mg2+o on outward conductance and Mg2+i on inward conductance of nAChR channels are debatable, as the currents are either not being affected [172] or being reduced [171]. Additionally, inward nAChR channel conductance is either not affected by varying [Mg2+]i [172], or slightly decreased with elevated [Mg2+]i [171]. The most likely explanation for the effects of both Mg2+i and Mg2+o on nAChR channel conductance is the screening of negative charges on both ends of the channel pore [172]. nAChRs are more sensitive to [Mg2+]i increase, blocking the outward currents, than to increasing [Mg2+]o blocking the inward currents, showing the asymmetrical effect of Mg2+ on nAChRs [171,172].
Figure 2 presents a visual summary of all major voltage-gated and ligand-gated ion channels in the nerve cell membrane, regulated by Mg2+.
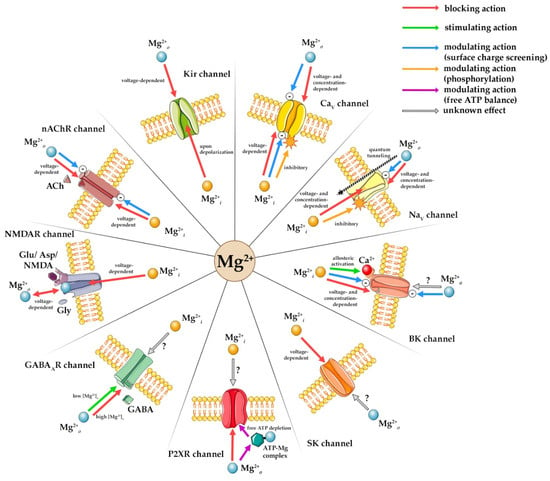
Figure 2.
Magnesium ions as modulators of ion channel function in the neuronal membrane. Mg2+i—intracellular Mg2+, Mg2+o—extracellular Mg2+, Kir—inward rectifier K+ channel, CaV—voltage-gated Ca2+ channel, NaV—voltage-gated Na+ channel, BK—large conductance Ca2+-activated K+ channel, SK—small conductance Ca2+-activated K+ channel, P2XRs—purinergic P2X receptors, ATP—adenosine triphosphate, NMDAR—N-methyl-D-aspartate Receptor, Gly—glycine, Glu—glutamate, Asp—aspartate, GABAAR—type A Gamma-Amino Butyric Acid Receptor, [Mg2+]o—concentration of extracellular Mg2+, nAChR—nicotinic ACh receptor; red arrow—blocking action, green arrow—stimulating action, blue arrow—modulating action (surface charge screening), orange arrow—modulating action (phosphorylation), magenta arrow—modulating action (free ATP balance), gray arrow—unknown mode of action.
Underlying mechanisms of Mg2+i and Mg2+o effects on neuronal ion channel function are concisely presented in Table 1.

Table 1.
Underlying mechanisms of Mg2+i and Mg2+o effects on ion channels in central neurons.
2.3. Other Mechanisms Contributing to Electrophysiological Effects of Mg2+ in Nerve Cells
All the interactions described evidence the critical Mg2+-dependent modulation of neuronal voltage-gated and ligand-gated ion channels. Some other mechanisms additionally contribute to the manyfold neuroactive effects through which Mg2+ interferes with the regulation of intrinsic nerve cell membrane excitability, ionic mechanisms, and chemical and electrical synapses in the brain. For example, ion channels gated by other types of signals can also be Mg2+-sensitive, such as some mechanosensitive and temperature-gated channels (e.g., transient receptor potential melastatin type 7–TRPM7 channel) [176], gap junction channels (e.g., connexin type 36–Cx36 channel) [177], intracellular ion channels (e.g., RyR channel) [178], and finally, the activity of the Na+/K+ pump is also dynamically regulated by Mg2+ as an essential cofactor of the Na+/K+-ATPase [179]. Mg2+ is also involved in regulating certain subthreshold pacemaker ion currents in central neurons. Although Mg2+ is not the main regulator of NaV channels, it can block the non-inactivating fraction of current through these channels–the persistent Na+ current (INaP) active in many pacemaker neurons [80]. Aside from affecting INaP, Mg2+ also helps regulate neuronal subthreshold pacemaker current through the hyperpolarization-activated cyclic nucleotide-gated (HCN) channels–the IHCN current. While Mg2+ is not their primary regulator, it can indirectly influence HCN channel function by interacting with the intracellular environment (including cyclic nucleotides and other ions such as K+ and Na+) [180].
3. Discussion
Magnesium is the fourth most abundant cation in the body and participates in the regulation of important processes in excitable tissues, such as membrane electrolyte flux and antagonism with Ca2+. Mg2+ is responsible for pivotal regulatory effects on voltage-gated and ligand-gated ion channels, determining cellular excitability and signal transduction. Taking into account inevitable electrolyte imbalances developing during ischemic or traumatic insults, both in the heart and the nervous system, it is important to recognize pathways that involve Mg2+ and lead to the disruption of membrane electrical properties and cell function.
Mg2+i essentially carries the inward rectification of K+ currents through Kir channels during membrane depolarization, since it enters the cytoplasmatic and transmembrane regions of the channel pore, binds to negatively charged amino acid residues in the pore lining, thereby blocking outward current flow. Mg2+o can exert a similar blocking effect, which is contingent on channel inactivation and direct blocking action, also by targeting negatively charged sites in the extracellular channel domains. The effects that Mg2+o and Mg2+i elicit on Kir channels can modify the resting membrane potential and cellular excitability, causing changes in normal cardiac rhythm, neurological disorders such as epilepsy or ataxia, and glial disorders or sensorineural hearing loss.
Depletion of Mg2+ weakens the voltage-dependent Kir channel block, preventing excitable cells from restoring [K+]i and maintaining [K+]o in the physiological range after APs. In return, the cells experience excessive depolarization, hyperexcitability, and late repolarization, which can be seen in cardiomyocytes [181]. Kir2.1 channelopathy stemming from KCNJ2 mutation manifests as Andersen–Tawill syndrome, where this channel is nonfunctional, and in addition to distinctive craniofacial features, patients suffer from periodic paralysis and long QT syndrome (LQT). Hyperexcitability in skeletal muscles leads to cramps and myotonia, episodically interrupted with muscle weakness, while the prolonged plateau phase of cardiac AP destabilizes membrane potential and can cause severe arrhythmias [181]. Since hypomagnesemia can exacerbate symptoms, administration of Mg2+ can be corrective for both the electrolyte imbalance and membrane stabilization. Severe Mg2+ deficit causes acquired LQT, which can be complicated with malignant arrhythmias, so urgent infusion of magnesium sulphate is indicated in these cases. Some Kir2.1 channel mutations are considered to be involved in retinopathy of prematurity, isolated arrhythmia with LQT phenotype (Thr 305 Ala substitution), or paroxysmal atrial fibrillation or ventricular tachycardia in patients with short QT syndrome (SQT3), where cardiac AP duration is shortened as well as the QT interval [14]. Mutations of KCNJ10 encoding the Kir4.1 channel leads to epileptic seizures, ataxia, sensorineural hearing loss and renal tubulopathy (EAST syndrome) [14,181]. Functional Kir4.1 channels balance extracellular K+ after neuronal firing, maintain endolymph [K+] and mediate reabsorption and electrolyte balance in renal tubules, while mutant channels become unstable, lose their control of the current, leading to EAST syndrome.
Elevated physiological or supraphysiological [Mg2+]i directly inhibits permeation through CaV channels in a voltage-dependent manner. The reduction in current flow is modulated by CaV channel phosphorylation, considering an interplay of channel gating kinetics shift due to Mg2+-induced phosphorylation and phosphorylation-induced channel susceptibility to Mg2+i blocking action. Mg2+i competes with Ca2+–CaM for binding sites on CaV channel regulatory domains, affecting CDI and VDI of overstimulated cells. Mg2+o directly blocks CaV channels, an effect which is potentiated with membrane hyperpolarization, and modifies channel gating kinetics through surface charge screening. CaV channels play a crucial role in excitation–contraction coupling in every muscle type, drive synaptic neurotransmitter release, support gene transcription, and plasticity. Changes in [Mg2+] can subsequently modify the effectiveness of Ca2+-dependent processes, leading to life-threatening cardiovascular or neurologic conditions.
As Mg2+ can affect vascular smooth muscle tone and regulate peripheral vascular resistance, this indirectly modifies blood pressure (BP). Acting as a blocker of CaV1 channels, Mg2+ reduces Ca2+ inward currents and myogenic tone, facilitating vasodilation. Depletion of Mg2+ severely impairs this effect, leading to vasoconstriction and hypertension [4]. On the other hand, diminished blocking of the CaV1 channels in cardiomyocytes due to hypomagnesemia enhances Ca2+ influx and prolongs the AP plateau phase, delaying repolarization. Consequently, hypomagnesemia can trigger an acquired LQT [4]. Mutations of the CaV1.1 or NaV1.4 channels are associated with hypokalemic periodic paralysis, a neurologic condition presenting with hypotonia and transient intervals of local or generalized paresis or paralysis [44] due to long-lasting membrane depolarization leading to NaV channel inactivation and loss of muscle excitability. CaV1.2 channels, highly expressed in hippocampal neurons, participate in long-term synaptic plasticity through the formation of spatial and fear memory and emotional behavior [41,44]. Dysregulation of these channels is related to forms of schizophrenia, bipolar affective disorder, and anxiety- and depression-like behaviors [41]. Mental disabilities, autism spectrum disorders, and severe cardiac arrhythmias are to some extent connected with CaV1.2 channel mutations [41]. Gain-of-function mutation of CaV1.2 in Timothy syndrome reduces the channel’s CDI and VDI, leading to excessive influx of Ca2+ and delayed cell repolarization, which destabilizes the cell membrane. LQT and neuronal hyperexcitability elicit severe arrhythmias and intellectual disability or autism in patients carrying this mutation [44]. Mutant or dysfunctional CaV1.3 channels can lead to SAN dysfunction–bradycardia, arrhythmias, and sensorineural hearing loss due to insufficient Ca2+i handling. Interestingly, CaV1.3 channels are an important gateway of [Ca2+]i increase in spontaneously active substantia nigra DA neurons, which are degenerated in Parkinson’s disease. DHP and related drugs–CaV channel blockers are commonly used to treat hypertension, but can be repurposed for the treatment of neuropsychiatric disorders, autism spectrum disorders, and neuroprotection in Parkinson’s disease [44]. Given its mechanism of action, it is conceivable that low [Mg2+]o can lead to increased CaV channel activity, leading to overexcitation of cells and manifesting similarly to disorders of different backgrounds with excessive CaV channel activation. Mg2+o blocks CaV2 channels on presynaptic nerve endings, which modulates neurotransmitter release. It is plausible that low [Mg2+]o alleviates this blocking action and facilitates the release of neurotransmitters, which in turn overstimulates postsynaptic cell membranes. Gain-of-function CaV2.1 channel mutation is linked to familial hemiplegic migraine, while loss-of-function mutation is connected with a form of episodic ataxia [42]. Although some CaV2 channel mutations have subtle presentation, a gain-of-function mutation to CaV2.2 channels can contribute to pain hypersensitivity or some forms of seizures [44]. In addition to its adjuvant analgesic effect mediated through NMDARs block, Mg2+o (magnesium sulphate) supports intraoperative neuromuscular blockade by reducing presynaptic CaV2 channel activity, thus suppressing ACh release in the motor end plate [182]. Neurotic disorders are a group of functional psychiatric disorders encompassing diverse symptomatology such as hyperexcitability, anxiety, panic, and phobic reactions, attention deficits, and sleep disorders, among others. Hyperactivity of glutamatergic neurotransmission–increased synthesis and/or presynaptic Glu release is one of the mechanisms underlying these disorders. Mg2+o reduces glutamatergic neurotransmission by blocking CaV2 channels on presynaptic neurons, alleviating anxiety, panic, and phobic reactions, and ameliorating sleeping deficits [183]. Mutations of the CaV3.2 are linked to generalized absence epilepsies and idiopathic generalized epilepsies, as they increase neuronal firing by reducing the threshold for rebound excitation. Inhibition of CaV3 channels protects from hyperexcitability disorders, such as absence epilepsy and certain forms of pain [42]. Additionally, mutations of all CaV3 channels have been linked to autism spectrum disorder [42].
Mg2+i acts as a direct, rapid NaV channel blocker, leading to flickering currents, but also modulates channel activity through channel phosphorylation. Elevated [Mg2+]o reduces NaV channel conduction in both concentration-and voltage-dependent manner, alters channel voltage sensing with surface charge screening, and, interestingly, is proposed to have the ability to depolarize the cell by traversing the closed channel by quantum tunneling. It is evident that minute changes of [Mg2+]i, even within the physiological range, can be detrimental to the cell’s electrical activity. NaV channels are essential in the initiation and propagation of APs in excitable cells. Perturbations in [Mg2+] can provoke severe, urgent disruptions of the fine-tuned cellular electrical activity, such as cardiac arrhythmias and bradycardias, muscle weakness, and epileptic seizures.
Mg2+o binds to the surface of skeletal muscle cell membrane, contributing to the electrical field. NaV channels are sensing and affecting their activation. Mg2+ deficiency alters the voltage dependence of NaV channel activation, allowing the channel to react to smaller membrane depolarization, leading to muscle spasms. Mutations of NaV1.1, NaV1.2, NaV1.3, or NaV1.6 are responsible for inherited epilepsy syndromes as inhibitory interneurons lose their tone, leading to hyperexcitability in the CNS [184]. SCN4A gain-of-function mutation encoding NaV1.4 channels can disable channel inactivation so that INaP in skeletal muscles elicits repetitive AP firing in some forms of myotonia or induces electrical silence in periodic paralysis [71,72]. Low [Mg2+]o may exacerbate myotonia in patients carrying SCN4A mutation, but supplementation with magnesium helps reduce weakness and myotonia [185]. Mg2+i can reduce NaV channel conductance of central neurons in a voltage- and concentration-dependent manner [73], so conditions where [Mg2+]i is low increase endogenous neuronal excitability, leading to epileptiform activity. Furthermore, gain-of-function SCN5A mutation encoding NaV1.5 channel abrogates fast channel inactivation, impairs channel closure, and prolongs AP due to INaP [184], eliciting LQT3 syndrome [71,72], while loss-of-function mutation in Brugada syndrome desynchronizes conduction in the ventricles [72,184]. Mutations of the SCN8A gene encoding the NaV1.6 channel are associated with cognitive deficits and susceptibility to bipolar disorder [184]. While the NaV1.6 channel is one of the most abundant Na+ channels in the CNS and PNS, responsible for repetitive neuronal firing due to resurgent currents [186], current evidence does not indicate a direct Mg2+ modulatory effect on these channels in central neurons. Furthermore, it is plausible that disturbances in Mg2+ homeostasis might indirectly alter neuronal excitability by influencing channels’ firing patterns. Sensory neurons bearing NaV1.7 channel mutations become hyperexcitable, leading to erythromelalgia, a rare vascular neuropathic pain disorder characterized by erythema, local hyperthermia, and burning pain in extremities. NaV1.7, NaV1.8, and NaV1.9 channels are present in DRG neurons, and they are important for the transportation of pain signals [184]. NaV1.8 channels mediate most of the inward Na+ currents during APs, and their mutations lead to abnormal firing of DRG neurons [184].
BK channels are important for the negative feedback control of Ca2+ influx and excitability in the nervous and cardiovascular systems, as they colocalize with CaV channels to form nanodomains [48] and form BK-GluN1 complexes with NMDARs [187]. Activation of BK channels in response to increasing [Ca2+]i decreases AP duration, enhances hyperpolarization potentials, limits neurotransmitter release, and time for Ca2+ influx. The BK-GluN1 complex has been described in the hippocampus, cerebellum, cortex, thalamus, and striatum, and it is proposed that the BK channel α-subunit S0–S1 loop interacts with the C-terminal domain of NMDAR. Activation of NMDAR by glutamate elicits outward currents through BK channels in dentate gyrus granule cells [187] and pyramidal neurons in the neocortex [188].
Dysfunction of BK channels is implicated in epilepsy, fragile X syndrome, intellectual disability, autism, movement disorders, and chronic pain [189]. A myriad of loss-of-function and gain-of-function mutations of the Slo1 (KCNMA1) gene generated in animal models help understand the role of BK channels in the etiopathogenesis of these disorders. The D434G mutant (Asp 434 Gly substitution) murine model shows generalized seizures and paroxysmal dyskinesia, most likely due to hyperexcitability of cortical and Purkinje neurons, as well as increased sensitivity of BK channels to Ca2+i [190]. A N995S mutation (Asn 995 Ser substitution) in the RCK2 domain that increases BK channel currents due to increased voltage sensitivity underlies paroxysmal non-kinesigenic dyskinesia [191]. Deletion of the KCNMB4 gene coding the β4 subunit [192] increases firing in hippocampal dentate granule cells, leading to temporal lobe epilepsy. Alteration in the regulatory β3-subunit as a result of a single base pair deletion in the KCNMB3 gene is associated with generalized epilepsy [193]. The BK channel G354S mutation (Gly 354 Ser substitution), which reduces channel conductance and ion selectivity, has been linked to congenital progressive cerebellar ataxia with cognitive impairment [194]. Single-nucleotide polymorphism in genes encoding α-subunit and β2-subunit (rs16934131 and rs637454, respectively), is associated with the risk of Alzheimer’s disease [195,196]. Release of excitatory neurotransmitters and calcitonin gene-related peptides from the trigeminal caudate nucleus is inhibited by BK channels, so they can be a potential target for the therapy of migraines [197]. The BK channels’ role in regulating neuronal firing and cell excitation is dependent on Ca2+i and membrane depolarization. Depletion of Mg2+i dampens BK channel activation, leaving neurons unprotected from overexcitation by increasing [Ca2+]i. BK channels are also an important regulator of smooth muscle cell excitability and vascular muscle tone since they mediate outward K+ currents. Mg2+i, in cooperation with Ca2+i, facilitates BK channel activation and reduces muscle cell excitation, leading to its relaxation and vasodilation. Vascular smooth muscle cells can have membrane microdomains where BK channels closely cooperate with CaV channels to regulate Ca2+i excitatory effect. Elevated [Mg2+]o is rare, but it can mediate a complex interplay between Mg2+o-mediated suppression of CaV channel inward Ca2+ current and Mg2+i-mediated activation of BK channel with large outward K+ current, leading to a decrease in smooth muscle membrane excitability and vasodilation [4]. It is then understandable why hypomagnesemia with both [Mg2+]o and [Mg2+]i reduction contributes to vasoconstriction and development of hypertension.
SK channels regulate membrane AHP, which is important for the regulation of intrinsic neuronal excitability and AP firing rate. Correspondingly to BK channels, they are located in the vicinity of CaV channels, NMDARs, or nAChRs and modify cell excitability upon activation by one of these ports of Ca2+ entry. In this manner, they affect synaptic plasticity in neurons and lead to AP completion in cardiomyocytes.
As SK channels are present on DA neurons in the midbrain, they control their firing patterns by counteracting the excitatory Ca2+ influx. Pathogenesis of Parkinson’s disease is related to these channels, which coordinate their activity with CaV channels to form patterns of DA release [198,199]. As the burst activity of these neurons releases DA [200], block of SK channels by Mg2+i or channel blockers (apamin) may alleviate symptoms of Parkinson’s disease [201,202]. This might be controversial since unregulated Ca2+ influx through activated NMDARs and CaV channels in DA neurons leads to their excitotoxic death and overall loss in the substantia nigra. Conversely, some studies show that SK channel activation results in afterhyperpolarization [203] in DA neurons, it reduces excitotoxicity [204] and enhances or preserves DA synthesis to mitigate long-term motor disorders in Parkinson’s disease. These contradictory findings are best explained by the effect of SK channel modulation in line with the stage of Parkinson’s disease. The gene KCNN3 encoding SK3 channels contains a sequence of trinucleotide CAG repeats, which is associated with schizophrenia and bipolar disorders [205,206]. Animal models with KCNN3 gene mutation present with suppressed SK3 channel activity in DA neurons, diminished counterbalance to NMDARs activation, which increases burst firing and release of DA, leading to attention deficits and sensory-motor alterations in behavior [207]. Recordings from Jurkat T cells expressing human mutant SK3 channel confirm the reduced channel activity previously described in animal models [208]. Moreover, a mutation of the CK3 channel resulting in the deletion of its N-terminal region was identified in patients with schizophrenia [209]. Mutations of SK channels have also been related to epilepsy. Expression of these channels in an animal model of epilepsy is shown to be significantly reduced [210]. Furthermore, in vitro [211], and in vivo [212,213] studies demonstrate that the use of SK channel activators reduces epileptiform activity. Activation of SK channels in atrial cardiomyocytes repolarizes them and reduces AP duration, acting as a negative feedback mechanism to Ca2+ influx during APs. Patients suffering from chronic atrial fibrillation exhibit a prominent shortening of AP duration, and it is known that AP shortening and prolongation may facilitate atrial arrhythmias in a similar fashion [118]. Expression of SK2 and SK3 channels in patients with chronic atrial fibrillation is reduced compared to healthy individuals [214].
NMDA receptors are inhibited by Mg2+i binding to GluN1 subunit amino acid residues deep within the channel pore, where Mg2+ can be “locked-in” by other cations binding distally in the channel. Mg2+o plays a crucial role in stabilizing cell excitability through voltage-dependent block of the NMDAR channels, binding to Asn residues (N-site) in adjacent GluN2 subunits and/or to TM1 and TM4 segments or TM2/TM3 linker in GluN2B subunits. NMDARs are crucial for synaptic plasticity, learning, and memory, but also orchestrate excitotoxicity in neurons in hypomagnesemia, leading to neurodegeneration in chronic settings or seizure activity in more acute cases. Hypermagnesemia impedes LTP as NMDARs are more resilient to depolarization, leading to learning deficits.
Mg2+o affects central excitatory glutamatergic synapses by modulating the activity of NMDARs, which at resting membrane potentials are blocked by these ions. A complex cascade of Glu-induced AMPAR activation and membrane depolarization removes the voltage-dependent Mg2+o block of NMDAR concomitantly stimulated by Glu and Gly and allows mostly an inward current flux through the NMDAR channel to elicit activation of neurons. The complex interplay of mechanisms occurring at low [Mg2+]o, which results in neuron overstimulation, is led by heightened NMDAR activation and excitotoxicity, where accumulation of Ca2+i expedites neuronal death. It is demonstrated that magnesium sulphate enters the cerebrospinal fluid and brain after systemic administration in an animal model. Consequent [Mg2+]o increase in the CNS disturbs binding of agonists (glutamate and glycine) to NMDARs in the hippocampus and cerebral cortex, providing insight into Mg2+ central anticonvulsant effect [215,216]. Mg2+o can disrupt the pathophysiological mechanism of NMDARs hyperactivity occurring in ischemic or traumatic brain injury that causes excitotoxic neuronal death [138,145], and it has been proven that magnesium therapy helps cognitive performance after brain injury. NMDAR activation can trigger cortical spreading depression (CSD), an electrical disturbance associated with seizures, migraine aura, and traumatic and ischemic brain injury, where localized intense depolarization of neurons and glial cells is followed by membrane ion flux change leading to an increase of [Ca2+]i while [Ca2+]o decreases [217]. CSD mechanism releases large amounts of Glu, causing a strong wave of depolarization spreading through the neighboring areas of the CNS. Hypomagnesemia can facilitate NMDAR activation and potentiate Glu effects, thus taking part in CSD initiation and spreading. In addition, physiological [Mg2+]o maintains Ca2+i balance by stabilizing NMDARs and blocks excessive Ca2+ influx and excitotoxicity, thus suppressing the production of substance P, one of the culprits in migraine pathogenesis [218]. Sensorineural hearing loss due to noise trauma is a result of NMDARs overstimulation in auditory neurons by large amounts of Glu released in ribbon synapses from cochlear inner hair cells reacting to higher amplitude of basilar membrane oscillation [219]. Several animal studies [220,221] demonstrate low [Mg2+] in the inner ear perilymph upon noise exposure and noise-induced hearing loss (NIHL), confirming the role of NMDAR involvement. In addition, studies on humans show that decreased serum [Mg2+] increases susceptibility to NIHL [222] and that magnesium supplementation can have therapeutic and prophylactic effects [223]. Another important role of Mg2+ is the prevention of NMDAR-mediated central sensitization following repetitive nociceptive inputs and the attenuation of pain hypersensitivity. Increase in neuronal [Ca2+]i upon repetitive stimulation is pivotal for central sensitization, but Mg2+o can help regulate this Ca2+ imbalance by antagonizing NMDARs, which reduces Ca2+ influx into neurons [224,225]. Considering its impact, Mg2+ (magnesium sulphate) is used as an adjuvant therapy for intra- and post-operative pain management and reduces analgesic requirements [226,227]. Interestingly, animal models exposed to dietary reduction in Mg2+ intake spanning a year present with atrophy and loss of pars compacta substantia nigra DA neurons [228,229,230]. Consistent with animal studies, a significant reduction of [Mg2+]i has been observed in patients with Parkinson’s disease [231]. Similarly, [Mg2+]o is reduced in the serum [232] and brain tissue (entorhinal cortex, hippocampal CA region, and globus pallidus) [233] of patients with Alzheimer’s disease. It is conceivable that Mg2+o might play a neuroprotective role in Parkinson’s disease and Alzheimer’s disease by modulating NMDAR activity [8], but these effects need to be studied further. Elevated activity of Glu stimulation i.e., Glu overstimulation of postsynaptic NMDARs, is one of the central mechanisms behind neurotic disorders. Most patients suffering from these disorders present with reduced intraerythocyte [Mg2+]i and [Mg2+]o. Mg2+o effects on Glu transmission are dual–suppression of presynaptic Glu release due to CaV blocking and direct competition with Ca2+ for NMDARs [183]. In light of these mechanisms, it is understandable that hypomagnesemia can increase neuronal vulnerability to various types of stressors in the development of neurotic disorders. More severe psychoses–acute schizophrenic episodes and bipolar disorder are accompanied by [Mg2+] decrease in the patient’s cerebrospinal fluid [234] or red blood cells [235], which steadily increases upon implementing antipsychotic therapy. Additionally, low plasma [Mg2+] is also present in patients with chronic schizophrenia [236]. It is proposed that Mg2+ reduces Glu release and Glu effects on NMDARs, as well as augments the activity of the GABAergic system.
Purinergic milieu in vivo varies across tissues in physiological states (homeostasis) and under pathophysiological conditions (inflammatory reaction, ischemia, etc.), depending on the concentrations of extracellular ATP and Mg2+o. Mg2+-dependent P2XR channel modulation contributes to the spatiotemporal tuning of P2XR signaling in the CNS, cardiovascular, and immune system, emphasizing Mg2+ status as a meaningful axis of variability in P2XR function in health and disease. Reviews synthesize P2XR ion channel dysfunctions contributing to nerve signaling, pain, inflammation, and different disease phenotypes. Several pathogenic variants of P2XRs and their disease associations have been documented, with the clearest human mutation–phenotype link in P2X2-related hearing loss. Broader receptor–disease connections are established by expression and functional studies across systems. Mutations of the human P2X2 receptor have been implicated in hereditary hearing loss, as these mutant variants alter P2X2 channel signaling in cochlear pathways, aligning with their role in auditory processing. Recent reports detail hearing loss-associated mutations affecting P2X2 channel function. Other than P2X2R association with auditory function, receptor–disease associations have been identified for other specific P2XR subtypes, P2X1 with platelet aggregation, P2X3 with asthma and sensory signaling, and P2X7 with vascular inflammation and immune responses. P2XR channelopathies are also being investigated in neurodegenerative conditions [237]. A study on murine substantia nigra DA neuron culture shows that P2X7Rs are directly involved in extracellular ATP-induced cell swelling and death [238]. Irregular activation of P2XRs mostly reported in microglial cells, can lead to a spectrum of mechanisms underlying Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis [239], yet diverse evidence of neuronal P2XR channelopathies in the etiopathogenesis of these diseases is lacking.
Concerning the effects of Mg2+ disorders, evidence directly connecting alterations of Mg2+ status to human P2X channelopathies is limited. In a context where a mutant receptor relies more on free ATP for efficient gating (P2X2, such as behavior), higher Mg2+o (favoring Mg-ATP2−) could reduce activation efficacy, potentially worsening functional deficits, and conversely, lower Mg2+o could increase free ATP availability and enhance receptor activation. More data are available on dysfunctions of standard, naturally occurring P2XR channel variants that arise from Mg2+ imbalances, which can perturb purinergic signaling in several ways, by shifting ATP speciation and allosteric modulation, producing subtype-dependent changes in P2XR activation, binding–gating coupling, and downstream cation flux. High Mg2+o elevates Mg-ATP2− levels and reduces free ATP levels, generally diminishing activation efficacy in subtypes like P2X2, while leaving binding relatively preserved; P2X3 subtype may show decreased activation with paradoxically increased binding, while heteromeric P2X2/3 displays mixed effects. Functional consequences include attenuated cation influx and altered desensitization dynamics, contingent on subtype composition [160,161].
Inhibitory effect of Mg2+o on GABAAR channels is concentration-dependent, as physiological Mg2+o levels allow maximal inward current flow through the channel, while higher concentrations reduce current amplitude and increase GABAA receptor susceptibility to Cl− channel blockers. GABAARs mediate inhibitory neurotransmission in the CNS and balance the excitatory signaling mediated by AMPARs and NMDARs. Mg2+ and GABA together achieve a neuroprotective synergistic effect through GABAARs activation. Mg2+ depletion can reduce GABAAR efficacy, leading to hyperexcitability, anxiety, insomnia, and seizure activity, whereas hypermagnesemia can amplify the intricate GABAergic inhibition, leading to cognitive impairment, sedation, and muscle weakness.
Acting as a GABAAR agonist, physiological [Mg2+]o causes neuron hyperpolarization, mediating Cl− influx and protecting it from excessive synaptic excitation from other sources. Mg2+o leads to concentration-dependent reversible suppression of epileptiform activity in hippocampal neurons induced by a GABAAR antagonist, bicuculine, through a direct block of CaV channel-mediated currents in postsynaptic neurites, as well as in presynaptic membranes to modulate synaptic transmission. Additionally, Mg2+o alters NaV channel activation while stimulating GABAARs [240]. Neurotic disorders can stem from a decrease in GABAergic activity, and Mg2+ helps reduce some of the symptomatology by enhancing the function of GABA-mediated neurotransmission and increasing GABA release [183]. The GABAergic system, which is suppressed in patients with schizophrenia, can be reactivated by increasing neuronal Mg2+ following antipsychotic drug therapy [164,241].
Mg2+o inhibits the activity of nACh receptors in a two-fold action, both being voltage-dependent. Interaction between Mg2+o and the binding sites in the channel pore lining impedes current flow, while interaction with the outer membrane surface through surface charge screening alters membrane voltage and channel gating properties. Nicotinic ACh receptors play a crucial role in neuromuscular signaling and motor control, autonomic nervous system output, and cognitive processing. Hypomagnesemia alleviates nAChRs from its blocking action, leading to muscle spasm, neuronal hyperexcitability, and autonomic nervous system hyperactivity. Conversely, high Mg2+ levels promote the attenuation of nAChR activity, leading to a suppression of electrical activity in most of the excitable tissues expressing these receptors.
By interacting with nAChRs, Mg2+ acts as a modulator of ion fluxes–centrally it reduces nAChR conductance, and peripherally it blocks the neuromuscular junction. Mg2+ potentiates intraoperative neuromuscular block elicited by known non-depolarizing nACh blockers (d-tubocurarine or vecuronium), possibly through bimodal action in the motor endplate–diminution of ACh-induced depolarizing action caused by nAChR block and suppression of ACh release from axon terminals due to CaV channel block [242,243].
4. Conclusions
Mg2+ ion is a homeostatic agent and one of the simple biological regulators of cell excitability. Both Mg2+i and Mg2+o exert pleotropic impacts on elements of synaptic and non-synaptic cell excitability. Their effects are most pronounced in central neurons, where a multitude of mechanisms contribute to the complex regulation of ion channel function, allowing Mg2+ to fine–tune cell membrane ion transport. Appreciating these cellular and tissue processes in the brain at which Mg2+ is interposed to maintain normal excitability as a vital body function can help us better understand and treat clinical conditions with neurological dysfunctions due to hyper-or hypoexcitability caused by disorders of Mg2+ homeostasis.
Magnesium ions are an integral part of the intricate mechanisms of membrane excitability control. Studying their impact on the function of voltage-gated and ligand-gated ion channels shows how delicate changes in the internal balance of Mg2+ provoke severe disruptions in cell excitability, function, and morphology. Despite extensive research already undertaken, some of the mechanisms of Mg2+i and Mg2+o effects on ion channels’ functions are still elusive and unknown.
5. Future Directions
Development of more precise imaging techniques, in silico molecular modeling, optogenetics, and ultra-high resolution electrophysiological recording combined with channel mutagenesis, warrants further discoveries concerning Mg2+-regulated ion channels and, more importantly, new targets for magnesium-based therapy for some inherited and acquired ion channel disturbances and subsequent clinical conditions.
Author Contributions
Literature review and original draft preparation: S.S. and M.S.; Writing: S.S., M.S. and S.L.; Conceptualization and supervision: M.S. and S.S.; Review and editing: S.S., M.S., M.B., N.J., S.L., S.K. and J.N.O.; Funding Acquisition: J.N.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia, project number: 451-03-66/2025-03/200110.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ER | endoplasmic reticulum |
| Mg2+ | magnesium ions |
| Na+ | sodium ions |
| K+ | potassium ions |
| Ca2+ | calcium ions |
| Cl− | chloride ions |
| Ca2+i | intracellular Ca2+ |
| iMg2+ | ionized Mg2+ fraction |
| Mg2+i | intracellular (inner) Mg2+ |
| Mg2+o | extracellular (outer) Mg2+ |
| [Mg2+] | concentration of Mg2+ |
| [Mg2+]i | concentration of intracellular Mg2+ |
| [Mg2+]o | concentration of extracellular Mg2+ |
| ATP | adenosine triphosphate |
| ADP | adenosine diphosphate |
| VGC | voltage-gated channel |
| VSD | voltage-sensing domain |
| Kir | inward rectifier potassium channel |
| TM | transmembrane |
| CTD | cytoplasmic domain |
| MEL | murine erythroleukaemia |
| Asp | Aspartate (D) |
| Asn | Asparagine (N) |
| Glu | Glutamate (E) |
| Gln | Glutamine (Q) |
| Gly | Glycine (G) |
| Ser | Serine (S) |
| [K+]i | concentration of intracellular K+ |
| [K+]o | concentration of extracellular K+ |
| His | Histidine (H) |
| KV | voltage-gated potassium |
| KCNQ | potassium voltage-gated channel subfamily Q |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| EAG | ether-à-go-go |
| hERG | human ether-à-go-go related gene |
| CaV | voltage-gated calcium |
| PD | pore domain |
| DHP | dihydropyridine |
| SAN | sinoatrial node |
| DHPR | dihydropyridine receptor |
| AP | action potential |
| HVA | high-voltage activated |
| LVA | low-voltage activated |
| [Ca2+]i | concentration of intracellular Ca2+ |
| [Ca2+]o | concentration of extracellular Ca2+ |
| VDI | voltage-dependent inactivation |
| CDI | Ca2+-dependent inactivation |
| IQ | isoleucine-glutamine |
| CaM | calmodulin |
| AID | α1-interacting domain |
| HEK | human embryonic kidney |
| Ala | Alanine (A) |
| Lys | Lysine (K) |
| DCT | distal C-terminal domain |
| PCT | proximal C-terminal domain |
| cAMP | cyclic adenosine monophosphate |
| PKA | protein kinase A |
| PP2A | phosphatase 2A |
| DRG | dorsal root ganglion |
| PC12 | rat pheochromocytoma cells |
| NaV | voltage-gated sodium channel |
| CNS | central nervous system |
| PNS | peripheral nervous system |
| [Na+]i | concentration of intracellular Na+ |
| [Na+]o | concentration of extracellular Na+ |
| TTX | tetrodotoxin |
| IC50 | half maximal inhibitory concentration |
| BK | large conductance Ca2+-activated potassium (channel) |
| NMDAR | N-methyl-D-aspartate Receptor |
| RyR | ryanodine receptor |
| PGD | pore-gate domain |
| RCK | regulator of K+ conductance |
| mSlo1 | mouse Slo1 |
| Cys | Cysteine (C) |
| G–V | Conductance–Voltage relationship |
| Arg | Arginine (R) |
| Trp | Tryptophan (W) |
| Ig | transient gating current |
| Leu | Leucine (L) |
| SK | small conductance Ca2+-activated potassium (channel) |
| AHP | afterhyperpolarization |
| CaMBD | CaM binding domain |
| DA | dopamine, dopaminergic |
| LTP | long-term potentiation |
| I–V | Current–Voltage relationship |
| rSK2 | rat SK2 (channel) |
| NMDA | N-methyl-D-aspartate |
| P2XR | purinergic P2X receptors |
| GABA | Gamma-Amino Butyric Acid |
| nAChR | nicotinic ACh receptor |
| iGluR | ionotropic glutamate receptor |
| ATD | amino-terminal domain |
| LBD | ligand-binding domain |
| AMPA | α-amino3-hydroxy-5-mathyl-4-isoxazole propionic acid |
| EPSC | excitatory postsynaptic current |
| AMPAR | α-amino3-hydroxy-5-mathyl-4-isoxazole propionic acid receptor |
| GABAAR | type A Gamma-Amino Butyric Acid Receptor |
| ECD | extracellular domain |
| ACh+ | acetylcholine oxyanion |
| ACh | acetylcholine |
| TRPM7 | transient receptor potential melastatine type 7 channel |
| Cx36 | connexin type 36 |
| INaP | persistent Na+ current |
| HCN | hyperpolarization-activated cyclic nucleotide-gated channels |
| IHCN | ion current through HCN channels |
| LQT | long QT |
| SQT | short QT |
| EAST | epileptic seizures, ataxia, sensorineural hearing loss, renal tubulopathy |
| BP | blood pressure |
| CSD | cortical spreading depression |
| NIHL | noise-induced hearing loss |
References
- Li, M.; Li, Y.; Lu, Y.; Li, J.; Lu, X.; Ren, Y.; Wen, T.; Wang, Y.; Chang, S.; Zhang, X.; et al. Molecular Basis of Mg2+ Permeation through the Human Mitochondrial Mrs2 Channel. Nat. Commun. 2023, 14, 4713. [Google Scholar] [CrossRef]
- Liu, M.; Dudley, S.C. Magnesium Homeostasis and Magnesium Transporters in Human Health. Nutrients 2025, 17, 920. [Google Scholar] [CrossRef]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The Involvement of Mg2+ in Regulation of Cellular and Mitochondrial Functions. Oxidative Med. Cell. Longev. 2017, 2017, 6797460. [Google Scholar] [CrossRef] [PubMed]
- Stanojević, M.; Djuricic, N.; Parezanovic, M.; Biorac, M.; Pathak, D.; Spasic, S.; Lopicic, S.; Kovacevic, S.; Nesovic Ostojic, J. The Impact of Chronic Magnesium Deficiency on Excitable Tissues—Translational Aspects. Biol. Trace Elem. Res. 2025, 203, 707–728. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C.; Bootman, M.D.; Scott, J.D. Second Messengers. Cold Spring Harb Perspect Biol. 2016, 8, a005926. [Google Scholar] [CrossRef]
- Iotti, S.; Malucelli, E. Free Magnesium Concentration in the Human Brain. In Magnesium in the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; ISBN 978-0-9870730-5-1. [Google Scholar]
- Segev, A.; Shechter, M.; Tsur, A.; Belkin, D.; Cohen, H.; Sharon, A.; Morag, N.; Grossman, E.; Maor, E. Serum Magnesium Is Associated with Long-Term Survival of Non-ST-Elevation Myocardial Infarction Patients. Nutrients 2023, 15, 4299. [Google Scholar] [CrossRef]
- Maier, J.A.M.; Locatelli, L.; Fedele, G.; Cazzaniga, A.; Mazur, A. Magnesium and the Brain: A Focus on Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2022, 24, 223. [Google Scholar] [CrossRef]
- Stanojević, M.; Parezanović, M.; Popović, A.; Spasić, S.; Lopičić, S.; Nedeljkov, V.; Jovanović, Z.; Vučković, S. Revising the Role of Magnesium in Epilepsy Research and Management. Serbian J. Med. Chamb. 2023, 4, 175–187. [Google Scholar] [CrossRef]
- Politi, H.C.; Preston, R.R. Is It Time to Rethink the Role of Mg2+ in Membrane Excitability? NeuroReport 2003, 14, 659–668. [Google Scholar] [CrossRef]
- Vandenberg, C.A. Inward Rectification of a Potassium Channel in Cardiac Ventricular Cells Depends on Internal Magnesium Ions. Proc. Natl. Acad. Sci. USA 1987, 84, 2560–2564. [Google Scholar] [CrossRef]
- Matsuda, H.; Saigusa, A.; Irisawa, H. Ohmic Conductance through the Inwardly Rectifying K Channel and Blocking by Internal Mg2+. Nature 1987, 325, 156–159. [Google Scholar] [CrossRef]
- Silver, M.R.; DeCoursey, T.E. Intrinsic Gating of Inward Rectifier in Bovine Pulmonary Artery Endothelial Cells in the Presence or Absence of Internal Mg2+. J. Gen. Physiol. 1990, 96, 109–133. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Jogini, V.; Jensen, M.Ø.; Shaw, D.E. Gating and Modulation of an Inward-Rectifier Potassium Channel. J. Gen. Physiol. 2023, 155, e202213085. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, X.; Liu, J.; Yu, H.; Zhang, S.; Zhan, Y.; Zhang, H.; Logothetis, D.E.; An, H. Lack of Negatively Charged Residues at the External Mouth of Kir2.2 Channels Enable the Voltage-Dependent Block by External Mg2+. PLoS ONE 2014, 9, e111372. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; MacKinnon, R. Electrostatic Tuning of Mg2+ Affinity in an Inward-Rectifier K+ channel. Nature 1994, 371, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H. Open-state Substructure of Inwardly Rectifying Potassium Channels Revealed by Magnesium Block in Guinea-pig Heart Cells. J. Physiol. 1988, 397, 237–258. [Google Scholar] [CrossRef]
- Matsuda, H. Effects of External and Internal K+ Ions on Magnesium Block of Inwardly Rectifying K+ Channels in Guinea-pig Heart Cells. J. Physiol. 1991, 435, 83–99. [Google Scholar] [CrossRef]
- Yang, L.; Frindt, G.; Palmer, L.G. Magnesium Modulates ROMK Channel–Mediated Potassium Secretion. J. Am. Soc. Nephrol. 2010, 21, 2109–2116. [Google Scholar] [CrossRef]
- Nichols, C.G.; Lopatin, A.N. Inward Rectifier Potassium Channels. Annu. Rev. Physiol. 1997, 59, 171–191. [Google Scholar] [CrossRef]
- Ishihara, K.; Mitsuiye, T.; Noma, A.; Takano, M. The Mg2+ Block and Intrinsic Gating Underlying Inward Rectification of the K+ Current in Guinea-pig Cardiac Myocytes. J. Physiol. 1989, 419, 297–320. [Google Scholar] [CrossRef]
- Stanfield, P.R.; Davies, N.W.; Shelton, P.A.; Khan, I.A.; Brammar, W.J.; Standen, N.B.; Conley, E.C. The Intrinsic Gating of Inward Rectifier K+ Channels Expressed from the Murine IRK1 Gene Depends on Voltage, K+ and Mg2+. J. Physiol. 1994, 475, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Elam, T.R.; Lansman, J.B. The Role of Mg2+ in the Inactivation of Inwardly Rectifying K+ Channels in Aortic Endothelial Cells. J. Gen. Physiol. 1995, 105, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Wible, B.A.; Taglialatela, M.; Ficker, E.; Brown, A.M. Gating of Inwardly Rectifying K+ Channels Localized to a Single Negatively Charged Residue. Nature 1994, 371, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, P.R.; Davies, N.W.; Shelton, P.A.; Sutcliffe, M.J.; Khan, I.A.; Brammar, W.J.; Conley, E.C. A Single Aspartate Residue Is Involved in Both Intrinsic Gating and Blockage by Mg2+ of the Inward Rectifier, IRK1. J. Physiol. 1994, 478, 1–6. [Google Scholar] [CrossRef]
- Taglialatela, M.; Ficker, E.; Wible, B.A.; Brown, A.M. C-Terminus Determinants for Mg2+ and Polyamine Block of the Inward Rectifier K+ Channel IRK1. EMBO J. 1995, 14, 5532–5541. [Google Scholar] [CrossRef] [PubMed]
- Tai, K.; Stansfeld, P.J.; Sansom, M.S.P. Ion-Blocking Sites of the Kir2.1 Channel Revealed by Multiscale Modeling. Biochemistry 2009, 48, 8758–8763. [Google Scholar] [CrossRef]
- Lopatin, A.N.; Makhina, E.N.; Nichols, C.G. Potassium Channel Block by Cytoplasmic Polyamines as the Mechanism of Intrinsic Rectification. Nature 1994, 372, 366–369. [Google Scholar] [CrossRef]
- Yang, L.; Edvinsson, J.; Sackin, H.; Palmer, L.G. Ion Selectivity and Current Saturation in Inward-Rectifier K+ Channels. J. Gen. Physiol. 2012, 139, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.M.; Quinn, C.C.; Leach, R.; Findlay, J.B.C.; Boyett, M.R. Effect of Extracellular Cations on the Inward Rectifying K+ Channels Kir2.1 and Kir3.1/Kir3.4. Exp. Physiol. 1999, 84, 471–488. [Google Scholar] [CrossRef]
- Murata, Y.; Fujiwara, Y.; Kubo, Y. Identification of a Site Involved in the Block by Extracellular Mg2+ and Ba2+ as Well as Permeation of K+ in the Kir2.1 K+ Channel. J. Physiol. 2002, 544, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Tammaro, P.; Smirnov, S.V.; Moran, O. Effects of Intracellular Magnesium on Kv1.5 and Kv2.1 Potassium Channels. Eur. Biophys. J. 2005, 34, 42–51. [Google Scholar] [CrossRef]
- Harris, R.E.; Isacoff, E.Y. Hydrophobic Mutations Alter the Movement of Mg2+ in the Pore of Voltage-Gated Potassium Channels. Biophys. J. 1996, 71, 209–219. [Google Scholar] [CrossRef]
- Tammaro, P.; Smith, A.; Crowley, B.; Smirnov, S. Modulation of the Voltage-Dependent K Current by Intracellular Mg in Rat Aortic Smooth Muscle Cells. Cardiovasc. Res. 2005, 65, 387–396. [Google Scholar] [CrossRef]
- Suh, B.-C.; Hille, B. Electrostatic Interaction of Internal Mg2+ with Membrane PIP2 Seen with KCNQ K+ Channels. J. Gen. Physiol. 2007, 130, 241–256. [Google Scholar] [CrossRef]
- Terlau, H.; Ludwig, J.; Steffan, R.; Pongs, O.; Stühmer, W.; Heinemann, S.H. Extracellular Mg2+ Regulates Activation of Rat Eag Potassium Channel. Pflüg. Arch. Eur. J. Physiol. 1996, 432, 301–312. [Google Scholar] [CrossRef]
- Schönherr, R.; Hehl, S.; Terlau, H.; Baumann, A.; Heinemann, S.H. Individual Subunits Contribute Independently to Slow Gating of Bovine EAG Potassium Channels. J. Biol. Chem. 1999, 274, 5362–5369. [Google Scholar] [CrossRef]
- Silverman, W.R.; Tang, C.-Y.; Mock, A.F.; Huh, K.-B.; Papazian, D.M. Mg2+ Modulates Voltage-Dependent Activation in Ether-à-Go-Go Potassium Channels by Binding between Transmembrane Segments S2 and S3. J. Gen. Physiol. 2000, 116, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Po, S.S.; Wang, D.W.; Yang, I.C.-H.; Johnson, J.P.; Nie, L.; Bennett, P.B. Modulation of HERG Potassium Channels by Extracellular Magnesium and Quinidine. J. Cardiovasc. Pharmacol. 1999, 33, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yu, Z.; Wang, L.; Li, X.; Li, N.; Bai, Q.; Wang, Y.; Li, R.; Meng, Y.; Xu, H.; et al. Structural Bases of Inhibitory Mechanism of CaV1.2 Channel Inhibitors. Nat. Commun. 2024, 15, 2772. [Google Scholar] [CrossRef]
- Simms, B.A.; Zamponi, G.W. Neuronal Voltage-Gated Calcium Channels: Structure, Function, and Dysfunction. Neuron 2014, 82, 24–45. [Google Scholar] [CrossRef]
- Yao, X.; Gao, S.; Yan, N. Structural Biology of Voltage-Gated Calcium Channels. Channels 2024, 18, 2290807. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef] [PubMed]
- Llinás, R.; Sugimori, M. Electrophysiological Properties of in Vitro Purkinje Cell Somata in Mammalian Cerebellar Slices. J. Physiol. 1980, 305, 171–195. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.; Niesen, C.; So, A.; Carlen, P.; Spigelman, I.; Jones, O. N-Type Ca2+ Channels Are Located on Somata, Dendrites, and a Subpopulation of Dendritic Spines on Live Hippocampal Pyramidal Neurons. J. Neurosci. 1994, 14, 6815–6824. [Google Scholar] [CrossRef]
- Indriati, D.W.; Kamasawa, N.; Matsui, K.; Meredith, A.L.; Watanabe, M.; Shigemoto, R. Quantitative Localization of Cav 2.1 (P/Q-Type) Voltage-Dependent Calcium Channels in Purkinje Cells: Somatodendritic Gradient and Distinct Somatic Coclustering with Calcium-Activated Potassium Channels. J. Neurosci. 2013, 33, 3668–3678. [Google Scholar] [CrossRef] [PubMed]
- Berkefeld, H.; Sailer, C.A.; Bildl, W.; Rohde, V.; Thumfart, J.-O.; Eble, S.; Klugbauer, N.; Reisinger, E.; Bischofberger, J.; Oliver, D.; et al. BKCa-Cav Channel Complexes Mediate Rapid and Localized Ca2+-Activated K+ Signaling. Science 2006, 314, 615–620. [Google Scholar] [CrossRef]
- Rehak, R.; Bartoletti, T.M.; Engbers, J.D.T.; Berecki, G.; Turner, R.W.; Zamponi, G.W. Low Voltage Activation of KCa1.1 Current by Cav3-KCa1.1 Complexes. PLoS ONE 2013, 8, e61844. [Google Scholar] [CrossRef]
- Huang, J.; Pan, X.; Yan, N. Structural Biology and Molecular Pharmacology of Voltage-Gated Ion Channels. Nat. Rev. Mol. Cell Biol. 2024, 25, 904–925, Correction in Nat. Rev. Mol. Cell Biol. 2024, 25, 947. [Google Scholar] [CrossRef]
- Lory, P.; Nicole, S.; Monteil, A. Neuronal Cav3 Channelopathies: Recent Progress and Perspectives. Pflüg. Arch. Eur. J. Physiol. 2020, 472, 831–844. [Google Scholar] [CrossRef]
- Van Petegem, F.; Minor, D.L. The Structural Biology of Voltage-Gated Calcium Channel Function and Regulation. Biochem. Soc. Trans. 2006, 34, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.Z.; Lee, J.S.; Mulle, J.G.; Wang, Y.; De Leon, M.; Yue, D.T. Critical Determinants of Ca2+-Dependent Inactivation within an EF-Hand Motif of L-Type Ca2+ Channels. Biophys. J. 2000, 78, 1906–1920. [Google Scholar] [CrossRef]
- Agus, Z.S.; Kelepouris, E.; Dukes, I.; Morad, M. Cytosolic Magnesium Modulates Calcium Channel Activity in Mammalian Ventricular Cells. Am. J. Physiol.-Cell Physiol. 1989, 256, C452–C455. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Berlin, J.R. Channel Phosphorylation and Modulation of L-Type Ca2+ Currents by Cytosolic Mg2+ Concentration. Am. J. Physiol.-Cell Physiol. 2006, 291, C83–C92. [Google Scholar] [CrossRef]
- Wang, M.; Berlin, J.R. Voltage-Dependent Modulation of L-Type Calcium Currents by Intracellular Magnesium in Rat Ventricular Myocytes. Arch. Biochem. Biophys. 2007, 458, 65–72. [Google Scholar] [CrossRef]
- Zhang, J.; Berra-Romani, R.; Sinnegger-Brauns, M.J.; Striessnig, J.; Blaustein, M.P.; Matteson, D.R. Role of Cav 1.2 L-Type Ca2+ Channels in Vascular Tone: Effects of Nifedipine and Mg2+. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H415–H425. [Google Scholar] [CrossRef]
- Brunet, S.; Scheuer, T.; Klevit, R.; Catterall, W.A. Modulation of CaV1.2 Channels by Mg2+ Acting at an EF-Hand Motif in the COOH-Terminal Domain. J. Gen. Physiol. 2005, 126, 311–323. [Google Scholar] [CrossRef]
- White, R.E.; Hartzell, H.C. Effects of Intracellular Free Magnesium on Calcium Current in Isolated Cardiac Myocytes. Science 1988, 239, 778–780. [Google Scholar] [CrossRef]
- Agus, Z.S.; Morad, M. Modulation of Cardiac Ion Channels by Magnesium. Annu. Rev. Physiol. 1991, 53, 299–307. [Google Scholar] [CrossRef]
- Hartzell, H.C.; White, R.E. Effects of Magnesium on Inactivation of the Voltage-Gated Calcium Current in Cardiac Myocytes. J. Gen. Physiol. 1989, 94, 745–767. [Google Scholar] [CrossRef] [PubMed]
- Brunet, S.; Scheuer, T.; Catterall, W.A. Cooperative Regulation of Cav1.2 Channels by Intracellular Mg2+, the Proximal C-Terminal EF-Hand, and the Distal C-Terminal Domain. J. Gen. Physiol. 2009, 134, 81–94. [Google Scholar] [CrossRef]
- Pelzer, S.; La, C.; Pelzer, D.J. Phosphorylation-Dependent Modulation of Cardiac Calcium Current by Intracellular Free Magnesium. Am. J. Physiol.-Heart Circ. Physiol. 2001, 281, H1532–H1544. [Google Scholar] [CrossRef]
- Wang, M.; Tashiro, M.; Berlin, J.R. Regulation of L-Type Calcium Current by Intracellular Magnesium in Rat Cardiac Myocytes. J. Physiol. 2004, 555, 383–396. [Google Scholar] [CrossRef]
- Bünemann, M.; Gerhardstein, B.L.; Gao, T.; Hosey, M.M. Functional Regulation of L-Type Calcium Channels via Protein Kinase A-Mediated Phosphorylation of the β2 Subunit. J. Biol. Chem. 1999, 274, 33851–33854. [Google Scholar] [CrossRef]
- Lansman, J.B.; Hess, P.; Tsien, R.W. Blockade of Current through Single Calcium Channels by Cd2+, Mg2+, and Ca2+. Voltage and Concentration Dependence of Calcium Entry into the Pore. J. Gen. Physiol. 1986, 88, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, P.G.; Mironov, S.L.; Shuba, Y.M. Two Ion-Selecting Filters in the Calcium Channel of the Somatic Membrane of Mollusc Neurons. J. Membr. Biol. 1983, 76, 83–93. [Google Scholar] [CrossRef]
- Carbone, E.; Lux, H.D.; Carabelli, V.; Aicardi, G.; Zucker, H. Ca2+ and Na+ Permeability of High-threshold Ca2+ Channels and Their Volt Age-dependent Block by Mg2+ Ions in Chick Sensory Neurones. J. Physiol. 1997, 504, 1–15. [Google Scholar] [CrossRef]
- Zhang, A.; Fan, S.H.; Cheng, T.P.; Altura, B.T.; Wong, R.K.; Altura, B.M. Extracellular Mg2+ Modulates Intracellular Ca2+ in Acutely Isolated Hippocampal CA1 Pyramidal Cells of the Guinea-Pig. Brain Res. 1996, 728, 204–208. [Google Scholar] [CrossRef]
- Shimosawa, T.; Takano, K.; Ando, K.; Fujita, T. Magnesium Inhibits Norepinephrine Release by Blocking N-Type Calcium Channels at Peripheral Sympathetic Nerve Endings. Hypertension 2004, 44, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Marban, E.; Yamagishi, T.; Tomaselli, G.F. Structure and Function of Voltage-gated Sodium Channels. J. Physiol. 1998, 508, 647–657. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated Sodium Channels at 60: Structure, Function and Pathophysiology. J. Physiol. 2012, 590, 2577–2589. [Google Scholar] [CrossRef]
- Lin, F.; Conti, F.; Moran, O. Competitive Blockage of the Sodium Channel by Intracellular Magnesium Ions in Central Mammalian Neurones. Eur. Biophys. J. 1991, 19, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Pusch, M.; Conti, F.; Stühmer, W. Intracellular Magnesium Blocks Sodium Outward Currents in a Voltage- and Dose-Dependent Manner. Biophys. J. 1989, 55, 1267–1271. [Google Scholar] [CrossRef]
- Pusch, M. Open-Channel Block of Na+ Channels by Intracellular Mg2+. Eur. Biophys. J. 1990, 18, 317–326. [Google Scholar] [CrossRef]
- Cantrell, A.R.; Catterall, W.A. Neuromodulation of Na+ Channels: An Unexpected Form of Cellular Platicity. Nat. Rev. Neurosci. 2001, 2, 397–407. [Google Scholar] [CrossRef]
- Bastidas, A.C.; Wu, J.; Taylor, S.S. Molecular Features of Product Release for the PKA Catalytic Cycle. Biochemistry 2015, 54, 2–10. [Google Scholar] [CrossRef]
- Messner, D.J.; Catterall, W.A. The Sodium Channel from Rat Brain. Separation and Characterization of Subunits. J. Biol. Chem. 1985, 260, 10597–10604. [Google Scholar] [CrossRef]
- Dribben, W.H.; Eisenman, L.N.; Mennerick, S. Magnesium Induces Neuronal Apoptosis by Suppressing Excitability. Cell Death Dis. 2010, 1, e63. [Google Scholar] [CrossRef] [PubMed]
- Sang, N.; Meng, Z. Blockade by Magnesium of Sodium Currents in Acutely Isolated Hippocampal CA1 Neurons of Rat. Brain Res. 2002, 952, 218–221. [Google Scholar] [CrossRef]
- Isaev, D.; Ivanchick, G.; Khmyz, V.; Isaeva, E.; Savrasova, A.; Krishtal, O.; Holmes, G.L.; Maximyuk, O. Surface Charge Impact in Low-Magnesium Model of Seizure in Rat Hippocampus. J. Neurophysiol. 2012, 107, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Vastani, N.; Seifert, B.; Spahn, D.R.; Maurer, K. Sensitivities of Rat Primary Sensory Afferent Nerves to Magnesium: Implications for Differential Nerve Blocks. Eur. J. Anaesthesiol. 2013, 30, 21–28. [Google Scholar] [CrossRef]
- Barjas Qaswal, A. Magnesium Ions Depolarize the Neuronal Membrane via Quantum Tunneling through the Closed Channels. Quantum Rep. 2020, 2, 57–63. [Google Scholar] [CrossRef]
- Cui, J.; Yang, H.; Lee, U.S. Molecular Mechanisms of BK Channel Activation. Cell. Mol. Life Sci. 2009, 66, 852–875. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, G.; Cui, J. BK Channels: Multiple Sensors, One Activation Gate. Front. Physiol. 2015, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Sah, P.; Louise Faber, E.S. Channels Underlying Neuronal Calcium-Activated Potassium Currents. Prog. Neurobiol. 2002, 66, 345–353. [Google Scholar] [CrossRef]
- Contet, C.; Goulding, S.P.; Kuljis, D.A.; Barth, A.L. BK Channels in the Central Nervous System. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 128, pp. 281–342. ISBN 978-0-12-803619-8. [Google Scholar]
- Lee, U.S.; Cui, J. BK Channel Activation: Structural and Functional Insights. Trends Neurosci. 2010, 33, 415–423. [Google Scholar] [CrossRef]
- Van, N.T.H.; Kim, W.K.; Nam, J.H. Challenges in the Therapeutic Targeting of KCa Channels: From Basic Physiology to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 2965. [Google Scholar] [CrossRef]
- Xia, X.-M.; Zeng, X.; Lingle, C.J. Multiple Regulatory Sites in Large-Conductance Calcium-Activated Potassium Channels. Nature 2002, 418, 880–884. [Google Scholar] [CrossRef]
- Golowasch, J.; Kirkwood, A.; Miller, C. Allosteric Effects of Mg2+ on the Gating of Ca2+-Activated K+ Channels from Mammalian Skeletal Muscle. J. Exp. Biol. 1986, 124, 5–13. [Google Scholar] [CrossRef]
- Oberhauser, A.; Alvarez, O.; Latorre, R. Activation by Divalent Cations of a Ca2+-Activated K+ Channel from Skeletal Muscle Membrane. J. Gen. Physiol. 1988, 92, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.G.; Petersen, O.H. Modulation of Ca2+- and Voltage-Activated K+ Channels by Internal Mg2+ in Salivary Acinar Cells. Biochim. Biophys. Acta BBA-Biomembr. 1987, 899, 171–175. [Google Scholar] [CrossRef] [PubMed]
- McLarnon, J.G.; Sawyer, D. Effects of Divalent Cations on the Activation of a Calcium-Dependent Potassium Channel in Hippocampal Neurons. Pflügers Arch. Eur. J. Physiol. 1993, 424, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, W.B. Competitive Mg2+ Block of a Large-Conductance, Ca2+-Activated K+ Channel in Rat Skeletal Muscle. Ca2+, Sr2+, and Ni2+ Also Block. J. Gen. Physiol. 1991, 98, 163–181. [Google Scholar] [CrossRef]
- Zhang, X.; Puil, E.; Mathers, D.A. Effects of Intracellular Mg2+ on the Properties of Large-Conductance, Ca2+ -Dependent K+ Channels in Rat Cerebrovascular Smooth Muscle Cells. J. Cereb. Blood Flow Metab. 1995, 15, 1066–1074. [Google Scholar] [CrossRef]
- Snetkov, V.; Gurney, A.; Ward, J.; Osipenko, O. Inward Rectification of the Large Conductance Potassium Channel in Smooth Muscle Cells from Rabbit Pulmonary Artery. Exp. Physiol. 1996, 81, 743–753. [Google Scholar] [CrossRef]
- Morales, E.; Cole, W.C.; Remillard, C.V.; Leblane, N. Block of Large Conductance Ca2+-activated K+ Channels in Rabbit Vascular Myocytes by Internal Mg2+ and Na+. J. Physiol. 1996, 495, 701–716. [Google Scholar] [CrossRef]
- Wachter, C.; Turnheim, K. Inhibition of High-Conductance, Calcium-Activated Potassium Channels of Rabbit Colon Epithelium by Magnesium. J. Membr. Biol. 1996, 150, 275–282. [Google Scholar] [CrossRef]
- Shi, J.; Cui, J. Intracellular Mg2+ Enhances the Function of Bk-Type Ca2+-Activated K+ Channels. J. Gen. Physiol. 2001, 118, 589–606. [Google Scholar] [CrossRef]
- Zhang, X.; Solaro, C.R.; Lingle, C.J. Allosteric Regulation of Bk Channel Gating by Ca2+ and Mg2+ through a Nonselective, Low Affinity Divalent Cation Site. J. Gen. Physiol. 2001, 118, 607–636. [Google Scholar] [CrossRef]
- Hu, L.; Yang, H.; Shi, J.; Cui, J. Effects of Multiple Metal Binding Sites on Calcium and Magnesium-Dependent Activation of BK Channels. J. Gen. Physiol. 2006, 127, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Niu, X.; Brelidze, T.I.; Magleby, K.L. Ring of Negative Charge in BK Channels Facilitates Block by Intracellular Mg2+ and Polyamines through Electrostatics. J. Gen. Physiol. 2006, 128, 185–202. [Google Scholar] [CrossRef]
- Horrigan, F.T.; Ma, Z. Mg2+ Enhances Voltage Sensor/Gate Coupling in BK Channels. J. Gen. Physiol. 2008, 131, 13–32. [Google Scholar] [CrossRef]
- Chen, R.-S.; Geng, Y.; Magleby, K.L. Mg2+ Binding to Open and Closed States Can Activate BK Channels Provided That the Voltage Sensors Are Elevated. J. Gen. Physiol. 2011, 138, 593–607. [Google Scholar] [CrossRef]
- Shi, J.; Krishnamoorthy, G.; Yang, Y.; Hu, L.; Chaturvedi, N.; Harilal, D.; Qin, J.; Cui, J. Mechanism of Magnesium Activation of Calcium-Activated Potassium Channels. Nature 2002, 418, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, L.; Shi, J.; Cui, J. Tuning Magnesium Sensitivity of BK Channels by Mutations. Biophys. J. 2006, 91, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hu, L.; Shi, J.; Delaloye, K.; Horrigan, F.T.; Cui, J. Mg2+ Mediates Interaction between the Voltage Sensor and Cytosolic Domain to Activate BK Channels. Proc. Natl. Acad. Sci. USA 2007, 104, 18270–18275. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shi, J.; Zhang, G.; Yang, J.; Delaloye, K.; Cui, J. Activation of Slo1 BK Channels by Mg2+ Coordinated between the Voltage Sensor and RCK1 Domains. Nat. Struct. Mol. Biol. 2008, 15, 1152–1159. [Google Scholar] [CrossRef]
- Hu, L.; Shi, J.; Ma, Z.; Krishnamoorthy, G.; Sieling, F.; Zhang, G.; Horrigan, F.T.; Cui, J. Participation of the S4 Voltage Sensor in the Mg2+-Dependent Activation of Large Conductance (BK) K+ Channels. Proc. Natl. Acad. Sci. USA 2003, 100, 10488–10493. [Google Scholar] [CrossRef]
- MacKinnon, R.; Latorre, R.; Miller, C. Role of Surface Electrostatics in the Operation of a High-Conductance Calcium-Activated Potassium Channel. Biochemistry 1989, 28, 8092–8099. [Google Scholar] [CrossRef]
- Adelman, J.P.; Maylie, J.; Sah, P. Small-Conductance Ca2+-Activated K+ Channels: Form and Function. Annu. Rev. Physiol. 2012, 74, 245–269. [Google Scholar] [CrossRef]
- Wolfart, J.; Roeper, J. Selective Coupling of T-Type Calcium Channels to SK Potassium Channels Prevents Intrinsic Bursting in Dopaminergic Midbrain Neurons. J. Neurosci. 2002, 22, 3404–3413. [Google Scholar] [CrossRef]
- Cangiano, L.; Wallén, P.; Grillner, S. Role of Apamin-Sensitive KCa Channels for Reticulospinal Synaptic Transmission to Motoneuron and for the Afterhyperpolarization. J. Neurophysiol. 2002, 88, 289–299. [Google Scholar] [CrossRef]
- Ngo-Anh, T.J.; Bloodgood, B.L.; Lin, M.; Sabatini, B.L.; Maylie, J.; Adelman, J.P. SK Channels and NMDA Receptors Form a Ca2+-Mediated Feedback Loop in Dendritic Spines. Nat. Neurosci. 2005, 8, 642–649. [Google Scholar] [CrossRef]
- Ikonen, S.; Riekkinen, P. Effects of Apamin on Memory Processing of Hippocampal-Lesioned Mice. Eur. J. Pharmacol. 1999, 382, 151–156. [Google Scholar] [CrossRef]
- Stackman, R.W.; Hammond, R.S.; Linardatos, E.; Gerlach, A.; Maylie, J.; Adelman, J.P.; Tzounopoulos, T. Small Conductance Ca2+-Activated K+ Channels Modulate Synaptic Plasticity and Memory Encoding. J. Neurosci. 2002, 22, 10163–10171. [Google Scholar] [CrossRef]
- Gu, M.; Zhu, Y.; Yin, X.; Zhang, D.-M. Small-Conductance Ca2+-Activated K+ Channels: Insights into Their Roles in Cardiovascular Disease. Exp. Mol. Med. 2018, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Soh, H.; Park, C.-S. Inwardly Rectifying Current-Voltage Relationship of Small-Conductance Ca2+-Activated K+ Channels Rendered by Intracellular Divalent Cation Blockade. Biophys. J. 2001, 80, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Soh, H.; Park, C.-S. Localization of Divalent Cation-Binding Site in the Pore of a Small Conductance Ca2+-Activated K+ Channel and Its Role in Determining Current-Voltage Relationship. Biophys. J. 2002, 83, 2528–2538. [Google Scholar] [CrossRef]
- Ledoux, J.; Bonev, A.D.; Nelson, M.T. Ca2+-Activated K+ Channels in Murine Endothelial Cells: Block by Intracellular Calcium and Magnesium. J. Gen. Physiol. 2008, 131, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Karakas, E.; Furukawa, H. Crystal Structure of a Heterotetrameric NMDA Receptor Ion Channel. Science 2014, 344, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, G.J.; Popescu, G.K. NMDA Receptors: Linking Physiological Output to Biophysical Operation. Nat. Rev. Neurosci. 2017, 18, 236–249. [Google Scholar] [CrossRef]
- Wollmuth, L.P.; Kuner, T.; Sakmann, B. Adjacent Asparagines in the NR2-subunit of the NMDA Receptor Channel Control the Voltage-dependent Block by Extracellular Mg2+. J. Physiol. 1998, 506, 13–32. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Lee, C.-H.; Kuo, C.-C. Ionic Flow Enhances Low-Affinity Binding: A Revised Mechanistic View into Mg2+ Block of NMDA Receptors: Ionic Flow-Dependent Mg2+ Block of NMDA Receptors. J. Physiol. 2010, 588, 633–650. [Google Scholar] [CrossRef]
- Johnson, J.W.; Ascher, P. Voltage-Dependent Block by Intracellular Mg2+ of N-Methyl-D-Aspartate-Activated Channels. Biophys. J. 1990, 57, 1085–1090. [Google Scholar] [CrossRef]
- Li-Smerin, Y.; Johnson, J.W. Effects of Intracellular Mg2+ on Channel Gating and Steady-state Responses of the NMDA Receptor in Cultured Rat Neurons. J. Physiol. 1996, 491, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Li-Smerin, Y.; Johnson, J.W. Kinetics of the Block by Intracellular Mg2+ of the NMDA-activated Channel in Cultured Rat Neurons. J. Physiol. 1996, 491, 121–135. [Google Scholar] [CrossRef]
- Wollmuth, L.P.; Kuner, T.; Sakmann, B. Intracellular Mg2+ Interacts with Structural Determinants of the Narrow Constriction Contributed by the NR1-subunit in the NMDA Receptor Channel. J. Physiol. 1998, 506, 33–52. [Google Scholar] [CrossRef]
- Kupper, J.; Ascher, P.; Neyton, J. Internal Mg2+ Block of Recombinant NMDA Channels Mutated within the Selectivity Filter and Expressed in Xenopus Oocytes. J. Physiol. 1998, 507, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Auerbach, A. Na+ Occupancy and Mg2+ Block of the N-Methyl-D-Aspartate Receptor Channel. J. Gen. Physiol. 2001, 117, 275–286. [Google Scholar] [CrossRef]
- Premkumar, L.S.; Auerbach, A. Identification of a High Affinity Divalent Cation Binding Site near the Entrance of the NMDA Receptor Channel. Neuron 1996, 16, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Antonov, S.M.; Johnson, J.W. Permeant Ion Regulation of N-Methyl-D-Aspartate Receptor Channel Block by Mg2+. Proc. Natl. Acad. Sci. USA 1999, 96, 14571–14576. [Google Scholar] [CrossRef]
- Zarei, M.M.; Dani, J.A. Ionic Permeability Characteristics of the N-Methyl-D-Aspartate Receptor Channel. J. Gen. Physiol. 1994, 103, 231–248. [Google Scholar] [CrossRef]
- Qian, A.; Antonov, S.M.; Johnson, J.W. Modulation by Permeant Ions of Mg2+ Inhibition of NMDA-activated Whole-cell Currents in Rat Cortical Neurons. J. Physiol. 2002, 538, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Caballero, M.; Robinson, H.P.C. Fast and Slow Voltage-Dependent Dynamics of Magnesium Block in the NMDA Receptor: The Asymmetric Trapping Block Model. J. Neurosci. 2004, 24, 6171–6180. [Google Scholar] [CrossRef]
- Nowak, L.; Bregestovski, P.; Ascher, P.; Herbet, A.; Prochiantz, A. Magnesium Gates Glutamate-Activated Channels in Mouse Central Neurones. Nature 1984, 307, 462–465. [Google Scholar] [CrossRef]
- Mayer, M.L.; Westbrook, G.L. The Action of N-Methyl-D-Aspartic Acid on Mouse Spinal Neurones in Culture. J. Physiol. 1985, 361, 65–90. [Google Scholar] [CrossRef] [PubMed]
- Alberi, S.; Dubois-Dauphin, M.; Dreifuss, J.J.; Raggenbass, M. Whole-Cell NMDA-Evoked Current in Suprachiasmatic Neurones of the Rat: Modulation by Extracellular Calcium Ions. Brain Res. 1997, 745, 55–66. [Google Scholar] [CrossRef]
- Wang, L.Y.; MacDonald, J.F. Modulation by Magnesium of the Affinity of NMDA Receptors for Glycine in Murine Hippocampal Neurones. J. Physiol. 1995, 486 Pt 1, 83–95. [Google Scholar] [CrossRef]
- Crunelli, V.; Mayer, M.L. Mg2+ Dependence of Membrane Resistance Increases Evoked by NMDA in Hippocampal Neurones. Brain Res. 1984, 311, 392–396. [Google Scholar] [CrossRef]
- Thomson, A.M. Comparison of Responses to Transmitter Candidates at an N-Methylaspartate Receptor Mediated Synapse, in Slices of Rat Cerebral Cortex. Neuroscience 1986, 17, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.M. A Magnesium-sensitive Post-synaptic Potential in Rat Cerebral Cortex Resembles Neuronal Responses to N-methylaspartate. J. Physiol. 1986, 370, 531–549. [Google Scholar] [CrossRef]
- Mayer, M.L.; Westbrook, G.L.; Guthrie, P.B. Voltage-Dependent Block by Mg2+ of NMDA Responses in Spinal Cord Neurones. Nature 1984, 309, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Hablitz, J.J. Action of Excitatory Amino Acids and Their Antagonists on Hippocampal Neurons. Cell. Mol. Neurobiol. 1985, 5, 389–405. [Google Scholar] [CrossRef]
- Garthwaite, G.; Garthwaite, J. Receptor-Linked Ionic Channels Mediate Neurotoxicity in Rat Cerebellar Slices. Neurosci. Lett. 1987, 83, 241–246. [Google Scholar] [CrossRef]
- Cox, J.A.; Lysko, P.G.; Henneberry, R.C. Excitatory Amino Acid Neurotoxicity at the N-Methyl-D-Aspartate Receptor in Cultured Neurons: Role of the Voltage-Dependent Magnesium Block. Brain Res. 1989, 499, 267–272. [Google Scholar] [CrossRef]
- Frandsen, A.; Schousboe, A. Effect of Magnesium on NMDA Mediated Toxicity and Increases in [Ca2+]i and cGMP in Cultured Neocortical Neurons: Evidence for Distinct Regulation of Different Responses. Neurochem. Int. 1994, 25, 303–308. [Google Scholar] [CrossRef]
- Ascher, P.; Nowak, L. The Role of Divalent Cations in the N-methyl-D-aspartate Responses of Mouse Central Neurones in Culture. J. Physiol. 1988, 399, 247–266. [Google Scholar] [CrossRef]
- Jahr, C.; Stevens, C. A Quantitative Description of NMDA Receptor-Channel Kinetic Behavior. J. Neurosci. 1990, 10, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Burnashev, N.; Schoepfer, R.; Monyer, H.; Ruppersberg, J.P.; Günther, W.; Seeburg, P.H.; Sakmann, B. Control by Asparagine Residues of Calcium Permeability and Magnesium Blockade in the NMDA Receptor. Science 1992, 257, 1415–1419. [Google Scholar] [CrossRef]
- Mori, H.; Masaki, H.; Yamakura, T.; Mishina, M. Identification by Mutagenesis of a Mg2+-Block Site of the NMDA Receptor Channel. Nature 1992, 358, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Kuner, T.; Schoepfer, R. Multiple Structural Elements Determine Subunit Specificity of Mg2+ Block in NMDA Receptor Channels. J. Neurosci. 1996, 16, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Robinson, H.P.C. Effects of Divalent Cations on Slow Unblock of Native NMDA Receptors in Mouse Neocortical Pyramidal Neurons. Eur. J. Neurosci. 2011, 34, 199–212. [Google Scholar] [CrossRef]
- Clarke, R.J.; Glasgow, N.G.; Johnson, J.W. Mechanistic and Structural Determinants of NMDA Receptor Voltage-Dependent Gating and Slow Mg2+ Unblock. J. Neurosci. 2013, 33, 4140–4150. [Google Scholar] [CrossRef]
- Retchless, B.S.; Gao, W.; Johnson, J.W. A Single GluN2 Subunit Residue Controls NMDA Receptor Channel Properties via Intersubunit Interaction. Nat. Neurosci. 2012, 15, 406–413. [Google Scholar] [CrossRef]
- Oken, A.C.; Krishnamurthy, I.; Savage, J.C.; Lisi, N.E.; Godsey, M.H.; Mansoor, S.E. Molecular Pharmacology of P2X Receptors: Exploring Druggable Domains Revealed by Structural Biology. Front. Pharmacol. 2022, 13, 925880. [Google Scholar] [CrossRef]
- Nicke, A.; Grutter, T.; Egan, T.M. P2X Receptors. In The Oxford Handbook of Neuronal Ion Channels; Bhattacharjee, A., Ed.; Oxford University Press: Oxford, UK, 2018; pp. 458–485. ISBN 978-0-19-066916-4. [Google Scholar]
- Li, M.; Silberberg, S.D.; Swartz, K.J. Subtype-Specific Control of P2X Receptor Channel Signaling by ATP and Mg2+. Proc. Natl. Acad. Sci. USA 2013, 110, E3455–E3463. [Google Scholar] [CrossRef]
- Brünings, X.; Schmauder, R.; Mrowka, R.; Benndorf, K.; Sattler, C. Subtype-Specific Ligand Binding and Activation Gating in Homomeric and Heteromeric P2X Receptors. Biomolecules 2024, 14, 942. [Google Scholar] [CrossRef]
- Li, M.; Silberberg, S.D.; Swartz, K.J. Magnesium Modulation of P2X Receptor Channels. Biophys. J. 2018, 114, 126a–127a. [Google Scholar] [CrossRef]
- Barnard, E.A.; Skolnick, P.; Olsen, R.W.; Mohler, H.; Sieghart, W.; Biggio, G.; Braestrup, C.; Bateson, A.N.; Langer, S.Z. International Union of Pharmacology. XV. Subtypes of Gamma-Aminobutyric acidA Receptors: Classification on the Basis of Subunit Structure and Receptor Function. Pharmacol. Rev. 1998, 50, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Möykkynen, T.; Uusi-Oukari, M.; Heikkilä, J.; Lovinger, D.M.; Lüddens, H.; Korpi, E.R. Magnesium Potentiation of the Function of Native and Recombinant GABAA Receptors. Neuroreport 2001, 12, 2175–2179. [Google Scholar] [CrossRef]
- Dani, J.A. Neuronal Nicotinic Acetylcholine Receptor Structure and Function and Response to Nicotine. Int. Rev. Neurobiol. 2015, 124, 3–19. [Google Scholar] [CrossRef]
- Taylor, D.B.; Spivak, C.E. The Activation of the Nicotinic Acetylcholine Receptor by the Transmitter. J. Theor. Biol. 1985, 112, 653–666. [Google Scholar] [CrossRef]
- Takeda, K.; Gage, P.W.; Barry, P.H. Effects of Divalent Cations on Toad End-Plate Channels. J. Membr. Biol. 1982, 64, 55–66. [Google Scholar] [CrossRef]
- Connor, E.A.; Neel, D.S.; Parsons, R.L. Influence of the Extracellular Ionic Environment on Ganglionic Fast Excitatory Postsynaptic Currents. Brain Res. 1985, 339, 227–235. [Google Scholar] [CrossRef]
- Sands, S.B.; Barish, M.E. Neuronal Nicotinic Acetylcholine Receptor Currents in Phaeochromocytoma (PC12) Cells: Dual Mechanisms of Rectification. J. Physiol. 1992, 447, 467–487. [Google Scholar] [CrossRef] [PubMed]
- Mathie, A.; Colquhoun, D.; Cull-Candy, S.G. Rectification of Currents Activated by Nicotinic Acetylcholine Receptors in Rat Sympathetic Ganglion Neurones. J. Physiol. 1990, 427, 625–655. [Google Scholar] [CrossRef] [PubMed]
- Ifune, C.K.; Steinbach, J.H. Voltage-Dependent Block by Magnesium of Neuronal Nicotinic Acetylcholine Receptor Channels in Rat Phaeochromocytoma Cells. J. Physiol. 1991, 443, 683–701. [Google Scholar] [CrossRef]
- Neuhaus, R.; Cachelin, A.B. Changes in the Conductance of the Neuronal Nicotinic Acetylcholine Receptor Channel Induced by Magnesium. Proc. Biol. Sci. 1990, 241, 78–84. [Google Scholar] [CrossRef]
- Nenov, A.P.; Norris, C.; Bobbin, R.P. Acetylcholine Response in Guinea Pig Outer Hair Cells. II. Activation of a Small Conductance Ca2+-Activated K+ Channel. Hear. Res. 1996, 101, 149–172. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.R.; Castro, N.G.; Alkondon, M.; Reinhardt, S.; Schröder, H.; Maelicke, A. Nicotinic Receptor Function in the Mammalian Central Nervous System. Ann. N. Y. Acad. Sci. 1995, 757, 48–72. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-H.; Xia, X.-M.; Lingle, C.J. Divalent Cation Sensitivity of BK Channel Activation Supports the Existence of Three Distinct Binding Sites. J. Gen. Physiol. 2005, 125, 273–286. [Google Scholar] [CrossRef]
- Paravicini, T.M.; Chubanov, V.; Gudermann, T. TRPM7: A Unique Channel Involved in Magnesium Homeostasis. Int. J. Biochem. Cell Biol. 2012, 44, 1381–1384. [Google Scholar] [CrossRef]
- Rimkute, L.; Kraujalis, T.; Snipas, M.; Palacios-Prado, N.; Jotautis, V.; Skeberdis, V.A.; Bukauskas, F.F. Modulation of Connexin-36 Gap Junction Channels by Intracellular pH and Magnesium Ions. Front. Physiol. 2018, 9, 362. [Google Scholar] [CrossRef]
- Nayak, A.R.; Rangubpit, W.; Will, A.H.; Hu, Y.; Castro-Hartmann, P.; Lobo, J.J.; Dryden, K.; Lamb, G.D.; Sompornpisut, P.; Samsó, M. Interplay between Mg2+ and Ca2+ at Multiple Sites of the Ryanodine Receptor. Nat. Commun. 2024, 15, 4115. [Google Scholar] [CrossRef]
- Apell, H.-J.; Hitzler, T.; Schreiber, G. Modulation of the Na,K-ATPase by Magnesium Ions. Biochemistry 2017, 56, 1005–1016. [Google Scholar] [CrossRef]
- Vemana, S.; Pandey, S.; Larsson, H.P. Intracellular Mg2+ Is a Voltage-Dependent Pore Blocker of HCN Channels. Am. J. Physiol.-Cell Physiol. 2008, 295, C557–C565. [Google Scholar] [CrossRef]
- Reilly, L.; Eckhardt, L.L. Cardiac Potassium Inward Rectifier Kir2: Review of Structure, Regulation, Pharmacology, and Arrhythmogenesis. Heart Rhythm 2021, 18, 1423–1434. [Google Scholar] [CrossRef]
- Fisher, D. Clinical Pharmacology of Neuromuscular Blocking Agents. Am. J. Health. Syst. Pharm. 1999, 56, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Vink, R.; Nechifor, M. (Eds.) Magnesium in the Central Nervous System; University of Adelaide Press: Adelaide, Australia, 2011; ISBN 978-0-9870730-5-1. [Google Scholar]
- Li, Z.; Chen, L.; Li, H. Voltage-Gated Sodium Channels and Blockers: An Overview and Where Will They Go? Curr. Med. Sci. 2019, 39, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Mankodi, A.; Grunseich, C.; Skov, M.; Cook, L.; Aue, G.; Purev, E.; Bakar, D.; Lehky, T.; Jurkat-Rott, K.; Pedersen, T.H.; et al. Divalent Cation-Responsive Myotonia and Muscle Paralysis in Skeletal Muscle Sodium Channelopathy. Neuromuscul. Disord. 2015, 25, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.; Tan, Z.-Y.; Wang, R.; Xie, W.; Strong, J.A.; Patel, R.R.; Vasko, M.R.; Zhang, J.-M.; Cummins, T.R. Navβ4 Regulates Fast Resurgent Sodium Currents and Excitability in Sensory Neurons. Mol. Pain 2015, 11, 60. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, X.; Li, Q.; Meredith, A.L.; Pan, H.-L.; Yan, J. Glutamate-Activated BK Channel Complexes Formed with NMDA Receptors. Proc. Natl. Acad. Sci. USA 2018, 115, E9006–E9014. [Google Scholar] [CrossRef]
- Gómez, R.; Maglio, L.E.; Gonzalez-Hernandez, A.J.; Rivero-Pérez, B.; Bartolomé-Martín, D.; Giraldez, T. NMDA Receptor–BK Channel Coupling Regulates Synaptic Plasticity in the Barrel Cortex. Proc. Natl. Acad. Sci. USA 2021, 118, e2107026118. [Google Scholar] [CrossRef]
- Ancatén-González, C.; Segura, I.; Alvarado-Sánchez, R.; Chávez, A.E.; Latorre, R. Ca2+- and Voltage-Activated K+ (BK) Channels in the Nervous System: One Gene, a Myriad of Physiological Functions. Int. J. Mol. Sci. 2023, 24, 3407. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, Y.; Hunanyan, A.S.; Mikati, M.A.; Cui, J.; Yang, H. Neuronal Mechanism of a BK Channelopathy in Absence Epilepsy and Dyskinesia. Proc. Natl. Acad. Sci. USA 2022, 119, e2200140119. [Google Scholar] [CrossRef]
- Park, S.M.; Roache, C.E.; Iffland, P.H.; Moldenhauer, H.J.; Matychak, K.K.; Plante, A.E.; Lieberman, A.G.; Crino, P.B.; Meredith, A. BK Channel Properties Correlate with Neurobehavioral Severity in Three KCNMA1-Linked Channelopathy Mouse Models. eLife 2022, 11, e77953. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.; Chen, Q.H.; Vilaythong, A.; Toney, G.M.; Noebels, J.L.; Aldrich, R.W. BK Channel Β4 Subunit Reduces Dentate Gyrus Excitability and Protects against Temporal Lobe Seizures. Nat. Neurosci. 2005, 8, 1752–1759. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, S.; Heils, A.; Kasper, J.M.; Sander, T. Allelic Association of a Truncation Mutation of the KCNMB3 Gene with Idiopathic Generalized Epilepsy. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 10–13. [Google Scholar] [CrossRef]
- Du, X.; Carvalho-de-Souza, J.L.; Wei, C.; Carrasquel-Ursulaez, W.; Lorenzo, Y.; Gonzalez, N.; Kubota, T.; Staisch, J.; Hain, T.; Petrossian, N.; et al. Loss-of-Function BK Channel Mutation Causes Impaired Mitochondria and Progressive Cerebellar Ataxia. Proc. Natl. Acad. Sci. USA 2020, 117, 6023–6034. [Google Scholar] [CrossRef]
- Beecham, G.W.; Schnetz-Boutaud, N.; Haines, J.L.; Pericak-Vance, M.A. CALHM1 Polymorphism Is Not Associated with Late-onset Alzheimer Disease. Ann. Hum. Genet. 2009, 73, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Beecham, G.W.; Hamilton, K.; Naj, A.C.; Martin, E.R.; Huentelman, M.; Myers, A.J.; Corneveaux, J.J.; Hardy, J.; Vonsattel, J.-P.; Younkin, S.G.; et al. Genome-Wide Association Meta-Analysis of Neuropathologic Features of Alzheimer’s Disease and Related Dementias. PLoS Genet. 2014, 10, e1004606. [Google Scholar] [CrossRef]
- Samengo, I.; Currò, D.; Barrese, V.; Taglialatela, M.; Martire, M. Large Conductance Calcium-Activated Potassium Channels: Their Expression and Modulation of Glutamate Release from Nerve Terminals Isolated from Rat Trigeminal Caudal Nucleus and Cerebral Cortex. Neurochem. Res. 2014, 39, 901–910. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Tang, J.; Lin, J.; Cai, X.; Wang, X.; Zheng, G. Potassium Channels: Possible New Therapeutic Targets in Parkinson’s Disease. Med. Hypotheses 2008, 71, 546–550. [Google Scholar] [CrossRef]
- Chen, X.; Xue, B.; Wang, J.; Liu, H.; Shi, L.; Xie, J. Potassium Channels: A Potential Therapeutic Target for Parkinson’s Disease. Neurosci. Bull. 2018, 34, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Waroux, O.; Massotte, L.; Alleva, L.; Graulich, A.; Thomas, E.; Liégeois, J.; Scuvée-Moreau, J.; Seutin, V. SK Channels Control the Firing Pattern of Midbrain Dopaminergic Neurons in Vivo. Eur. J. Neurosci. 2005, 22, 3111–3121. [Google Scholar] [CrossRef] [PubMed]
- Liegeois, J.; Mercier, F.; Graulich, A.; Graulich-Lorge, F.; Scuvee-Moreau, J.; Seutin, V. Modulation of Small Conductance Calcium-Activated Potassium (SK) Channels: A New Challenge in Medicinal Chemistry. Curr. Med. Chem. 2003, 10, 625–647. [Google Scholar] [CrossRef]
- Alvarez-Fischer, D.; Noelker, C.; Vulinović, F.; Grünewald, A.; Chevarin, C.; Klein, C.; Oertel, W.H.; Hirsch, E.C.; Michel, P.P.; Hartmann, A. Bee Venom and Its Component Apamin as Neuroprotective Agents in a Parkinson Disease Mouse Model. PLoS ONE 2013, 8, e61700. [Google Scholar] [CrossRef]
- Ji, H.; Shepard, P.D. SK Ca2+-Activated K+ Channel Ligands Alter the Firing Pattern of Dopamine-Containing Neurons in Vivo. Neuroscience 2006, 140, 623–633. [Google Scholar] [CrossRef]
- Dolga, A.M.; De Andrade, A.; Meissner, L.; Knaus, H.-G.; Höllerhage, M.; Christophersen, P.; Zischka, H.; Plesnila, N.; Höglinger, G.U.; Culmsee, C. Subcellular Expression and Neuroprotective Effects of SK Channels in Human Dopaminergic Neurons. Cell Death Dis. 2014, 5, e999. [Google Scholar] [CrossRef] [PubMed]
- Chandy, K.G.; Fantino, E.; Wittekindt, O.; Kalman, K.; Tong, L.-L.; Ho, T.-H.; Gutman, G.A.; Crocq, M.-A.; Ganguli, R.; Nimgaonkar, V.; et al. Isolation of a Novel Potassium Channel Gene hSKCa3 Containing a Polymorphic CAG Repeat: A Candidate for Schizophrenia and Bipolar Disorder? Mol. Psychiatry 1998, 3, 32–37. [Google Scholar] [CrossRef]
- Ritsner, M.; Modai, I.; Ziv, H.; Amir, S.; Halperin, T.; Weizman, A.; Navon, R. An Association of CAG Repeats at the KCNN3 Locus with Symptom Dimensions of Schizophrenia. Biol. Psychiatry 2002, 51, 788–794. [Google Scholar] [CrossRef]
- Soden, M.E.; Jones, G.L.; Sanford, C.A.; Chung, A.S.; Güler, A.D.; Chavkin, C.; Luján, R.; Zweifel, L.S. Disruption of Dopamine Neuron Activity Pattern Regulation through Selective Expression of a Human KCNN3 Mutation. Neuron 2013, 80, 997–1009. [Google Scholar] [CrossRef]
- Miller, M.J.; Rauer, H.; Tomita, H.; Rauer, H.; Gargus, J.J.; Gutman, G.A.; Cahalan, M.D.; Chandy, K.G. Nuclear Localization and Dominant-Negative Suppression by a Mutant SKCa3 N-Terminal Channel Fragment Identified in a Patient with Schizophrenia. J. Biol. Chem. 2001, 276, 27753–27756. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.; Williams, N.; Norton, N.; Spurlock, G.; Wittekindt, O.H.; Morris-Rosendahl, D.J.; Williams, H.; Brzustowicz, L.; Hoogendoorn, B.; Zammit, S.; et al. Mutation Screening of the KCNN3 Gene Reveals a Rare Frameshift Mutation. Mol. Psychiatry 2001, 6, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Skinner, F.; Arshadmansab, M.F.; Garcia, I.; Mello, C.F.; Knaus, H.-G.; Ermolinsky, B.S.; Otalora, L.F.P.; Garrido-Sanabria, E.R. Altered Expression and Function of Small-Conductance (SK) Ca2+-Activated K+ Channels in Pilocarpine-Treated Epileptic Rats. Brain Res. 2010, 1348, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Lappin, S.C.; Dale, T.J.; Brown, J.T.; Trezise, D.J.; Davies, C.H. Activation of SK Channels Inhibits Epileptiform Bursting in Hippocampal CA3 Neurons. Brain Res. 2005, 1065, 37–46. [Google Scholar] [CrossRef]
- Khandai, P.; Forcelli, P.A.; N’Gouemo, P. Activation of Small Conductance Calcium-Activated Potassium Channels Suppresses Seizure Susceptibility in the Genetically Epilepsy-Prone Rats. Neuropharmacology 2020, 163, 107865. [Google Scholar] [CrossRef]
- Anderson, N.J.; Slough, S.; Watson, W.P. In Vivo Characterisation of the Small-Conductance KCa (SK) Channel Activator 1-Ethyl-2-Benzimidazolinone (1-EBIO) as a Potential Anticonvulsant. Eur. J. Pharmacol. 2006, 546, 48–53, Correction in Eur. J. Pharmacol. 2008, 592, 167. [Google Scholar] [CrossRef]
- Skibsbye, L.; Poulet, C.; Diness, J.G.; Bentzen, B.H.; Yuan, L.; Kappert, U.; Matschke, K.; Wettwer, E.; Ravens, U.; Grunnet, M.; et al. Small-Conductance Calcium-Activated Potassium (SK) Channels Contribute to Action Potential Repolarization in Human Atria. Cardiovasc. Res. 2014, 103, 156–167. [Google Scholar] [CrossRef]
- Hallak, M.; Berman, R.F.; Irtenkauf, S.M.; Janusz, C.A.; Cotton, D.B. Magnesium Sulfate Treatment Decreases N-Methyl-D-Aspartate Receptor Binding in the Rat Brain: An Autoradiographic Study. J. Soc. Gynecol. Investig. 1994, 1, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Hallak; Irtenkauf; Cotton. Effect of Magnesium Sulfate on Excitatory Amino Acid Receptors in the Rat Brain. Am. J. Obstet. Gynecol. 1996, 175, 575–581. [Google Scholar] [CrossRef]
- Gorji, A.; Scheller, D.; Straub, H.; Tegtmeier, F.; Köhling, R.; Höhling, J.-M.; Tuxhorn, I.; Ebner, A.; Wolf, P.; Werner Panneck, H.; et al. Spreading Depression in Human Neocortical Slices. Brain Res. 2001, 906, 74–83. [Google Scholar] [CrossRef]
- Dominguez, L.; Veronese, N.; Sabico, S.; Al-Daghri, N.; Barbagallo, M. Magnesium and Migraine. Nutrients 2025, 17, 725. [Google Scholar] [CrossRef]
- Pujol, R.; Puel, J.-L.; D’aldin, C.G.; Eybalin, M. Pathophysiology of the Glutamatergic Synapses in the Cochlea. Acta Otolaryngol. 1993, 113, 330–334. [Google Scholar] [CrossRef]
- Joachims, Z.; Babisch, W.; Ising, H.; Günther, T.; Handrock, M. Dependence of Noise-Induced Hearing Loss upon Perilymph Magnesium Concentration. J. Acoust. Soc. Am. 1983, 74, 104–108. [Google Scholar] [CrossRef]
- Ising, H.; Handrock, M.; Günther, T.; Fischer, R.; Dombrowski, M. Increased Noise Trauma in Guinea Pigs through Magnesium Deficiency. Arch. Otorhinolaryngol. 1982, 236, 139–146. [Google Scholar] [CrossRef]
- Attias, J.; Weisz, G.; Almog, S.; Shahar, A.; Wiener, M.; Joachims, Z.; Netzer, A.; Ising, H.; Rebentisch, E.; Guenther, T. Oral Magnesium Intake Reduces Permanent Hearing Loss Induced by Noise Exposure. Am. J. Otolaryngol. 1994, 15, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Attias, J.; Sapir, S.; Bresloff, I.; Reshef-Haran, I.; Ising, H. Reduction in Noise-Induced Temporary Threshold Shift in Humans Following Oral Magnesium Intake. Clin. Otolaryngol. Allied Sci. 2004, 29, 635–641. [Google Scholar] [CrossRef]
- Woolf, C.J.; Thompson, S.W.N. The Induction and Maintenance of Central Sensitization Is Dependent on N-Methyl-d-Aspartic Acid Receptor Activation; Implications for the Treatment of Post-Injury Pain Hypersensitivity States. Pain 1991, 44, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Chong, M.-S. Preemptive Analgesia—Treating Postoperative Pain by Preventing the Establishment of Central Sensitization. Anesth. Analg. 1993, 77, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Telci, L.; Esen, F.; Akcora, D.; Erden, T.; Canbolat, A.T.; Akpir, K. Evaluation of Effects of Magnesium Sulphate in Reducing Intraoperative Anaesthetic Requirements. Br. J. Anaesth. 2002, 89, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Kang, M.-H.; Park, K.-S.; Do, S.-H. Effects of Magnesium Sulphate on Intraoperative Anaesthetic Requirements and Postoperative Analgesia in Gynaecology Patients Receiving Total Intravenous Anaesthesia. Br. J. Anaesth. 2008, 100, 397–403. [Google Scholar] [CrossRef]
- Oyanagi, K.; Makifuchi, T.; Ohtoh, T.; Chen, K.-M.; Gajdusek, D.C.; Chase, T.N. Distinct Pathological Features of the Gallyas- and Tau-Positive Glia in the Parkinsonism-Dementia Complex and Amyotrophic Lateral Sclerosis of Guam. J. Neuropathol. Exp. Neurol. 1997, 56, 308–316. [Google Scholar] [CrossRef]
- Oyanagi, K. The Nature of the Parkinsonism-Dementia Complex and Amyotrophic Lateral Sclerosis of Guam and Magnesium Deficiency. Park. Relat. Disord. 2005, 11, S17–S23. [Google Scholar] [CrossRef]
- Oyanagi, K.; Kawakami, E.; Kikuchi-Horie, K.; Ohara, K.; Ogata, K.; Takahama, S.; Wada, M.; Kihira, T.; Yasui, M. Magnesium Deficiency over Generations in Rats with Special References to the Pathogenesis of the Parkinsonism–Dementia Complex and Amyotrophic Lateral Sclerosis of Guam. Neuropathology 2006, 26, 115–128. [Google Scholar] [CrossRef]
- Barbiroli, B.; Martinelli, P.; Patuelli, A.; Lodi, R.; Iotti, S.; Cortelli, P.; Montagna, P. Phosphorus Magnetic Resonance Spectroscopy in Multiple System Atrophy and Parkinson’s Disease. Mov. Disord. 1999, 14, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Vural, H.; Demirin, H.; Kara, Y.; Eren, I.; Delibas, N. Alterations of Plasma Magnesium, Copper, Zinc, Iron and Selenium Concentrations and Some Related Erythrocyte Antioxidant Enzyme Activities in Patients with Alzheimer’s Disease. J. Trace Elem. Med. Biol. 2010, 24, 169–173. [Google Scholar] [CrossRef]
- Andrási, E.; Páli, N.; Molnár, Z.; Kösel, S. Brain Aluminum, Magnesium and Phosphorus Contents of Control and Alzheimer-Diseased Patients. J. Alzheimers Dis. 2005, 7, 273–284. [Google Scholar] [CrossRef]
- Levine, J.; Rapoport, A.; Mashiah, M.; Dolev, E. Serum and Cerebrospinal Levels of Calcium and Magnesium in Acute versus Remitted Schizophrenic Patients. Neuropsychobiology 1996, 33, 169–172. [Google Scholar] [CrossRef]
- Nechifor, M.; Vaideanu, C.; Palamaru, I.; Borza, C.; Mindreci, I. The Influence of Some Antipsychotics on Erythrocyte Magnesium and Plasma Magnesium, Calcium, Copper and Zinc in Patients with Paranoid Schizophrenia. J. Am. Coll. Nutr. 2004, 23, 549S–551S. [Google Scholar] [CrossRef]
- Kirov, G.K.; Tsachev, K.N. Magnesium, Schizophrenia and Manic-Depressive Disease. Neuropsychobiology 1990, 23, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Wand, P.-L.; Brünings, X.; Mrowka, R.; Zimmer, T.; Benndorf, K.; Sattler, C. BPS2025—Hearing Loss Mutations in Human P2X2 Receptor Channels. Biophys. J. 2025, 124, 281a–282a. [Google Scholar] [CrossRef]
- Jun, D.-J.; Kim, J.; Jung, S.-Y.; Song, R.; Noh, J.-H.; Park, Y.-S.; Ryu, S.-H.; Kim, J.-H.; Kong, Y.-Y.; Chung, J.-M.; et al. Extracellular ATP Mediates Necrotic Cell Swelling in SN4741 Dopaminergic Neurons through P2X7 Receptors. J. Biol. Chem. 2007, 282, 37350–37358. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Huang, L.; Kuang, Y.; Wu, X.; Ma, X.; Zhao, B.; Lan, J. Unlocking the Therapeutic Potential of P2X7 Receptor: A Comprehensive Review of Its Role in Neurodegenerative Disorders. Front. Pharmacol. 2024, 15, 1450704. [Google Scholar] [CrossRef]
- Saft, C.; Speckmann, E.-J. Antiepileptic Effects of Cobalt, Manganese and Magnesium on Bicuculline-Induced Epileptiform Activity in Hippocampal Neurons. Brain Res. 2020, 1732, 146684. [Google Scholar] [CrossRef]
- Staley, K.J.; Soldo, B.L.; Proctor, W.R. Ionic Mechanisms of Neuronal Excitation by Inhibitory GABAA Receptors. Science 1995, 269, 977–981. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; Long, L.P. The Interaction between Magnesium and Other Neuromuscular Blocking Agents. Anesthesiology 1970, 32, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Buder, T.; Wilder-Smith, O.H.G.; Borgeat, A.; Tassonyi, E. Interaction of Magnesium Sulphate with Vecuronium-Induced Neuromuscular Blockt. Br. J. Anaesth. 1995, 74, 405–409. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).