Abstract
Antioxidants are essential bioactive compounds widely recognized for their health benefits in preventing oxidative stress-related diseases. However, many lipophilic antioxidants suffer from poor aqueous solubility, low chemical stability, and limited bioavailability, restricting their application in food, nutraceutical, and pharmaceutical industries. Cyclodextrins (CDs), a class of cyclic oligosaccharides with a hydrophilic exterior and lipophilic interior, present an effective strategy to encapsulate and deliver these compounds by improving their solubility, stability, and therapeutic efficacy. This review critically examines the structural features and derivatives of cyclodextrins relevant for antioxidant encapsulation, mechanisms and thermodynamics of inclusion complex formation, and advanced characterization techniques. It evaluates the influence of CD encapsulation on the oral bioavailability and antioxidant activity of various lipophilic antioxidants supported by recent in vitro and in vivo studies. Moreover, sustainable preparation methods for CD complexes are discussed alongside safety and regulatory considerations. The comprehensive synthesis of current knowledge contributes to guiding the rational design and development of CD-based antioxidant nutraceuticals, addressing formulation challenges while promoting efficacy and consumer safety.
Keywords:
cyclodextrin; inclusion complex; antioxidant; carotenoid; retinoid; tocopherol; curcumin; capsaicin 1. Introduction
Lipophilic antioxidants such as carotenoids, tocopherols, retinoids, curcumin (CUR), capsaicin (CAP), and coenzyme Q10 (CoQ10) are extensively studied for their crucial role in maintaining human health by counteracting oxidative stress, which is linked to numerous chronic diseases and ageing processes [1,2,3]. However, their practical application in food, pharmaceutical, and nutraceutical industries is severely limited by poor water solubility, chemical instability, and low bioavailability. These challenges call for innovative formulation strategies to enhance their stability, solubility, and therapeutic efficiency [4].
Cyclodextrins (CDs) are cyclic oligosaccharides with a hydrophilic exterior and a lipophilic cavity, making them effective carriers for lipophilic antioxidants [5]. This encapsulation not only enhances the aqueous solubility and chemical stability of these compounds but also improves their bioavailability and antioxidant efficacy. Over the past decade, advances in CD chemistry, including novel derivatives and polymeric forms, have expanded the potential of CD-based delivery systems in overcoming the intrinsic limitations of lipophilic antioxidants [5] (Figure 1).
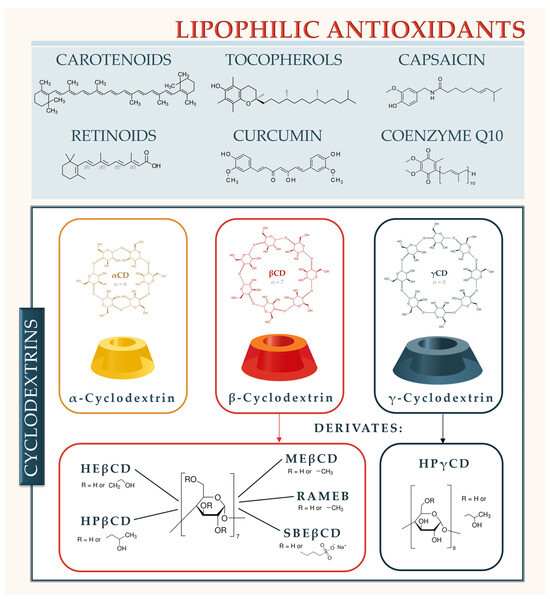
Figure 1.
Lipophilic antioxidants and cyclodextrins analyzed in this study. HEβCD (hydroxyethyl-β-cyclodextrin), HPβCD (hydroxypropyl-β-cyclodextrin), MEβCD (methyl-β-cyclodextrin), RAMEB (randomly methylated-β-cyclodextrin), SBEβCD (sulphobuthylether-β-cyclodextrin), and HPγCD (Hydroxypropyl-γ-cyclodextrin). The present study aims to comprehensively explore the benefits and challenges associated with CD-encapsulation of antioxidants in the context of nutraceutical development focusing primarily on per os application. Namely, parenteral applications face limitations due to safety and toxicology; therefore, at present, only hydroxypropyl-beta-cyclodextrin (HPβCD) and sulfobutylether-beta-cyclodextrin (SBEβCD) are classified as safe for parenteral administration [6].
By systematically reviewing the structural features, physicochemical properties, complexation mechanisms, and characterization techniques of CDs and their inclusion complexes, this work provides critical scientific insight into optimizing formulation approaches (Figure 2). Additionally, it evaluates the biological impacts, including enhanced antioxidant activity and bioavailability, supported by cutting-edge in vitro and in vivo studies.
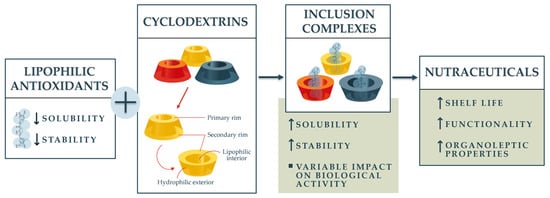
Figure 2.
Conceptual framework of the study.
This article integrates recent advancements in CD technology with the specific needs of lipophilic antioxidant formulation, thereby promoting sustainable and efficient nutraceutical products. It addresses existing gaps in mechanistic understanding and practical challenges such as scalability, safety, and regulatory considerations, setting a foundation for future research and application in functional foods, dietary supplements, and therapeutic agents.
2. Cyclodextrins in Nutraceutical Development
2.1. Cyclodextrins—Structural Features and Novel Cyclodextrin Derivatives
CDs are a group of nonreducing cyclic oligosaccharides known for more than 120 years [5,7]. Natural αCD, βCD, and γCD, produced by bacterial starch degradation, are assembled of 6, 7, or 8 α-1,4-linked D-glucopyranose units, respectively. Recently, small CDs consisting of 3 and 4 glucopyranose units were synthesized [8], while large CDs with more than 8 glucopyranose units have been known for some time [9] but are not relevant for nutraceutical and drug delivery. CDs have a hollow truncated cone structure, with hydroxyl groups oriented to the exterior of the molecule, giving it a hydrophilic character. Primary hydroxyl groups are directed toward the narrower edge of the cone, while secondary ones are located on the broader rim of the molecule. The central cavity of the CD is lipophilic, presenting the same polarity as diluted ethanol, and its size is determined by the number of glucopyranose units (Table 1) [10,11,12].

Table 1.
Physicochemical properties, safety, and application of pharmaceutically relevant cyclodextrins [13,14,15,16,17].
Inter-and intra-molecular hydrogen bonding between vicinal C2 and C3 hydroxyl groups of adjacent glucopyranose units diminishes CD’s ability to form hydrogen bonds with the surrounding water molecules and contributes to a highly stable crystal lattice formation. Because of that, natural CDs, and especially βCD, are less soluble in water than corresponding linear maltodextrins. Substitution of any of the hydroxyl groups in the CD molecule disrupts the hydrogen-bond formation and results in a dramatic increase in the CD solubility. Numerous CD derivatives with increased solubility improved functionality, and a more acceptable toxicological profile with respect to that of natural CDs have been synthesized [7,11,18]. However, to gain optimal solubility and complexation ability, the average number of substituents per glucopyranose unit should be low [8]. The list of pharmaceutically most important CDs is given in Table 1; however, novel CD derivatives are now emerging. Among them, thiolated CDs, as mucoadhesive compounds able to prolong gastrointestinal residence time [18,19], and a wide selection of polymeric CD derivatives, like CD-based polyrotaxanes, grafted CD polymers, crosslinked CD polymers etc., that are stimuli-responsive and are able to form well-defined aggregated nanostructures [20] are the most prominent.
2.2. Metabolism and Safety of Cyclodextrins
After oral administration, γCD and its derivatives are completely digested in the gastrointestinal tract (GIT) by salivary and pancreatic α-amylases. In contrast, αCD, βCD, and their hydrophilic derivatives are resistant towards α-amylases and are dominantly digested by bacteria in the colon. Furthermore, high molecular weight, numerous hydrogen bond donors, and acceptors in the CDs’ structure, as well as pronounced hydrophilicity, restrict their permeability across biological membranes by passive diffusion [7,12]. All this contributes to the limited oral bioavailability of CDs in animals and humans, ranging from 0.1 to 3% of the applied dose, so they are practically nontoxic when administered orally. The maximum recommended oral daily doses of α-cyclodextrin (αCD), β-cyclodextrin (βCD), γ-cyclodextrin (γCD), and hydroxypropyl-β-cyclodextrin (HPβCD) are 6 g, 0.5 g, 10 g, and 8 g, respectively. Administration of higher doses may result in diarrhea, attributable to the osmotic activity of CDs [21]. An interaction between orally ingested CDs and the absorption of fat-soluble vitamins or other lipophilic nutrients is not anticipated, as the formation of inclusion complexes is reversible. Furthermore, γCD is readily digested in the small intestine, and studies with βCD, a non-digestible CD, have shown that the bioavailability of A, D, and E vitamins is not impaired [15]. RAMEB, a more lipophilic derivative, shows an oral bioavailability of 12% in rats. Both αCD and βCD are unsuitable for parenteral administration due to renal toxicity, while among CD derivatives, methylated CDs as surface-active agents cause significant hemolysis [10]. HPβCD, SBEβCD, γCD, and HPγCD are considered safe even for parenteral administration, displaying a small volume of distribution and rapid clearance in intact form by glomerular filtration. Pharmacokinetic studies show that more than 90% of parenterally administered HPβCD and SBEβCD are eliminated from the body within 6 h, and over 99.9% within 24 h, posing no risk of accumulation in individuals with normal kidney function. However, in patients with severe renal impairment (renal creatinine clearance < 10 mL/min), CD accumulation will be observed, but its clinical significance is still to be determined [7,22]. Administration of natural and hydrophilic CDs on nasal, buccal, vaginal, or rectal mucosa is safe in a wide range of concentrations, while for RAMEB, the concentration (~5%) and exposure time should be limited [10,23]. Natural CDs and their hydrophilic derivatives are not able to permeate skin in significant amounts (0.02% and 0.3% of the applied dose for HPβCD and RAMEB, respectively) and are generally considered to be non-irritant to the skin. However, under occlusive conditions, transdermal absorption of CDs could be more pronounced [21].
2.3. Regulatory Status
The regulatory status of CDs has continuously developed over the past 40 years [11]. Natural αCD, βCD, and γCD are listed in the Generally Regarded as Safe list of the Food and Drug Administration (FDA) for their use as food additives and, along with HPβCD, have monographs in the European Pharmacopeia and the United States Pharmacopeia and the National Formulary (USP/NF). SBEβCD has a monograph only in USP/NF, while other CD derivatives are considered related substances. The main problem with CD derivatives from a regulatory standpoint relates to the homogeneity of the final product. For example, methylated βCD derivatives are commercially available in various qualities, with isomeric purity of 50%, 80%, or 95%, but there is no 100% pure 2,6-dimethyl-βCD, making it difficult to set the quality standards [23]. In the European Union, βCD is approved as a food additive (E459), with an acceptable daily intake of 5 mg/kg/day [11,21], and αCD is regulated as a dietary fibre with an authorized health claim “for contributing to the reduction of postprandial glycemic responses” [24]. Nowadays, in drug formulations, CDs are considered as excipients and not as part of the drug substance. Thus, βCD, HPβCD, SBEβCD, γCD, and HPγCD are introduced into the FDA’s list of Inactive Pharmaceutical Ingredients [7] but have not yet been recognized as ingredients for the use in food [25]. In the context of a new drug application, when a given CD derivative is used for the first time in humans, it is treated as an active ingredient. A complete safety evaluation may not be needed if a given CD derivative has been previously used at the same concentration and route of administration as in the marketed product [11].
2.4. Inclusion Complex Formation
An inclusion complex is formed by the reversible entrapment of a poorly soluble compound or, more often, a sterically compatible lipophilic functional moiety of the molecule into the lipophilic CD central cavity. This causes the displacement of energy-rich water molecules from the CD central cavity, accompanied by a whole set of intermolecular drug/CD interactions, including hydrogen bonding, van der Waals forces, and hydrophobic interactions as well as ionic interactions (in case of charged drugs and CD derivatives), all contributing to steric and thermodynamic stabilization of the system. Therefore, inclusion complex formation occurs spontaneously in the aqueous media, and no covalent bonds are formed or broken during the inclusion complex formation [10,11,26]. Inclusion complex formation is a reversible process characterized by a dynamic equilibrium between the association and the dissociation of the drug/CD complex, described by inclusion complex association (i.e., stability) constant, ranging from 2 M−1 (fenofibrate) up to 40,000 M−1 (telmisartan) [27]. One molecule is usually included in one CD central cavity, presenting 1:1 molar stoichiometry. But in certain cases, higher-order complexes were described, where one drug molecule is included in two or more CDs, depending on the drug geometry [28].
Natural CDs self-assemble in an aqueous solution, forming aggregates in a concentration-dependent manner [29,30,31]. Aggregation is further promoted by the inclusion complex formation and chemically modified CDs, which alone have a low affinity for aggregation but self-assemble upon inclusion complexation with lipophilic drugs due to the surfactant-type structure of the complex formed [30]. CD aggregation should be considered in the development of liquid formulations containing high CD concentrations, as it could affect the inclusion complex formation, the physicochemical properties, and the in vivo performance of such products [32].
Absorption through the biological membrane and binding to the targeted receptor site are prerequisites for the drug/nutraceutical’s biological action. Therefore, when administered as an inclusion complex, the included compound must first be released from the complex formed. CDs will restrict compound permeation across biological membranes due to their high molecular weight and hydrophilic character, and they will sterically hinder their binding to their targeted sites. The compound release from the inclusion complex is mainly governed by simple media dilution, usually after oral or parenteral administration. Furthermore, other mechanisms can shift the equilibrium toward complex dissociation, such as the compound binding to proteins, competitive displacement from the CD cavity caused by bile salt in the GIT, and direct drug partitioning from the complex to the tissue. All this results in rapid and complete drug release from the complex after parenteral and oral administration, so CD complexation will not hamper its therapeutic effect. CD complexation will affect the drug pharmacokinetics after parenteral administration only in cases where the binding constant is higher than 100,000 M−1. In topical applications, media dilution is limited, and CDs can hamper drug release and absorption. Therefore, the use of excessive CD concentration in topical formulations should be avoided [11,28,33].
Inclusion complex formation enhances solubility, dissolution rate, chemical stability, and bioavailability of encapsulated molecule [7,34]. Thereby, CD complexation can be considered multifunctional technology to increase therapeutic potential and efficiency of drugs, especially as it could reduce or prevent certain side effects of the drugs [35], enhance drug permeation across the biological membranes [11,36], prevent drug-to-drug and drug-to-excipient interactions [37], favourably modify organoleptic characteristics of the included molecule [38], and even convert liquid and volatile compounds to technologically more acceptable free-flowing powders [39]. Because of such multifunctionality, CDs emerge as attractive excipients for the development of novel pharmaceuticals, including the reformulation of existing drug formulations and enabling an alternative application route for a given drug [28]. In some cases, the addition of polymers, organic acids, metal ions, or lipids may further enhance CD performance by a ternary or higher-order complex formation [11]. Presently, CD technology has been employed in the development of more than 120 pharmaceutical products available worldwide, while numerous formulations are in advanced phases of clinical testing [17].
In addition to pharmaceutical application in drug formulation development, certain CDs are used in the food industry to solubilize and/or chemically stabilize different food ingredients, remove unwanted or harmful food components like cholesterol or mycotoxins, improve antioxidant efficiency, mask unpleasant taste, improve sensory quality, preserve the colour of food, and are applied in food packaging [25,40,41].
2.5. Preparation and Characterization of Cyclodextrin Complexes—Sustainable Approaches
The development and preparation of CD complexes is a complex task requiring the simultaneous use of different preparation and characterization techniques to define optimal CD derivative and the preparation conditions for the molecule of interest. The usual approach is starting from the solution, using the phase solubility studies, where the effect of CD concentration on the drug solubility is assessed, usually by UV/VIS or HPLC analysis [38]. This phase aims to determine the type and concentration of the CD required to obtain the maximum solubilization of the investigated compound. This approach also enables the calculation of the association constant for each drug/CD combination. However, this step is rather time-consuming, as it should be performed in different media like water and other biorelevant buffered media, depending on the characteristics of the studied compound, such as solubility, chemical stability, pKa, etc. Depending on the obtained results, CD complexation efficiency and drug solubilization should be further enhanced by pH regulation, the addition of co-solvents, or by the formation of ternary complexes with suitable compounds, like amino acids, polyhydric acids, and polymers [7]. This, in turn, requires the development and validation of adequate drug quantification methods for each of the selected media. An additional problem arises with poorly soluble drugs, whose concentration in the samples without CDs may fall below the limit of quantification of the method employed. In the case when drug quantification is performed by HPLC, which is nowadays a gold standard, a large volume of solvents used as the mobile phase should be considered, requiring strict hazardous waste management. In this sense, capillary electrophoresis, as a more environmentally friendly method for drug/nutraceutical quantification, should also be employed [42]. Furthermore, molecular modelling methods, including molecular docking, molecular dynamics, and free energy binding analysis, may provide a helpful shortcut in selecting the optimal CD derivative to solubilize the compound of interest, significantly reducing the experimental time and waste production [43].
Depending on the chromophore presence in the structure of the examined molecule, the inclusion complex formation may change UV absorption spectra, enhance fluorescence, or lead to changes in circular dichroism spectra, providing additional analytical techniques to monitor drug/CD interactions in the solution [44]. Finally, perhaps the most important analytical technique to characterize the CD complexation in the solution is NMR spectroscopy, which provides direct evidence of the actual inclusion complex formation by observation of the chemical shift difference in proton (1H NMR) or carbon (13C NMR) between free guest and host species and the presumed complex. Furthermore, the measurement of chemical shift changes in the guest as a function of increased CD concentration (i.e., NMR titrations or Job’s plot) enables the determination of stoichiometry of the formed CD complex. Finally, two-dimensional NMR techniques like NOESY (Nuclear Overhauser Effect Spectroscopy) and ROESY (Rotational Overhauser Effect Spectroscopy) evidence the spatial proximity of host and guest protons, enabling precise determination of the drug/CD binding mode [44,45].
The other useful techniques to characterize the complex formation in the solution are dynamic light scattering, enabling monitoring of aggregation phenomena of CDs and complexes formed [31], and isothermal titration calorimetry, useful to precisely determine thermodynamic parameters of the complexation process, like complex stoichiometry, association constants, as well as enthalpy and entropy changes upon complexation in a single experiment [46,47].
After selecting the most suitable CDs for the compound of interest, a complex in solid state should be prepared. It must be underlined that demonstrating the actual inclusion complex formation in the solution does not automatically guarantee its existence in the solid state. Because of this, the complex preparation method should be carefully selected and optimized based on the characteristics of the drug and CD and the desired characteristics of the obtained product [47].
The traditional approach involves methods for the complex preparation in the solution. Here, the drug and CD are separately dissolved in adequate media. The critical properties are solubility and chemical stability of both drug and CD, directing the selection of the solvents for the complexation media and the drying technique employed for isolating the complexes in the solid state. For the drug, this mainly involves using organic solvents that are miscible with water. However, caution must be taken regarding the toxicological profile of the solvent used, as it may remain in the final product. This will be especially pronounced in the case of solvents having a high affinity for complexation with CDs, thereby competing with the drug for the inclusion complex formation. Because of that, the list of available solvents is mainly restricted to ethanol [35]. CDs are usually dissolved in water, and the complex formation occurs by mixing the ethanolic drug and aqueous CD solution. The resultant mixture may be further stirred, sonicated, or heated to promote drug/CD interaction. Heat must be used prudently, as excessive heat may lead to chemical degradation of the drug and/or inclusion complex dissociation due to high thermodynamic activity in the system at temperatures above 50 °C. In some cases, the drug may be dissolved directly in the CD aqueous solution. Also, other approaches like pH modification and salt formation may be beneficial in this regard, depending on the physicochemical characteristics of the drug. After the inclusion complexation is achieved in the solution, the solvent must be removed to isolate the CD complex in the solid state [37,38]. In the case of natural CDs, this may be obtained by precipitation or using other drying techniques like spray-drying and freeze-drying. Both techniques are suitable for the industrial production of CD complexes. The spray-drying technique is particularly suitable, as it ensures rapid solvent removal, thus preventing the dissociation of the complexes formed. Indeed, slow solvent removal in rotational vacuum evaporators may lead to complex dissociation, especially when the included compound has a very stable crystal lattice [48]. The drawback of the spray-drying technique is the fact that it is not suitable for thermolabile and volatile compounds, and the product yield is typically lower (40–79%), as some product particles are lost through the exhaust of the instrument or are attached to the walls of the drying chamber and cannot be collected [49]. Freeze-drying is a method of choice when dealing with CD complexes of the thermolabile compounds; however, in this case, the organic solvent (i.e., ethanol) must first be removed, as it interferes with the freezing step of this drying process. Finally, long duration and high energy consumption are other drawbacks of the freeze-drying process [50]. In this regard, new, more environmentally friendly, and sustainable methods must be considered for preparing CD complexes from the solution. One such method, preparing complexes using supercritical CO2, has great potential owing to its mild critical point that ensures the chemical stability of the drug, tunable solvent power, and the absence of solvent residue after depressurization [51]. Furthermore, the potential of green solvents, like ionic liquids and deep eutectic solvents as the complexation media should be further examined, as they provide an interesting opportunity to address the problem of the limited solubility of native CDs (i.e., the solubility of βCD may reach 1000 mg/mL at 20 °C) [52,53].
Kneading is a semi-solid state-based method where a drug/CD mixture is wetted with a small amount of water or water/ethanol mixture until the paste-like product is obtained. Such a product is then mixed (i.e., kneaded) for some time to promote drug/CD interaction. Finally, the added solvent is removed by drying at a reduced pressure, and the resultant product is milled and sieved. This method is usually used for compounds with limited solubility and results in the low content of the actual drug/CD complex [42].
Rising environmental awareness, the need for sustainability, and the implementation of a green chemistry approach are setting the focus on solid-state methods for the preparation of CD complexes. These methods eliminate problems related to the low solubility and chemical instability of drugs in solution, as well as the potential toxic effects of residual solvents. In this light, mechanochemical activation by grinding emerged as a rapid, highly efficient, solvent-free, sustainable approach [54,55]. Here, the complexation is obtained in high-energy vibrational or rotational mills, where the energy of the colliding balls (grinding bodies) is transferred to the treated material (equimolar drug/CD mixture) upon impact. This first causes the shredding of the treated materials up to a critical threshold, accompanied by intense mixing. Further energy supply by grinding leads to amorphization of the material and, consequently, its chemical activation, enabling drug/CD complexation in the solid state. However, parameters of the grinding process, like grinding frequency, time, and temperature at which the process is performed, must be carefully optimized to avoid degradation of the treated material [56]. Furthermore, the grinding process may raise unconventional supramolecular interactions that lead to novel molecular arrangements not observed in the solution, providing significant advantages in the formulation development [57].
It has been shown that the technique of CD complex preparation has an essential role on the physicochemical properties and the performance of the obtained product [47]. Furthermore, a comprehensive characterization of the solid product obtained is essential. This enables the selection of the most appropriate preparation technique and optimal technological parameters of the method applied to achieve the most effective drug/CD solid-state interaction in the product obtained, obtaining the desired increase in functionality [47,58].
Differential scanning calorimetry (DSC) is the most widely applied technique for characterizing CD complexes in the solid state [47,59]. This technique compares the thermal properties (most often melting temperature and fusion enthalpy) of the pure compounds (i.e., CDs and the drug of interest), their physical mixtures, and intended complexes. In general, reduction in the fusion enthalpy, shifting of the drugs’ melting peak to lower temperatures, or its complete disappearance is usually taken as an indication of the CD complexation in the solid state. However, it must be considered that the disappearance of the melting peak in the DSC thermogram of the analyzed product only indicates the drug amorphization that may be a consequence of many different non-inclusion phenomena during the CD complex preparation like fast solvent evaporation that disabled the crystal lattice formation or solid dispersion formation [60]. Also, heat-induced drug interaction brought up by thermal energy supply must be considered. Methylated CD derivatives appear especially prone to such interactions [48,61]. Because of that, DSC data must be further supported by complementary techniques like X-ray powder diffraction (XRPD). XRPD requires low sample quantity, is a non-destructive technique, and does not change the physicochemical properties of the sample during the spectra recording, providing additional insight into the solid state of the sample prepared. However, the sensitivity of XRPD in detecting the residual crystalline drug samples in the analyzed product is usually lower than that of DSC [62]. Regardless, the reduced crystallinity or complete amorphization of the drug in the sample observed by DSC and XRPD only indicates inclusion complexation. The only technique able to demonstrate the actual inclusion complex formation in the solid state is solid-state NMR (ssNMR). In particular, ssNMR can provide information on the orientation of the guest molecule inside the cavity and the complex stability in the solid state. Furthermore, it enables quantitative analysis of the phases, especially the complexed and non-complexed guest molecules. In addition, this technique allows for studying the local molecular dynamics of guest molecules and the nature of intermolecular interactions between the host and the guest [63]. The most widely applied ssNMR technique for the characterization of the CD complexes is the 13C cross-polarization magic-angle spinning (CP-MAS) analysis. However, this technique is somewhat laborious and expensive, requires long experimental time to obtain good-quality results, and signal assignment and data interpretation may be difficult, especially in the case of complexes of chemically modified CD derivatives [61].
Other techniques used for characterizing CD complexes in the solid state are Fourier transform infrared spectroscopy (FTIR), which provides insight into the chemistry of the solid-state interaction among included compounds and CDs, and scanning electron microscopy (SEM), a surface-imaging technique that detects changes in the sample morphology upon complexation with CDs [47]. Also, the drug content in the product obtained must be analyzed, as too harsh conditions of complex preparation (e.g., drying at too high temperatures or prolonged grinding at high grinding frequencies) may lead to drug degradation [56,64]. Furthermore, moisture, the content of residual solvents, and elemental impurities (for the complexes prepared by grinding) must be monitored, as they may impair the chemical stability and safety of the CD complexes [65]. Finally, there is a whole set of different functional tests, such as in vitro dissolution, permeation across biomimetic membranes or cell monolayers, antioxidant activity, particle size distribution, real-time and accelerated stability testing, pharmacokinetic studies etc., that enable the determination of the benefit obtained by CD complexation of the molecule of interest [33,61,64,66,67,68].
3. Antioxidants in Functional Foods and Nutraceuticals
3.1. Antioxidants and Their Mechanisms of Action
Antioxidants are compounds that can stop or slow down the oxidation of proteins, lipids, carbohydrates, and DNA in biological systems [69]. Superoxide dismutase (SOD), glutathione peroxidase, and catalase are examples of enzymatic antioxidants, while nonenzymatic antioxidants include glutathione, uric acid, alpha-lipoic acid, ubiquinones, bilirubin, metal chelators, vitamin C, carotenoids, tocopherols, and phenolics. Additionally, carotenoids, polyphenols, and tocopherols are examples of exogenous antioxidants, and the same should be sourced from the diet or supplementation [70]. Reducing oxidative stress and DNA damage are two of the primary benefits of antioxidants. In general, antioxidants work by interrupting chain reactions, mending damaged biomolecules, inactivating oxidants by adding H+, or transforming them into weaker molecules. Furthermore, they exhibit their effects by blocking lipid peroxidation chain reactions, accumulating reactive oxygen species, preventing lipid peroxidation, and actively participating in the transformation of peroxides into nonradical compounds [69]. Since antioxidants are essential for preserving health and preventing chronic diseases [71], the mechanisms of action by different groups of lipophilic antioxidants are shown in the following part.
Carotenoids are widespread natural pigments classified as bioactive compounds. They contribute to the yellow, orange, and red coloration of fruits and vegetables, such as tomato, carrot, apricot, and pepper, and the pigmentation of dark-green leafy vegetables, like spinach and kale [72]. Recognized as health promoters, carotenoids play an important role in human health and diet [73]. They reduce oxidative damage by capturing free radicals [74]. The same compounds play a significant part in the lipid oxidation process, scavenging singlet oxygen, peroxyl, sulfonyl, and NO2 radicals as well as providing defence against hydroxyl and superoxide radical attacks [70]. Furthermore, lycopene can scavenge superoxide radicals (LOO•) and singlet oxygen (1O2) [75]. It raises the concentrations of enzyme antioxidants as glutathione peroxidase, catalase, and superoxide dismutase. As a result, the antioxidant response element is activated, which is connected to nuclear factor E2 [76]. Additionally, non-enzymatic antioxidants like vitamins C and E can be renewed by lycopene. The cellular antioxidant defence system benefits from this [77], and it has been shown that lipids and DNA are two vital bodily components that lycopene can shield because of its antioxidant properties [78]. Furthermore, by immediately squelching reactive oxygen species, as well as by promoting glutathione synthesis in human retinal pigment epithelial cells, zeaxanthin demonstrates its antioxidant properties.
Vitamin E encompasses two families of lipophilic compounds: tocopherols and tocotrienols. Each family comprises four stereoisomers (α, β, γ, and δ), giving rise to eight distinct forms of vitamin E. Tocopherols are characterized by a saturated phytyl tail, whereas tocotrienols contain an unsaturated isoprenoid side chain, conferring unique biophysical and pharmacological properties to each isoform [79]. In typical diets, γ-tocopherol predominates, whereas most commercially available supplements deliver α-tocopherol, selected for its superior bioavailability and for establishing dietary reference intakes.
The primary role of vitamin E is to terminate lipid peroxidation by scavenging chain-propagating peroxyl radicals in cell membranes, thereby preventing oxidative damage to lipids, proteins, and DNA. It protects against lipid and lipoprotein peroxidation by acting as a potent scavenger of superoxide, peroxyl, and hydroxyl radicals [74,80]. Additionally, it has outstanding scavenging capabilities for reactive nitrogen species [81]. Beyond its classical antioxidant mechanism, individual tocopherols and tocotrienols exhibit divergent activities, including modulation of signal transduction pathways, gene expression, and inflammatory responses [82], which makes it an effective nutritional supplement and antioxidant with a wide range of potential in the food, pharmaceutical, and cosmetic [83]. Daily intake of this antioxidant will aid in raising superoxide dismutase levels [84] and promote the activity of several anti-oxidative enzymes, including peroxidase and catalase [80].
The ability of retinoids to quench 1O2, their high molar absorption coefficient, and their ability to lose protons when interacting with reactive species to form a less reactive radical centre stabilized by the polyene network are the three main ways that they shield cellular components from damage caused by photooxidation and reactive oxygen species (ROS) [85].
Retinoids include a family of molecules containing a 20-carbon structure with various chemical groups at the 15 carbon position. Variations at the 15 carbon position yield different retinoid forms, including retinol, retinal, retinoic acid, and retinyl ester. The most potent form of vitamin A, all-trans-retinol, is the form of retinol in the diet. It reverses signs and symptoms of vitamin A deficiency and is the standard for vitamin A activity [86]. Either pre-formed vitamin A from animal products (retinol) or provitamin A carotenoids from plant products is an acceptable vitamin A source, although provitamin A carotenoids must be consumed in larger quantities [87]. All-trans retinoic acid is the active form of vitamin A in almost all biological processes.
Retinoic acid regulates the expression of various genes that encode for structural proteins, such as keratins in the skin; enzymes, such as alcohol dehydrogenase; extracellular matrix proteins, such as the basement membrane protein laminin; and retinol binding proteins and receptors [88]. Vitamin A also regulates genes, including those of the dopaminergic system, in the brain, and central nervous system [89], and acts as a cofactor in mucopolysaccharide synthesis, cholesterol synthesis, hydroxysteroid metabolism, and glycoprotein glycosylation. Retinoids exhibit antioxidant activity primarily through their chemical structure, characterized by multiple conjugated double bonds that enable the quenching of ROS such as singlet oxygen and peroxyl radicals. This antioxidant action involves scavenging, inactivation, and termination of oxidative radical chain reactions, thereby protecting cellular components from oxidative damage. Mechanistically, retinoids stabilize free radicals by forming less reactive radical centres, aided by their polyene network. Additionally, retinoids influence antioxidant pathways by modulating enzymes such as SOD, catalase, and glutathione peroxidase, enhancing cellular defence against oxidative stress. These properties are important in various physiological contexts, including skin photoprotection and the mitigation of oxidative stress in disease states. However, the antioxidant effects can be complex, as retinoids may also induce oxidative stress under certain conditions, depending on dose and cellular context [85]. Vitamin A used in clinical research has included oral, topical, and injectable products. Most dosing recommendations for vitamin A are for pre-formed vitamin A (retinol). Vitamin A is required for vision, growth and bone development, reproduction, cell proliferation and differentiation, immune function, and the integrity of mucosal and epithelial surfaces.
CoQ10 is naturally found in all of our body’s cell membranes as the essential part of the mitochondria’s oxidative phosphorylation process [90]. Additionally, it is ideally situated near the unsaturated lipids in membranes, serving as a major scavenger of free radicals to prevent lipid peroxidation and shielding DNA and cellular membranes from oxidative damage [91]. Additionally, by maintaining nitric oxide, CoQ10 can enhance blood flow and protect blood vessels [90].
CUR exhibits potent antioxidant activity primarily due to its chemical structure, which contains phenolic hydroxyl groups and a conjugated diketone moiety. These structural features enable CUR to donate hydrogen atoms to neutralize ROS and free radicals, thus functioning as a chain-breaking antioxidant [92,93]. CUR enhances systemic indicators of oxidative stress by modifying the activity of the catalase, SOD, and GSH (glutathione), which are involved in the neutralization of free radicals [94]. It inhibits enzymes that produce ROS, including xanthine hydrogenase/oxidase and lipoxygenase/cyclooxygenase [95].
CAP exhibits significant antioxidant activity mainly through its free radical scavenging mechanisms. It primarily acts by hydrogen transfer (HT) from its phenolic OH group to neutralize ROS, effectively scavenging peroxyl and alkoxyl radicals and reducing lipid peroxidation in cellular membranes, mitochondria, and lipoproteins. This antioxidant effect helps prevent oxidative damage by preserving endogenous antioxidants like glutathione and restoring the activity of enzymes such as superoxide dismutase and glutathione reductase. CAP’s antioxidant capacity can surpass that of vitamin E, melatonin, and caffeine in some models, and its action is often mediated through the activation of the TRPV1 receptor and the Nrf2 signalling pathway, which enhances cellular defence mechanisms against oxidative stress. The aromatic benzene ring and the ortho-position methoxy and hydroxy groups in CAP’s structure play a crucial role in its radical scavenging efficacy. These combined mechanisms contribute to its protective effects against oxidative stress-related damage and inflammation in biological systems [96,97].
Table 2 presents the most important human intervention randomized clinical studies or meta-analyses performed in the past 10 years that investigated potential health effects that can be achieved by oral supplementation of lipophilic antioxidants indicating their relevance in contemporary clinical practice.

Table 2.
Health-promoting effects of oral supplementation of antioxidants.
Depending on the antioxidative mechanisms explained above, intake of carotenoids has shown positive benefits on cardiovascular, cognitive, and visual health. The two carotenoids that were evaluated the most in the clinical trials were lutein and zeaxanthin, compared to the β-carotene supplementation that is rarely studied as a single molecule [137]. Patients who took lutein supplements experienced a substantial increase in macular pigment optical density, according to the meta-analysis. The mentioned effect was determined by the supplementation in a daily dose of 20 mg for a period longer than 6 months [101]. Furthermore, lutein has been shown to improve cognition in both young and older adults [98]. According to Table 2, the improvement in visual memory and performance by lutein and zeaxanthin supplementation was also determined in a few other studies, in a recommended dose of 10 mg of lutein and 2 mg of zeaxanthin for six months [105]. Additionally, lutein has positive benefits on other tissues, including the brain, where it has been linked to enhanced cognitive function [138], but can also reduce skin inflammation, inhibit oncogenesis, and increase tolerance to UV exposure. Lycopene may be useful in the treatment of cardiovascular disease, according to studies by [109,110]. In a placebo-controlled, double-blinded, randomized study, performed by Grether-Beck and co-authors [103], three months of consumption of 5 mg of lycopene daily resulted in the protection of human skin against UV radiation. Wolak and co-authors [111] demonstrated efficacy in lowering systolic blood pressure in the hypertensive subject group. Supplementation with astaxanthin was monitored in a dose range from 2 to 12 mg up to six months.
A total of 400 IU daily was the dose of oral supplementation with vitamin E used in different human clinical studies. For example, Jaffary and co-authors [117] determined effectiveness with the four-month supplementation of patients with atopic dermatitis, without side effects. Vitamin E, a potent antioxidant, can help to safely alleviate clinical symptoms and lower oxidative stress conditions in people with late-stage knee osteoarthritis. The above-mentioned impact was determined following the two-month supplementation with 400 IU daily [118]. Moreover, it has been shown that oral retinoids, especially isotretinoin, are a successful treatment for acne vulgaris [139]. Isotretinoin has been indicated as a successful treatment for this inflammatory condition, particularly in instances that have left scars or have not responded to previous treatments. In that context, Dhaked and co-workers [121] have determined 20 mg for six months as an efficient therapy for mild to severe acne vulgaris. An evaluation of the pertinent literature indicates that oral isotretinoin is superior to oral antibiotics in achieving long-lasting remission following treatment [140].
CAP supplementation during high-intensity exercise could be employed to enhance muscular endurance and improve exercise performance in physically active adults in a dose of 12 mg 45 min before exercise [123,124,125].
CUR efficiency in the improvement of endothelial function was shown in the double-blinded randomized studies by Oliver and co-workers and Santos-Parker and co-workers [126,127], with the dose of 200–400 mg daily for 8–12 weeks. Furthermore, the potential antioxidative and anti-inflammatory effects of the CUR supplementation is shown in Table 2.
The beneficial effect of the oral CoQ10 supplementation was shown for a dose range from 100 to 600 mg daily, usually during a one-month to one-year period. Potential efficiency in the prevention of migraines and reduction in the frequency of headaches was detected by the consumption of 400 mg daily for three months, while a dose of 500 mg daily for one year relieves symptoms of depression and fatigue in patients with multiple sclerosis [90,134,136].
3.2. Bioavailability of Lipophilic Antioxidants
Oral administration is usually the recommended route of application for bioactive compounds with therapeutic or preventive advantages when patient compliance is essential. However, the hostile environment of the GIT can decrease the solubility, stability, and absorption of orally ingested bioactive substances [141]. Specifically, compounds with low water solubility do not dissolve easily in the GIT, and gastrointestinal barrier blocks penetration into the systemic circulation. Additionally, chemical and enzymatic degradation might decrease the quantity that is accessible for absorption [142]. In that context, antioxidant structure, half-life, interactions with other compounds, and delivery mechanisms all affect antioxidant bioavailability. Bioavailability refers to the portion of the compound that is absorbed into the bloodstream, distributed through the systemic circulation, exerts its post-metabolic biological effects, and is then eliminated [143]. Carotenoids’ low water solubility and vulnerability to light- and oxygen-driven deterioration limit their bioactivity [144]. Absorption can range from 5 to 50% and varies greatly based on other dietary components included in the meal [145]. Lycopene, as one of the main serum carotenoids found in human blood and tissues, has a half-life of two to three days within the body and a human plasma concentration that varies between 0.22 and 1.06 nmol/mL [146]. It has been shown that factors influencing lycopene bioavailability include cooking, food cutting or chopping, dosage, interactions of lycopene with other carotenoids, the presence of fats or oils, and the physiological state of the individual consuming the lycopene [147]. According to reports, lycopene is only 23% soluble when combined with oil and can reach 5% when ingested with tomato products or juice [148]. On the other hand, the usage of tocopherols is limited not only because of their low bioavailability, poor water solubility, and inconsistent absorption [149] but also stability influenced by exposure to light, oxygen, and high temperatures [150]. After 3–4 h, peak plasma levels of α-tocopherols are reached, whereas those of γ and δ tocotrienols are reached after 5 h of consumption [151]. Because of its higher binding affinity with the hepatic α-tocopherol transfer protein, only α-tocopherol has high levels in plasma and tissues among all isoforms. This allows it to remain in plasma for an extended period of time to exert its pharmacological effects, whereas other tocopherol isoforms are quickly metabolized and eliminated in feces [152]. Furthermore, isotretinoin is almost insoluble in water and extremely lipophilic. Even though the molecule easily passes across cell membranes, oral bioavailability of isotretinoin is limited due to poor solubility in the intestinal fluid environment [153]. Due to low water solubility, poor intestinal permeability, and hepatic metabolic biotransformation, over half of an oral dose of CUR is eliminated undisturbed in the feces, and very little CUR is detectable in plasma following low-dose supplementation [154,155]. According to research on humans, the peak plasma concentration of CUR is only 11.1 nmol/L 1–2 h after 3.6 g of CUR is consumed [156]. CoQ10’s bioavailability is also limited and is usually in the order of 5% or less, due to its unique chemical structure and poor water solubility [157,158].
In order to overcome the constraints of peroral administration and enhance the therapeutic effect resulting from poor stability/bioavailability, lipophilic antioxidants can be integrated into a biocompatible substrate to improve their stability, half-life, tissue-specific distribution, and bioavailability [91]. In that context, a lot of research has indicated that the encapsulation of antioxidants can result in advantageous effects, protection of the bioactive principle, controlled release, a reduction in the amount of biocompound needed, and increased antioxidant efficacy before intake [159,160,161,162].
3.3. Challenges in Formulating Lipophilic Antioxidants
The most common antioxidant groups, including carotenoids, tocopherols, and retinoids, comprise highly hydrophobic molecules which, with their aqueous solubility of around 0.01 mg mL−1, are considered practically insoluble [163,164]. As explained previously, it contributes to their low bioavailability, which is further affected by other factors such as food processing, meal composition, and interactions within the gastrointestinal system [165,166]. Furthermore, exposure to high temperatures, oxygen, and/or light may hinder the antioxidants’ stability, considering their susceptibility to chemical degradation in suboptimal conditions [167,168]. To overcome these challenges, many strategies are being employed, including optimizing the processing conditions, chemical and physical modification, and incorporating the molecules in various formulations, mostly lipid-based delivery systems [169]. The most commonly developed antioxidant-loaded delivery systems for food application are lipid-based nanosystems, including vesicular systems such as liposomes and niosomes, as well as solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), and nanoemulsions [170].
Liposomes are spherical nanoparticles composed of one or more phospholipid bilayers, whereas niosomes usually comprise synthetic, non-ionic surfactants, usually with only one hydrophobic chain [167,171]. Due to the amphiphilic nature of these constituents, both liposomes and niosomes may be used for the encapsulation and delivery of both hydrophilic and hydrophobic molecules [167]. In food industry, liposomes are mostly used as texture- and water retention-modulators, but recent trends lean towards utilizing liposomes for encapsulation of sensitive food molecules, including antioxidants such as resveratrol, CUR, β-carotene, and apigenin [172]. Similarly, niosomes are also employed for lipophilic antioxidants encapsulation [173] but are characterized by the lower production cost, simpler preparation methods, as well as a better stability profile compared to liposomes [171].
Lipid nanoparticles may be categorized depending on the state of matter of the comprising lipids: SLNs are nanoparticles, which consist of lipids in solid form at both physiological and storage/room temperature, dispersed in water, and stabilized by one or more surfactants [166], while NLCs comprise the combination of liquid and solid lipids, creating a less-ordered structure. Both of these nanosystems are under investigation for food optimization and lipophilic compound encapsulation, mainly carotenoids, tocopherols, and polyphenols [174].
Nanoemulsions used for the enhancement of delivery of lipophilic antioxidants in nutrition products are almost exclusively oil-in-water (o/w) emulsions, defined as colloid aqueous dispersions in which nanoscale oil droplets are stabilized by emulsifying agents such as surfactants and polymers [167]. So far, their use has been investigated for the incorporation of essential oils, alone or in combination with lipophilic bioactive compounds such as carotenoids, tocopherol, or retinol [175]. Compared to the nanoparticles such as liposomes, SLNs, and NLCs, preparation of nanoemulsion is simpler and less expensive; however, its addition to the food may alter organoleptic properties, potentially impacting consumer acceptance [167].
Lipid-based delivery systems are one of the main strategies for improvement of the solubility and bioavailability of water-insoluble antioxidants. They consist of biocompatible excipients that protect the encapsulated molecules from degradation during processing, storage, and digestion, while enabling improved dissolution in the GIT [176]. Nevertheless, despite their obvious advantages, lipid-based systems as delivery methods for lipophilic antioxidants are still not widely used in food technology. This is most likely due to the complexity and the high cost of manufacturing of such formulations [170]. Furthermore, the preparation of lipid-based delivery systems usually requires specialized equipment which then poses a challenge in the case of production scale-up. Also, it is important to take into consideration the influence of these forms on the appearance, smell, and taste of the food. Due to these limitations, some other formulation strategies for lipophilic antioxidants are being investigated, including the development of protein-based or polysaccharide-based delivery systems, the most common of which are CD-based formulations [167].
4. Cyclodextrin-Based Formulations of Lipophilic Antioxidants
As stated previously, CD encapsulation constitutes a pivotal strategy in the formulation of lipophilic compounds, notably antioxidants, by significantly enhancing their aqueous solubility, chemical stability, and bioavailability. When contrasted with alternative formulation approaches such as lipid-based nanoformulations—including liposomes and solid lipid nanoparticles—CD inclusion complexes present several fundamental advantages. Formulation of lipid-based carriers is often complex and expensive, requiring specialized equipment, which complicates the possibility of production scale-up. Additionally, their production necessitates the use of surfactants, organic solvents, and complex processing conditions that may raise concerns regarding biocompatibility and residual solvent toxicity. Conversely, CD complexation represents a simpler approach and typically proceeds via spontaneous, non-covalent interactions in aqueous media, circumventing the extensive use of potentially harmful solvents [177,178]. From an environmental and sustainability perspective, CD-based formulations offer substantial benefits since they are derived from renewable starch sources through enzymatic processes, ensuring their biodegradability and low ecological impact. CD complexation generally minimizes or eliminates the use of hazardous organic solvents, thereby reducing solvent waste and environmental emissions [179]. Furthermore, advancements in green chemistry have facilitated the development of environmentally benign synthesis methodologies for CD inclusion complexes. As explained previously, supercritical CO2 has emerged as a non-toxic solvent medium facilitating complex formation under mild conditions without solvent residues [51]. Other green solvents such as ionic liquids and deep eutectic solvents are under active investigation for their potential to further reduce the environmental footprint of complex preparation, and solid-state methods for the preparation of CD complexes are also emerging [52,53]. This aligns with principles of sustainable pharmaceutical and nutraceutical manufacturing, offering routes to minimize energy consumption and ecological burden without compromising product efficacy.
4.1. Cyclodextrin-Based Formulations of Carotenoids
Carotenoids are highly lipophilic compounds, soluble in organic solvents and insoluble in water. They are sensitive to pH, temperature, light, and oxygen [72]. When present within the food matrix in which they are synthesized, they are relatively stable, but once isolated, carotenoids become highly unstable, limiting their use as food ingredients due to degradation, colour changes, and loss of nutritional value [180]. To overcome such limitations and improve the stability, solubility, and bioavailability of carotenoids, CDs are used [72]. As mentioned previously, CDs are popular delivery systems, with a hydrophilic surface and a hydrophobic cavity, which makes them suitable for the encapsulation of lipophilic guest molecules. One of the newer delivery systems being used for carotenoids are nanosponges, which are mesh-like 3D structures with a hydrophobic interior and hydrophilic exterior, often formed by crosslinking CDs with various compounds [181]. Similarly to CDs, nanosponges improve the solubility, stability, and bioavailability of both water-soluble and lipid-soluble drugs.
Table 3 provides a detailed overview of recent studies on inclusion complexes formed between various carotenoids and CDs over the past decade. It summarizes the types of carotenoids examined, the types of CDs used, as well as the preparation methods applied. As shown in Table 3, βCD is one of the most commonly used CDs, and β-carotene is one of the most studied carotenoids. The primary objectives of the presented studies were to enhance solubility, improve stability (storage, heat, colour, and photostability), bioavailability, antioxidant activity, and other biological effects. Literature data show that CD complexation increased carotenoid solubility and slowed down the intestinal release of β-carotene in vitro, leading to improved bioaccessibility. Additionally, all conducted studies showed significantly improved stability of carotenoid-CD complexes, compared to native compounds (Table 3). On the other hand, CD complexation either increased or decreased measured antioxidant activity. For this purpose, various methodologies were employed by the authors, including simple in vitro assays (DPPH, TEAC, FRAP, CUPRAC), cytotoxicity testing, and animal studies (Table 3). Obtained results varied depending on guest, CD derivative, solvent/media, and applied assay. Variability of results in simple in vitro assays may arise from the antioxidant’s ability to interact with specific reagents in the reaction mixture, influenced by the polarity of the solvent or precise structure of the cyclodextrin complex. In cell-based or in vivo assays, factors such as the type of cell line used, gastrointestinal stability, or cell membrane permeability may affect results. Therefore, interpretation of these findings should be approached with caution. Given the well-documented limitations of in vitro and cell-based antioxidant assays—including their lack of physiological relevance and failure to account for absorption, metabolism, and bioavailability—it is essential to prioritize the findings of in vivo studies. Results from in vitro investigations should primarily be used to compare the relative antioxidant capacities of different cyclodextrin derivatives or methods of inclusion complex preparation and should not be extrapolated to predict in vivo effects.

Table 3.
Cyclodextrin inclusion complexes with carotenoids.
As presented in Table 3, the inclusion complex of β-carotene and βCD prepared by co-precipitation, as well as the complex prepared by kneading and drying at 50 °C in 1:1, 1:2, and 1:3 molar ratios, showed more negative values of the zeta potential compared to free β-carotene, indicating that the formation of the inclusion complex led to an increased stability [182,191]. The solubility test of the β-carotene/βCD complex formed by extended co-precipitation showed twice the concentration (1.2 µg of β-carotene per mg of complex) compared to the complex formed by co-precipitation (0.6 µg of β-carotene per mg of complex) [186]. β-carotene encapsulated in three derivatives of βCD, methyl-β-cyclodextrin (MeβCD), HPβCD, and 2-hydroxyethyl-β-cyclodextrin (HEβCD), prepared by the precipitation method and vacuum drying, showed lower antioxidative and radical scavenging activity compared to free β-carotene. The highest antioxidant activity was observed in the complex with HEβCD and the lowest in the complex with MeβCD, which can be related to the deeper and more hydrophobic cavity. Çelik and co-workers and Puebla-Duarte and co-workers [72,191] reported similar antioxidant activity of the complex β-carotene/βCD compared to free β-carotene.
Regarding the inclusion complex of β-carotene and βCD, in vitro availability of β-carotene from the inclusion complex was investigated and evaluated through in vitro digestion, using rice as a food matrix. The complex was prepared by physical blending, kneading, and co-precipitation, as well as by co-precipitation followed by freeze-drying [179,181]. Unchanged or increased release of β-carotene was observed at particular stages of digestion, possibly due to the potential interference of rice as a food matrix. In vitro release from the inclusion complexes prepared with water-dispersible β-carotene extracted with absolute ethanol decreased consistently and was not influenced by the presence of rice as a food matrix, whereas hexane-soluble β-carotene extracted with ethanol:hexane mixture exhibited gradual release during intestinal digestion, but its release was reduced in the presence of rice as a food matrix [185,194]. They used three types of βCD-based nanosponges and reported improved in vitro release under physiological conditions. Furthermore, the complex exhibited an increased cytotoxic effect in both normal and kidney cancer cells compared to free β-carotene [194].
CD-based inclusion complexes with other types of carotenoids were investigated as well, including bixin, astaxanthin, fucoxanthin, lutein, zeaxanthin, and crocetin. Pinzon-Garcia and co-workers [190] investigated the effects of oral administration of the inclusion complex of bixin and βCD, prepared by mixing solutions and freeze-drying, on insulin resistance in high-fat-fed C57BL/6 obese mice. Oral administration of the inclusion complex not only resulted in a reduction in body weight, Lee’s index, and relative adipose tissue weight, but also significantly decreased serum cholesterol (CHT), triglycerides (TG), the CHT/HDL-c ratio, and glucose levels. The inclusion complexes of astaxanthin/MeβCD exhibited improved inhibition activity compared to pure astaxhantin, possibly due to enhanced solubility of astaxanthin and higher transport through the cell membrane [189]. Similar observations were made for fucoxanthin/HPβCD complex [192]. The investigation of applicability of lutein- and zeaxanthin-CD inclusion complexes showed improved solubility and permeability compared to native compounds. Among different tested CDs, RAMEB demonstrated the highest solubilizing efficiency, reaching solubility values of 572 ± 55 μM for lutein and 1470 ± 103 μM for zeaxanthin. Both in vitro and ex vivo porcine eye models showed that RAMEB-based formulations significantly enhanced the permeability of the carotenoid derivatives by increasing their solubility, while Raman mapping confirmed that CDs can enhance corneal permeability, even for highly lipophilic compounds [195].
Crocetin has been recognized as an active compound with pharmacological activities, particularly relevant for Alzheimer’s disease. γCD-based inclusion complex with crocetin was formed by dissolution and ultrasonic homogenization, followed by membrane extrusion. The use of γCD has facilitated the passage of crocetin through the blood–brain barrier, which makes it a potential delivery system to treat Alzheimer’s disease [196].
4.2. Cyclodextrin-Based Formulations of Tocopherols and Tocotrienols
Despite its recognized therapeutic potential, vitamin E (particularly α-tocopherol) is chemically labile, exhibiting high susceptibility to degradation under heat, light, oxygen exposure, and alkaline conditions [198]. Additionally, its strong lipophilicity leads to poor aqueous solubility, which limits bioavailability and poses significant challenges for formulation in both food and pharmaceutical applications [199]. Co-administration with dietary lipids has been shown to enhance intestinal absorption, likely through micelle-mediated transport mechanisms [200]. To overcome these physicochemical limitations—specifically poor water solubility and chemical instability—various esterified derivatives, such as tocopheryl acetate, have been developed. These derivatives offer improved oxidative and thermal stability and are less susceptible to isomerization [201]. However, recent evidence suggests that complexed free tocopherol, the natural and biologically active form, may exhibit greater stability than its esterified counterparts under certain conditions [202]. This may explain why most studies involving CD inclusion complexes have utilized tocopherol rather than tocopheryl acetate.
Several review articles have already summarized earlier studies, which predominantly involved simple CD-based formulations of tocopherols. For instance, López-Nicolás [203] reviewed the literature on complexes formed between CDs and various antioxidant compounds, including tocopherols. The review highlighted inconsistencies in the reported data, including conflicting findings on the antioxidant activity of host–guest complexes, variations in complexation constants for ostensibly identical systems, and differences in the bioavailability of antioxidant compounds upon complexation with CDs. The study also discussed recommendations regarding the use of natural versus chemically modified CDs. Subsequent reviews by Uekaji and co-workers and Wupper and co-workers [204,205] focused specifically on the influence of γCD on the bioavailability, stability, and bioactivity of nutraceutical compounds.
Different CDs were investigated for encapsulating tocopherols, including all native CDs, and their hydroxypropylated versions, as well as octenyl succinic βCD. In several studies, large ring CD were used which included up to 54 glucose units. More recent studies incorporated CDs in more complex formulations such as copolymers, Pickering emulsions, and hydrogels in order to improve tocopherol solubility/bioavailability (Table 4).
Native βCD and HPβCD are the most extensively studied derivatives for tocopherol inclusion. Iaconinotoand and co-workers [206] demonstrated that βCD–α tocopherol complexes prepared by kneading did not alter photostability or antioxidant activity, whereas HPβCD afforded improved storage stability at the expense of enhanced photostability. Conversely, Lange and co-workers [207] reported that lyophilized complexes of βCD and HPβCD markedly enhanced α-tocopherol thermal stability, with native βCD surpassing HPβCD, highlighting the critical influence of complexation methodology on protective efficacy. They also reported that the kneading method was ineffective for preparing the inclusion complex with the hydroxypropyl derivative of β-CD. This confirms that the method of complex preparation critically determines the protective effect of CD on tocopherol.
It seems that lyophilization enables more complete encapsulation, which results in enhanced thermal stability, whereas kneading may not sufficiently protect tocopherol during storage due to incomplete complexation. That is in line with studies comparing preparation methods for CD complexes that consistently report that lyophilization and solvent evaporation produce inclusion complexes with superior stability and dissolution properties compared to kneading [47]. Moreover, Celebioglu [208] showed improvement of stability, solubility, antioxidative activity, and photostability after electrospinning of inclusion complex between HPβCD without using a polymeric matrix to produce nanofibers. This simple approach could enable the usage of HPβCD for tocopherol applications in food, pharmaceuticals, and healthcare, thanks to the efficient antioxidant activity along with enhanced water solubility, prolonged shelf-life, and high photostability of Vitamin E.
Large-ring CDs (LRCDs) consisting of 22–54 glucose units and amphiphilic derivatives such as octenyl succinate/βCD have been explored for enhanced solubilization and emulsification of vitamin E. Kuttiyawong and co-workers [209] achieved an 800-fold increase in water solubility of vitamin E acetate using large ring-CD22–CD54 (10:1 ratio), albeit with a 30% reduction in radical-scavenging activity. Cao and co-workers [210], on the other hand, used LRCDs (C9–C22) and a completely different tocopherol:CD ratio (1:2) but also showed great potential of LRCD to improve the thermal stability and antioxidant activity of tocopherol. These studies could indeed serve as a reference for the more effective use of LRCDs as antioxidant carriers in the future. Recent studies employed octenyl succinic derivatives of βCD. In study of Ke and co-workers [211], encapsulation in octenyl succinic βCD enabled significantly better antioxidative activity of tocopherol, and it was able to improve the physical and oxidative stability of the emulsion, which is of great significance to the food industry since the complex addition to the emulsions significantly improved emulsifying properties (particle size distribution, z-potential, and creaming index).
As previously mentioned, βCD complexes with tocopherol were also used to form more complex formulations. Singh and co-workers [212] successfully formulated hyaluronic acid/βCD grafted copolymer to improve tocopherol solubility, while two recent studies also investigated different formulations with βCD as a component to examine formulation impact on tocopherol bioavailability in vivo. These highly valuable results confirmed that both approaches succeeded in improving tocopherol bioavailability.
Eid and co-workers [213] used an approach targeted towards the development of βCD hydrogel nanocomposites, which enhanced the controlled release and bioavailability of vitamin E. Additionally, the oral administration of these CDs with vitamin E to rats resulted in a sustained increase in the vitamin levels in plasma, even after 12 h post-administration, which proves the increase in the pharmacological bioavailability of vitamin E when incorporated into this type of delivery system [214]. However, no control sample using only βCD complex was included in the study.
Cheng and co-workers [215] formulated cinnamaldehyde/βCD Pickering emulsions and enhanced oral bioavailability of α-tocopherol in vivo. However, this study also did not include a control sample (pure α-tocopherol), and only the βCD complex was included.

Table 4.
Cyclodextrin inclusion complexes with tocopherols (years 2015–2025).
Table 4.
Cyclodextrin inclusion complexes with tocopherols (years 2015–2025).
| Tocopherol | Cyclodextrin; Tocopherol:Cyclodextrin Ratio (molar) | Technology of Preparation | Characterization | Target Functionality Aspect | Effect | Reference |
|---|---|---|---|---|---|---|
| alpha tocopherol | βCD | kneading | NMR | Photostability | ≡ | [206] |
| Stability | ≡ | |||||
| Antioxidative activity | ≡ | |||||
| HPβCD; 1:1 | Photostability | ↘ photostability | ||||
| Stability | ↗ stability | |||||
| Antioxidative activity | ≡ | |||||
| HPγCD | Photostability | ↘ photostability | ||||
| Stability | ↗ stability | |||||
| Antioxidative activity | ≡ | |||||
| βCD; 1:1 | freeze-drying | DSC, FTIR, NMR | Thermal stability | ↗ thermal stability | [207] | |
| HPβCD; 1:1 | ||||||
| HPβCD; 1:1; 1:2 | nanofibres; electrospinning | NMR, XRD, FTIR, DSC | Stability | ↗ stability | [208] | |
| Solubility | ↗ solubility | |||||
| Antioxidative activity | ↗ antioxidative activity | |||||
| Photostability | ↗ photostability | |||||
| osβCD; 1:12 | freeze-drying | FTIR, NMR, SEM, AFM | Antioxidative activity | ↗ antioxidative activity | [211] | |
| Emulsifying properties (particle size distribution, ζ-potential, and creaming index) | ↗ emulsifying properties | |||||
| LR-CD (CD9–CD22); 1:2 | coprecipitation | SEM, FTIR, NMR | Thermal stability | ↗ thermal stability | [210] | |
| Stability | ↗ stability | |||||
| Hyaluronic acid-βCD Grafted Copolymer | freeze-drying | FTIR, NMR, SEM, XRD, | Solubility | ↗ solubility | [212] | |
| βCD Hydrogel nanocomposites; 1:8 | coprecipitation; suspension chemical crosslinking | FTIR, DSC, XRD, SEM, TEM, AFM, CLSM | Bioavailavility | ↗ bioavailability | [213] | |
| βCD/cinnamaldehyde based Pickering emulsions | freeze-drying; high speed shear emulsification | / | Bioavailavility | ↗ bioavailability | [214] | |
| tocopheryl acetate | βCD; 1:1 | freeze-drying | IR, TGA, DSC | Stability | ↗ stability | [202] |
| Photostability | ↗ photostability | |||||
| LR-CD (CD22–CD54); 10:1 | coprecipitation | FTIR | Solubility | ↗ solubility | [209] | |
| Antioxidative activity | ↘ antioxidative activity |
βCD (beta cyclodextrin); HPβCD (hydroxypropyl beta cyclodextrin); HPγCD (hydroxypropyl gamma cyclodextrin); osβCD (octenyl succinic beta cyclodextrin); LR-CD (large rig cyclodextrin); FTIR (Fourier transform infrared spectroscopy); TGA (thermogravimetric analysis); NMR (nuclear magnetic resonance); DSC (differential scanning calorimetry); XRD (X-ray Diffraction); SEM (scanning electron microscopy); AFM (Atomically Resolved Imaging); TEM (transmission electron microscopy); CLSM (Confocal Laser Scanning Microscopy); IR (Infrared Resonance); ≡ (no effect); ↗ (increased); ↘ (decreased).
4.3. Cyclodextrin-Based Formulations of Retinoids
Retinoids exhibit high sensitivity to light and heat, primarily due to their conjugated polyene structure. This configuration renders it susceptible to decomposition, structural rearrangement, and oxidative degradation in the presence of UV radiation and atmospheric oxygen [216]. Additionally, vitamin A demonstrates poor solubility and limited dispersibility in aqueous environments. These physicochemical limitations—namely, low water solubility and instability—significantly restrict its application in the food additive and cosmetics industries [199]. To address these issues, ester derivatives of vitamin A, such as retinyl acetate and retinyl palmitate, have been developed. These derivatives exhibit improved chemical and thermal stability and are less prone to oxidation and isomerization. Consequently, they are commonly employed as alternative sources of vitamin A. However, esterification does not alter the inherent polyenic structure of the molecule, and thus challenges related to stability and water solubility persist. To overcome these limitations, various strategies have been explored to enhance the stability of retinoids [216]. Among these, CD encapsulation has emerged as one of the most extensively studied approaches (Table 5).

Table 5.
Cyclodextrin inclusion complexes with carotenoids (years 2015–2025).
Studies that investigated retinoid CD encapsulation usually focused on retinyl esters, acetate, and palmitate, and less frequently on retinol and retinoic acid. βCD, in particular, has garnered significant attention in presented studies, while simple CD derivatives such as HPβCD and HPγCD were used less frequently. More complex fatty amide-CD conjugates were also investigated. γCD was incorporated into metal–organic frameworks (MOFs), complex systems composed of metal clusters and organic linkers, as well as ternary complexes, were obtained using βCD and HPγCD and the amino acid arginine (Table 5).
Vilanova and co-workers [217] and Xu and Fathalla [218] successfully applied βCD for formation of inclusion complexes with retinoids. In all studies, a 1:1 molar ratio of retinoid to CD was used, except for retinol, which required a 1:2 ratio. Encapsulation led to enhanced solubility of the compounds, and, in Vilanova’s study [217], improved photostability was also observed.
Celebioglu and co-workers [219] demonstrated that electrospun inclusion complexes of retinyl acetate with HPβCD and HPγCD, prepared without a polymeric matrix, significantly improved the compound’s thermal stability, solubility, and antioxidant activity. Notably, the HPβCD complex exhibited superior solubilizing capacity, higher release efficiency, and stronger antioxidant potential compared to the HPγCD complex. Based on these findings, the authors proposed the use of electrospun CD-based nanofibers as a novel carrier system for food and dietary supplements, offering improved delivery and rapid oral dissolution characteristics.
More complex chemical systems involving covalently modified CDs—resulting in discrete or extended supramolecular entities—have also been employed for the encapsulation of retinoids. Kim and co-workers [220] developed amphiphilic βCD derivatives capable of forming micellar aggregates or vesicular structures, which are particularly advantageous for applications in surface activity enhancement, solubilization, and drug delivery. Their approach involved the covalent conjugation of fatty acids to the primary hydroxyl side of βCD, aiming to improve both the encapsulation efficiency and photostability of all-trans-retinol. Specifically, stearic and oleic acids were selected as model lipophilic moieties. The resulting mono-substituted CD derivatives—mono[6-deoxy-6-(octadecanamido)]-βCD and mono[6-deoxy-6-(octadecenamido)]-βCD—were shown to self-assemble into nano-vesicles in aqueous environments. These vesicles effectively encapsulated all-trans-retinol and significantly enhanced its stability against photo-degradation. Due to their amphiphilic nature and self-assembling capabilities, these modified CDs present a promising platform for retinoid delivery in both cosmetic and pharmaceutical formulations.
Zhang and co-workers [221] explored the use of MOF carriers for the stabilization and delivery of retinyl palmitate in food and pharmaceutical applications. For that purpose, MOFs were constructed using γCD as the organic ligand and potassium ions as the metal centres, forming highly porous, biocompatible materials suitable for bioactive compound delivery. Notably, retinyl palmitate encapsulated within the γCD-based MOFs (γCD/MOFs) exhibited significantly improved stability compared to commercial reference formulations, and an enhanced protective effect was attributed to the molecular conformation of retinyl palmitate within the MOF structure, which provided a physical barrier against environmental degradation.
In summary, CD encapsulation of different retinoids has been clearly shown to be an effective way to boost solubility and stability in vitro. The remaining task is to deepen our mechanistic understanding, demonstrate clear in vivo benefits, and develop scalable formulations. If these can be achieved, CD–retinoid complexes could advance to practical products for dermatology, ophthalmology, and nutrition.
4.4. Cyclodextrin-Based Formulations of Capsaicin, Coenzyme Q10, and Curcumin
CAP is the main active component of chilli peppers and is widely explored for pain management and as a dietary supplement. Its broader use as a dietary ingredient is limited by irritation of the GIT and high lipophilicity, which results in very low water solubility [222]. To address these limitations, advanced pharmaceutical formulations are being developed, including CD-based complexes (Table 6).
The effect of complexation on GI irritation was examined by Zhao and co-workers [223], who fed rats either free CAP or HPβCD/CAP. Histopathological analysis showed reduced gastric mucosal irritation with the complex, indicating its potential as a safer dietary formulation. The same study also demonstrated significantly higher bioavailability of HPβCD/CAP compared with free CAP, though human trials remain necessary. Chen and co-workers [224] evaluated CAP bioavailability following subcutaneous administration in rats. Plasma CAP was detectable at 10 min for HPβCD/CAP, compared with 80 min for free CAP, and overall bioavailability increased 2.36-fold. CAP is also used clinically for pain relief, either alone or as an adjuvant. Couto and co-workers [225] reported improved analgesia in mice when HPβCD/CAP was combined with mepivacaine; however, the study lacked a mepivacaine + free CAP group, preventing a direct comparison between the inclusion complex and pure CAP. To mitigate CAP cytotoxicity, Kadian and co-workers [226] developed βCD-based nanosponges for potential arthritis therapy. In vitro studies on macrophage cell lines showed reduced cytotoxicity relative to free CAP, alongside comparable anti-arthritic effects. Statistical comparisons with free CAP were not fully reported, leaving the magnitude of difference unclear.
Overall, these studies demonstrate that CDs can successfully form CAP inclusion complexes, with improved physicochemical properties, reduced GI irritation, and increased bioavailability.
The major pharmaceutical issue of CoQ10 delivery is its high molecular weight and poor water solubility, leading to poor oral bioavailability. To overcome these limitations, different advanced drug delivery systems were designed, with special emphasis on pharmacokinetic perspectives and clinical relevance. They include nanoparticles, solid dispersions, liposomes, nanoemulsions, self-emulsifying drug delivery systems, nanostructured lipid carriers, CDs, and nanocapsules [227]. CD-based formulations are presented in Table 6.
Gao and co-workers [228] explored αCD, βCD, γCD, and HPβCD complexes, followed by an in vivo dog study. Among the tested systems, γCD/CoQ10 showed the most favourable physicochemical profile. Beagle dogs administered γCD/CoQ10 exhibited significantly higher plasma CoQ10 levels than the γCD/CoQ10 physical mixture or free CoQ10, suggesting that γCD is promising for dietary formulations. Takahashi and co-workers [229] examined αCD, βCD, and γCD complexes in 20 fasting female volunteers. Bioavailability was assessed by plasma Cmax and AUC, again identifying γCD-CoQ10 as optimal. A separate clinical trial compared three CoQ10 formulations: βCD-CoQ10 liquid (commercial Q10VITAL®), βCD-CoQ10 powder, and CoQ10 in soybean oil. βCD-CoQ10 in liquid form produced the highest absorption and bioavailability, exceeding both the powder and oil formulations. CD complexation can also enhance CoQ10 stability. Fir and co-workers [230] reported that βCD and γCD complexes improved light and temperature resistance, with βCD showing superior photoprotection. Phase-solubility tests likewise indicated that βCD achieved stronger CoQ10 solubilization than γCD. Taken together, these findings support the use of CDs—especially γCD and βCD—as effective carriers to improve CoQ10 solubility, bioavailability, and stability.
CUR is another important lipophilic antioxidant, the primary bioactive in turmeric. It has anti-inflammatory and anticancer potential but suffers from high lipophilicity and low bioavailability. To overcome these limitations, it is often incorporated into advanced pharmaceutical formulations, including CD-based formulations (Table 6).
Paramera and co-workers [231] developed βCD/CUR complexes to evaluate stability under light, heat, and humidity, as well as behaviour in simulated gastric fluid (SGF) and simulated intestinal fluids (SIFs). The dissolution rates in SGF were high and the complex degraded in SIF, indicating it cannot withstand gastrointestinal conditions long enough to reach the small intestine intact for efficient absorption. Thermal stability also declined relative to free CUR, although preservation under elevated temperature and humidity was improved. Huand and co-workers [232] and Xu and co-workers [233] addressed these shortcomings using succinylated βCD complexes. Testing under natural light/temperature and SGF/SIF conditions revealed markedly improved storage, thermal, and photochemical stability, as well as retained gastrointestinal stability. This highlights how chemical modification of CDs can modulate complex properties. Furthermore, Song and co-workers [234] conducted an in vivo study in beagle dogs, showing that βCD/CUR achieved 231.9% relative oral bioavailability compared to free CUR. Zhang and co-workers [235] examined nasal delivery for Alzheimer’s therapy: in mice, CUR plasma and brain concentrations were 2.57-fold and 1.12-fold higher, respectively, compared to conventional CUR formulation, suggesting enhanced uptake.
Difluorinated curcumin (CDF) has also been tested. In mice, intravenous βCD/CDF improved bioavailability and pancreatic accumulation, while in vitro studies showed lower IC50 values in cancer cell lines. Zhang and co-workers [236] reported stronger antitumor activity of βCD/CUR compared to CUR in a mouse hepatoma model. Mechanistic studies in lung tumour cells indicated that βCD/CUR up-regulated p53/p21 pathway, down-regulated the CyclinE-CDK2 combination, and increased Bax/caspase 3 expression through the regulation of MAPK/NF-κB pathway, resulting in induced cellular apoptosis. Patro and co-workers [237] compared αCD, βCD, and γCD/CUR complexes in rabbits. Relative bioavailability was highest for αCD (460%) and βCD (365%), with γCD showing minimal improvement (99%). Advanced CD-based formulations continue to emerge. βCD nanosponges enhance solubility and stability, with crosslinker content strongly influencing properties [238], as do HPβCD and HPγCD/CUR nanofibers [239]. Celebioglu and co-workers demonstrated greater solubility, antioxidant activity, and complete disintegration in simulated saliva, supporting food or packaging applications.
γCD MOFs prepared by Sun and co-workers [240] exhibited good stability under light, heat, gastric pH (1.2), and PBS. Cai and co-workers [241] further evaluated γCD-MOF/CUR for fruit preservation, reporting improved light and oxygen barrier properties, though reduced water barrier capacity. Mechanical parameters (tensile strength, elongation at break) were favourable, and antibacterial activity with good biocompatibility was observed, leading to convincing evidence of enhanced fruit preservation. Nanotechnological approaches also improve anticancer efficacy. Wei and co-workers [242] demonstrated βCD nanoparticles reduced tumour volume and size in mice, reflecting a stronger tumour inhibition rate.

Table 6.
Cyclodextrin inclusion complexes with capsaicin, CoQ, and curcumin (years 2015–2025).
Table 6.
Cyclodextrin inclusion complexes with capsaicin, CoQ, and curcumin (years 2015–2025).
| Cyclodextrin | Technology of Preparation | Characterization | Target Functionality Aspect | Effect | Reference |
|---|---|---|---|---|---|
| Capsaicin | |||||
| HPβCD | Magnetic stirring | UV, IR, DSC | solubility, oral bioavailability, gastric irritation | ↑ solubility ↑ oral bioavailability ↓ gastric irritation | [223] |
| HPβCD | freeze-drying | DSC, XRD, ITC, NMR SEM, | solubility | ↑ solubility sustained release kinetics ↑ pain control in combination with mepivacaine compared to mepivacaine alone | [225] |
| HPβCD | saturation method | DSC, XRD | bioavailability, pharmacokinetics | ↑ bioavailability faster absorption | [224] |
| βCD nanosponges | melt method, freeze-drying | UV, DSC, FTIR, XRD, NMR, RS, SEM, ELS | solubility, toxicity, anti-arthritic activity | ↑ solubility ↓ toxicity = anti-arthritic activity | [226] |
| Coenzyme Q10 | |||||
| αCD βCD γCD HPβCD | kneading method | XRD, DSC | oral bioavailability | ↑ bioavailability of q10 in γCD complexes | [228] |
| βCD γCD | precipitation, freeze-drying | FTIR, DSC, TGA, XRD | solubility, thermal stability, photochemical stability | ↑ solubility (γCD > βCD) ↑ thermal stability (βCD > γCD) ↑ photochemical stability (βCD > γCD) | [230] |
| αCD βCD γCD | high-pressure homogenization, spray drying | DSC | solubility, oral bioavailability | ↑ solubility (γCD) ↑ bioavailability (γCD) | |
| βCD (Q10VITAL®) | precipitation, evaporation, thermal drying | DSC, XRD, IR | oral bioavailability | ↑ bioavailability | [229] |
| Curcumin | |||||
| βCD | freeze drying, kneading, co-evaporation, co-precipitation | DSC | dissolution, thermal stability, photochemical stability, storage stability | ↑ dissolution in SGF degradation of the complex in SPF ↓ photochemical stability ↑ thermal stability ↑ storage (humidity) stability | [233] |
| sβCD | freeze-drying | XRD, TGA, FTIR, NMR | solubility, photochemical, thermal, storage, gastrointestinal stability | ↑ solubility ↑ storage stability ↑ thermal stability ↑ photochemical stability ↑ stability in gastrointestinal conditions | [232] |
| sβCD/chitosan | freeze-drying | XRD, TGA, FTIR, NMR | gastrointestinal stability, controlled release | ↑ solubility ↑ stability in gastrointestinal conditions | [233] |
| αCD βCD γCD | pulverization | FTIR, DSC, XRD, NMR | solubility, oral bioavailability | ↑ solubility ↑ bioavailability in order αCD > βCD > γCD | [237] |
| βCD | freeze-drying | FTIR | anticancer activity (liver, lungs) | ↑ anticancer activity | [236] |
| βCD | co-precipitation, freeze-drying | FTIR, XRD | nasal bioavailability, in vitro cellular uptake | ↑ bioavailability ↑ cellular uptake | [235] |
| βCD | precipitation | FTIR, XRD, SEM | oral bioavailability | ↑ bioavailability | [234] |
| βCD | Kneading | FTIR, DSC, XRD, NMR, SEM, CVS | solution, intravenous bioavailability, anticancer activity in pancreas | ↑ solubility ↑ bioavailability and concentration in pancreas ↑ anticancer activity in vitro | [243] |
| βCD βCD nanosponges | sonication, solvent-free melting method (nanosponge) | DSC, XRD, FTIR, SEM | solubility, dissolution | ↑ solubility =dissolution rate | [238] |
| γ-MOF | impregnation | XRD, FTIR | Thermal stability, photochemical stability, pH stability | ↑ thermal stability ↑ photochemical stability ↑ stability under different pH | [240] |
| γ-MOF composite films | vapour diffusion (multiple step preparation) | NMR, FTIR, XRD | mechanical properties, barrier properties, antioxidative activity, antimicrobial activity, biocompatibility, fruit preservation | ↑ light barrier ↑ mechanical properties ↓ water barrier ↑oxygen barrier significant antibacterial activity good biocompatibility (80% zebrafish survival) ↑ fruit preservation | [241] |
| HPβCD HPγ nanofibers | electrospinning | NMR, FTIR, XRD, DSC, TGA | solubility, dissolution, antioxidative activity | ↑ solubility (HPγCD) complete disintegration of fibre structure and dissolution in SSF ↑ antioxidative activity (HPγCD) | [239] |
| βCD nanoparticles | freeze-drying | NMR, FTIR, DSC, XRD, DLS; TEM | anticancer activity | ↑ anticancer activity | [242] |
αCD (alpha-cyclodextrin); βCD (beta-cyclodextrin); γCD (gamma cyclodextrin); HPβCD (hydroxypropyl-beta-cyclodextrin); sβCD (succinylated-beta-cyclodextrin); FTIR (Fourier transform infrared spectroscopy); SEM (scanning electron microscopy); DSC (differential scanning calorimetry); NMR (nuclear magnetic resonance spectroscopy); ELS (electrophoretic light scattering); IR (Infrared Resonance); XRD (X-ray diffraction study); TGA (thermogravimetric analysis); ITC (isothermal titration calorimetry); CVS (cyclic voltametric study); RS (Raman spectroscopy); UV-VIS (Ultraviolet-Visible Spectroscopy); MOF (metal–organic framework); SGF (simulated gastric fluid); SPF (simulated pancreatic fluid); ↑ (increased); ↓ (decreased); = (similar effect).
5. Conclusions
CD-mediated formulation of lipophilic antioxidants represents a promising strategy to overcome intrinsic limitations of lipophilicity and chemical instability and offers significant advantages over alternative formulation strategies, considering different aspects of sustainability. Selection of CD type, chemical modification, host–guest stoichiometry, and complexation technique profoundly influences the physicochemical properties and functional performance of the resulting inclusion complexes. Future work should prioritize systematic head-to-head comparisons—including cost–benefit analyses—between native, modified, and large-ring CDs, as well as between simple inclusion complexes versus advanced polymeric or particulate formulations, to guide the rational design of effective and scalable nutraceutical products.
Author Contributions
Conceptualization, D.V.Č. and M.J. (Mario Jug); investigation, K.R., L.N.N., M.J. (Marina Jurić), N.G., T.P., E.G. and M.J. (Mario Jug); resources D.V.Č. and I.J.; writing—original draft preparation, M.J. (Mario Jug), K.R., E.G., M.J. (Marina Jurić), L.N.N. and T.P.; writing—review and editing, D.V.Č., N.G. and I.J.; visualization, N.G.; supervision, D.V.Č.; project administration, D.V.Č. and I.J.; funding acquisition, D.V.Č. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Science Foundation under the project “Application of sustainable extraction and formulation principles in development of tomato waste-derived nutraceuticals” [HRZZ-IP-2022-10-4597], and by the project “Development of innovative formulations of clinical nutrition” under the call Capacity Building for Research, Development, and Innovation [OP-KK.01.1.1.07.0040] within the Operational Programme Competitiveness and Cohesion 2014–2020. The work of the doctoral student Nikolina Golub was supported through the “Young researchers’ career development project—training of doctoral students” [HRZZ-DOK-2021-02-6801]. The work of the doctoral student Tea Petković was supported by “Young researchers’ career development project—training of doctoral students” of the Croatian Science Foundation [HRZZ-DOK-NPOO-2023-10-6543].
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CD | cyclodextrin |
| αCD | α-cyclodextrin |
| βCD | β-cyclodextrin |
| γCD | γ-cyclodextrin |
| HPβCD | hydroxypropyl-β-cyclodextrin |
| RAMEB | randomly methylated β-cyclodextrin |
| SBEβCD | sulphobuthylether-β-cyclodextrin |
| HPγCD | hydroxypropyl-γ-cyclodextrin |
| MeβCD | methyl-β-cyclodextrin |
| HEβCD | hydroxyethyl-β-cyclodextrin |
| LD | lethal dose |
| NOEL | no observed effect level |
| FDA | Food and Drug Administration |
| USP/NF | United States Pharmacopeia and the National Formulary |
| DSC | differential scanning spectroscopy |
| XRPD | X-ray powder diffraction |
| ssNMR | solid state NMR |
| SOD | superoxide dismutase |
| ROS | reactive oxygen species |
| CoQ10 | coenzyme Q10 |
| GIT | gastrointestinal tract |
| SLN | solid lipid nanoparticle |
| NLC | nanostructured lipid carrier |
| LRCDs | large-ring cyclodextrins |
| CAP | capsaicin |
| CUR | curcumin |
| SGF | simulated gastric fluid |
| SIF | simulated intestinal fluid |
| MOF | metal–organic framework |
References
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Blagov, A.V.; Summerhill, V.I.; Sukhorukov, V.N.; Zhigmitova, E.B.; Postnov, A.Y.; Orekhov, A.N. Potential use of antioxidants for the treatment of chronic inflammatory diseases. Front. Pharmacol. 2024, 15, 1378335. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- Tan, Y.; McClements, D.J. Improving the bioavailability of oil-soluble vitamins by optimizing food matrix effects: A review. Food Chem. 2021, 348, 129148. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Duarte, F.Í.C.; Heimfarth, L.; Quintans, J.D.S.S.; Quintans-Júnior, L.J.; Júnior, V.F.D.V.; De Lima, Á.A.N. Cyclodextrin-Drug Inclusion Complexes: In Vivo and in Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Loftsson, T. Cyclodextrins in parenteral formulations. J. Pharm. Sci. 2021, 110, 654–664. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Haimhoffer, A.; Rusznyák, A.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in Drug Delivery Systems and their Effects on Biological Barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef]
- Larsen, K.L. Large Cyclodextrins. J. Inclus. Phenom. Macrocycl. Chem. 2002, 43, 1–13. [Google Scholar] [CrossRef]
- Duchêne, D.; Bochot, A. Thirty Years with Cyclodextrins. Int. J. Pharm. 2016, 514, 58–72. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Saokham, P.; Loftsson, T. γ-Cyclodextrin. Int. J. Pharm. 2017, 516, 278–292. [Google Scholar] [CrossRef]
- Kiss, T.; Fenyvesi, F.; Bácskay, I.; Váradi, J.; Fenyvesi, É.; Iványi, R.; Szente, L.; Tósaki, Á.; Vecsernyés, M. Evaluation of the Cytotoxicity of β-Cyclodextrin Derivatives: Evidence for the Role of Choles-Terol Extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef]
- Munro, I.C.; Newberne, P.M.; Young, V.R.; Bär, A. Safety Assessment of γ-Cyclodextrin. Regul. Toxicol. Pharmacol. 2004, 39, 3–13. [Google Scholar] [CrossRef]
- Musuc, A.M. Cyclodextrins: Advances in Chemistry, Toxicology, and Multifaceted Applications. Molecules 2024, 29, 5319. [Google Scholar] [CrossRef]
- Puskás, I.; Szente, L.; Szőcs, L.; Fenyvesi, É. Recent List of Cyclodextrin-Containing Drug Products. Period. Polytech. Chem. Eng. 2023, 67, 11–17. [Google Scholar] [CrossRef]
- Kali, G.; Haddadzadegan, S.; Laffleur, F.; Bernkop-Schnürch, A. Per-Thiolated Cyclodextrins: Nanosized Drug Carriers Providing a Prolonged Gastrointestinal Residence Time. Carbohydr. Polym. 2023, 300, 120275. [Google Scholar] [CrossRef]
- Hussain Asim, M.; Ijaz, M.; Rösch, A.C.; Bernkop-Schnürch, A. Thiolated Cyclodextrins: New Perspectives for Old Excipients. Coord. Chem. Rev. 2020, 420, 213433. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z. Cyclodextrin Polymers: Structure, Synthesis, and Use as Drug Carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- European Medicines Agency Committee for Human Medicinal Products (EMA/CHMP) Cyclodextrins Used as Excipients Report Published in Support of the ‘Questions and Answers on Cyclodextrins Used as Excipients in Medicinal Products for Human Use’ (EMA/CHMP/495747/2013). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf. (accessed on 30 October 2025).
- Hoover, R.K.; Alcorn, H.; Lawrence, L.; Paulson, S.K.; Quintas, M.; Luke, D.R.; Cammarata, S.K. Clinical Pharmacokinetics of Sulfobutylether-β-Cyclodextrin in Patients With Varying Degrees of Renal Impairment. J. Clin. Pharmacol. 2018, 58, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Cal, K.; Centkowska, K. Use of Cyclodextrins in Topical Formulations: Practical Aspects. Eur. J. Pharm. Biopharm. 2008, 68, 467–478. [Google Scholar] [CrossRef]
- Wittkowski, K.M. The Effect of Alpha-Cyclodextrin on Postprandial Glucose Excursions: A Systematic Meta-Analysis. Cureus 2022, 14, 31160. [Google Scholar] [CrossRef] [PubMed]
- Arruda, T.R.; Marques, C.S.; Soares, N.F.F. Native Cyclodextrins and Their Derivatives as Potential Additives for Food Packaging: A Review. PSA 2021, 2, 825–842. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Kurkov, S.V.; Madden, D.E.; Carr, D.; Loftsson, T. The Effect of Parenterally Administered Cy-clodextrins on the Pharmacokinetics of Coadministered Drugs. J. Pharm. Sci. 2012, 101, 4402–4408. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Do, T.T.; Van Hooghten, R.; Van den Mooter, G. A Study of the Aggregation of Cyclodextrins: Determination of the Critical Aggregation Concentration, Size of Aggregates and Thermodynamics Using Isodesmic and K2–K Models. Int. J. Pharm. 2017, 521, 318–326. [Google Scholar] [CrossRef]
- Ryzhakov, A.; Do Thi, T.; Stappaerts, J.; Bertoletti, L.; Kimpe, K.; Sá Couto, A.R.; Saokham, P.; Van den Mooter, G.; Augustijns, P.; Somsen, G.W.; et al. Self-Assembly of Cyclodextrins and Their Complexes in Aqueous Solutions. J. Pharm. Sci. 2016, 105, 2556–2569. [Google Scholar] [CrossRef]
- Saokham, P.; Sá Couto, A.; Ryzhakov, A.; Loftsson, T. The self-assemble of Natural Cyclodextrins in Aqueous Solutions: Application of Miniature Permeation Studies for Critical Aggregation Concentration (cac) Determinations. J. Pharm. Sci. 2016, 505, 187–193. [Google Scholar] [CrossRef]
- Loftsson, T.; Sigurdsson, H.H.; Jansook, P. Anomalous Properties of Cyclodextrins and Their Complexes in Aqueous Solutions. Materials 2023, 16, 2223. [Google Scholar] [CrossRef]
- Loftsson, T.; Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A. Pharmacokinetics of Cyclodextrins and Drugs after Oral and Parenteral Administration of Drug/Cyclodextrin Complexes. J. Pharm. Pharmacol. 2016, 68, 544–555. [Google Scholar] [CrossRef]
- Popielec, A.; Loftsson, T. Effects of Cyclodextrins on the Chemical Stability of Drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins: Basic Science and Product Development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical Applications of Cyclodextrins: Effects on Drug Permeation through Biological Membranes. J. Pharm. Pharmacol. 2011, 63, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Conceição, J.; Adeoye, O.; Cabral-Marques, H.M.; Lobo, J.M.S. Cyclodextrins as excipients in tablet formulations. Drug. Discov. Today 2018, 23, 1274–1284. [Google Scholar] [CrossRef]
- Arima, H.; Higashi, T.; Motoyama, K. Improvement of the Bitter Taste of Drugs by Complexation with Cyclodextrins: Applications, Evaluations and Mechanisms. Ther. Deliv. 2012, 3, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Helena, M.; Marques, C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour. Frag. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Gao, X.; Fu, J.; Hu, L. Application of Cyclodextrin in Food Industry. Crit. Rev. FSNT 2022, 62, 2627–2640. [Google Scholar] [CrossRef]
- Pereira, A.G.; Carpena, M.; Oliveira, P.G.; Mejuto, J.C.; Prieto, M.A.; Gandara, J.S. Main Ap-plications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef]
- Szente, L.; Szemán, J.; Sohajda, T. Analytical Characterization of Cyclodextrins: History, Official Methods and Recommended New Techniques. J. Pharm. Biomed. Anal. 2016, 130, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, M.A.; Zoppi, A. Current trends in molecular modeling methods applied to the study of cyclodextrin complexes. J. Inclus. Phenom. Macrocycl. Chem. 2017, 90, 1–14. [Google Scholar] [CrossRef]
- Mura, P. Analytical Techniques for Characterization of Cyclodextrin Complexes in Aqueous Solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef]
- Haouas, M.; Falaise, C.; Leclerc, N.; Floquet, S.; Cadot, E. NMR Spectroscopy to Study Cyclodextrin-Based Host–guest Assemblies with Polynuclear Clusters. Dalton Trans. 2023, 52, 13467–13481. [Google Scholar] [CrossRef]
- Lima Cavalcanti, I.D.; Xavier Junior, F.H.; Santos Magalhães, N.S.; Nogueira, M.C.d.B.L. Isothermal Titration Calorimetry (ITC) as a Promising Tool in Pharmaceutical Nanotechnology. Int. J. Pharm. 2023, 641, 123063. [Google Scholar] [CrossRef]
- Mura, P. Analytical Techniques for Characterization of Cyclodextrin Complexes in the Solid State: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Jug, M.; Maestrelli, F.; Bragagni, M.; Mura, P. Preparation and Solid-State Characterization of Bupivacaine Hydrochloride Cyclodextrin Complexes Aimed for Buccal Delivery. J. Pharm. Biomed. Anal. 2010, 52, 9–18. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray Drying of Pharmaceuticals and Biopharmaceuticals: Critical Parameters and Experimental Process Optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef]
- Abla, K.K.; Mehanna, M.M. Freeze-Drying: A Flourishing Strategy to Fabricate Stable Pharmaceutical and Biological Products. Int. J. Pharm. 2022, 628, 122233. [Google Scholar] [CrossRef]
- Banchero, M. Supercritical Carbon Dioxide as a Green Alternative to Achieve Drug Complexation with Cyclodextrins. Pharmaceuticals 2021, 14, 562. [Google Scholar] [CrossRef] [PubMed]
- Moufawad, T.; Moura, L.; Ferreira, M.; Bricout, H.; Tilloy, S.; Monflier, E.; Costa Gomez, M.; Landy, D.; Fourmentin, S. First Evidence of Cyclodextrin Inclusion Complexes in a Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2019, 7, 6345–6351. [Google Scholar] [CrossRef]
- Kfoury, M.; Fourmentin, S. State of the art in Cyclodextrin Solubility Enhancement. Are Green Solvents the Solution? J. Mol. Liq. 2024, 410, 125599. [Google Scholar] [CrossRef]
- Jug, M.; Mura, P.A. Grinding as Solvent-Free Green Chemistry Approach for Cyclodextrin Inclusion Complex Preparation in the Solid State. Pharmaceutics 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, C.D.; de Sá Haddad Queiroz, M.; de Souza Lima, C.G.; de Carvalho da Silva, F.; Futuro, D.O.; Ferreira, V.F. An Improved Method for the Preparation of β-Lapachone:2-Hydroxypropyl-β-Cyclodextrin Inclusion Complexes. J. Drug Deliv. Sci. Technol. 2020, 58, 101777. [Google Scholar] [CrossRef]
- Zanolla, D.; Perissutti, B.; Passerini, N.; Invernizzi, S.; Voinovich, D.; Bertoni, S.; Melegari, C.; Millotti, G.; Albertini, B. Milling and Comilling Praziquantel at Cryogenic and Room Temperatures: As-Sessment of the Process-Induced Effects on Drug Properties. J. Pharm. Biomed. Anal. 2018, 153, 82–89. [Google Scholar] [CrossRef]
- Cabrera-Quiñones, N.C.; López-Méndez, L.J.; Guadarrama, P. Inclusion and Non-Inclusion Complexes between Curcumin and β-Cyclodextrin with High-Curcumin Loading and Enhanced Aque-ous Solubility Obtained by Mechanochemistry. ChemistrySelect 2023, 8, e202303254. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins Inclusion Complex: Preparation Methods, Analytical Techniques and Food Industry Applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; David, I.; Hădărugă, D.I. A Review on Thermal Analyses of Cyclodextrins and Cyclodextrin Complexes. Environ. Chem. Lett. 2019, 17, 349–373. [Google Scholar] [CrossRef]
- Baird, J.A.; Taylor, L.S. Evaluation of Amorphous Solid Dispersion Properties Using Thermal Analysis Techniques. Adv. Drug Deliv. Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef]
- Jablan, J.; Szalontai, G.; Jug, M. Comparative Analysis of Zaleplon Complexation with Cyclodex-Trins and Hydrophilic Polymers in Solution and in Solid State. Adv. Drug Deliv. Rev. 2012, 71, 35–44. [Google Scholar] [CrossRef]
- Dedroog, S.; Pas, T.; Vergauwen, B.; Huygens, C.; Van den Mooter, G. Solid-State Analysis of Amorphous Solid Dispersions: Why DSC and XRPD May not Be Regarded as Stand-Alone Techniques. J. Pharm. Biomed. Anal. 2020, 178, 112937. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł. A Review of Applications of Solid-State Nuclear Magnetic Resonance (ssNMR) for the Analysis of Cyclodextrin-Including Systems. Int. J. Mol. Sci. 2023, 24, 3648. [Google Scholar] [CrossRef]
- Jurmanović, S.; Jug, M.; Safner, T.; Radić, K.; Domijan, A.M.; Pedisić, S.; Šimić, S.; Jablan, J.; Vitali Čepo, D. Utilization of Olive Pomace as a Source of Polyphenols: Optimization of Microwave-Assisted Extraction and Characterization of Spray-Dried Extract. J. Food Nutr. Res. 2019, 58, 51–62. [Google Scholar]
- Jablan, J.; Marguí, E.; Posavec, L.; Klarić, D.; Cinčić, D.; Galić, N.; Jug, M. Product Contamination During Mechanochemical Synthesis of Praziquantel Co-Crystal, Polymeric Dispersion and Cyclodextrin Complex. J. Pharm. Biomed. Anal. 2024, 238, 115855. [Google Scholar] [CrossRef]
- Maestrelli, F.; Cirri, M.; Mennini, N.; Zerrouk, N.; Mura, P. Improvement of Oxaprozin Solubil-Ity and Permeability by the Combined Use of Cyclodextrin, Chitosan, and Bile Components. Eur. J. Pharm. Biopharm. 2011, 78, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of Phytochemicals with Cyclodextrin Derivatives–An Insight. Biomed. Pharmacother. 2017, 88, 1122–1144. [Google Scholar] [CrossRef]
- Vitali Čepo, D.; Jug, M.; Grdić Rajković, M.; Jablan, J. Formulation of a Nutraceutical Derived from Carob: Β-Cyclodextrin Encapsulation of Antioxidants from Carob Pod. JFNR 2017, 6, 48–60. [Google Scholar]
- Kıran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative Stress and Antioxidants in Health and Disease. J. Lab. Med. 2023, 47, 1–11. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative Stress Mitiga-Tion by Antioxidants-An Overview on Their Chemistry and Influences on Health Status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A update: Forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Celik, S.E.; Bekdeser, B.; Tufan, A.N.; Apak, R. Modified Radical Scavenging and Antioxidant Activity Measurement of Β-Carotene with Β-Cyclodextrins Complexation in Aqueous Medium. Anal. Sci. 2017, 33, 299–305. [Google Scholar] [CrossRef]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular Carotenoid Complexes of Enhanced Solubility and Stability—The Way of Bioavailability Improvement. Molecules 2019, 24, 3947. [Google Scholar] [CrossRef]
- Meng, L.; Liu, S.; Luo, J.; Tu, Y.; Li, T.; Li, P.; Yu, J.; Shi, L. Oxidative Stress and Reactive Oxygen Species in Otorhinolaryngological Diseases: Insights from Pathophysiology to Targeted Antioxidant Therapies. Redox Rep. 2025, 30, 2458942. [Google Scholar] [CrossRef]
- Joshi, B.; Kar, S.K.; Yadav, P.K.; Yadav, S.; Shrestha, L.; Bera, T.K. Therapeutic and Medicinal Uses of Lycopene: A Systematic Review. Int. J. Res. Med. Sci. 2020, 8, 1195. [Google Scholar] [CrossRef]
- Sun, X.; Jia, H.; Xu, Q.; Zhao, C.; Xu, C. Lycopene Alleviates H2O2-Induced Oxidative Stress, Inflammation and Apoptosis in Bovine Mammary Epithelial Cells via the NFE2L2 Signaling Pathway. Food Funct. 2019, 10, 6276–6285. [Google Scholar] [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxid. Med. Cell. Longev. 2021, 19, 2713511. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and Lycopene and Multiple Health Outcomes: Umbrella Review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Kwak, S.G.; Kwak, S. Effect of Dietary Vitamins C and E on The Risk of Parkinson’s Disease: A Meta-Analysis. Clin. Nutr. 2021, 40, 3922–3930. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of Food Antioxidants in Modulating Gut Microbial Communities: Novel Understandings in Intestinal Oxidative Stress Damage and Their Impact on Host Health. Antioxidants 2021, 10, 1563. [Google Scholar] [CrossRef]
- Niki, E. Role of Vitamin E as a Lipid-Soluble Peroxyl Radical Scavenger: In Vitro and in Vivo Evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Schneider, C. Chemistry and Biology of Vitamin E. Mol. Nutr. Food Res. 2005, 49, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Luo, X.; Wang, Z.; McClements, D.J.; Huang, W.; Fu, H.; Zhu, K. Dual Role of Polyglycerol Vitamin E Succinate in Emulsions: An Efficient Antioxidant Emulsifier. Food Chem. 2023, 416, 135776. [Google Scholar] [CrossRef]
- Debbabi, M.; Nury, T.; Zarrouk, A.; Mekahli, N.; Bezine, M.; Sghaier, R.; Grégoire, S.; Martine, L.; Durand, P.; Camus, E.; et al. Protective Effects of α-Tocopherol, γ-Tocopherol and Oleic Acid, Three Compounds of Olive Oils, and No Effect of Trolox, on 7-Ketocholesterol-Induced Mitochondrial and Peroxisomal Dysfunction in Microglial BV-2 Cells. Int. J. Mol. Sci. 2016, 17, 1973. [Google Scholar] [CrossRef]
- Vašková, J.; Stupák, M.; Vidová Ugurbaş, M.; Židzik, J.; Mičková, H. Therapeutic uses of retinol and retinoid-related antioxidants. Molecules 2025, 30, 2191. [Google Scholar] [CrossRef]
- Lips, P. Hypervitaminosis A and Fractures. N. Engl. J. Med. 2003, 348, 347–349. [Google Scholar] [CrossRef]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2002; Available online: https://nap.nationalacademies.org/read/10026/chapter/1#ii (accessed on 30 October 2025).
- Baineni, R.; Gulati, R.; Delhi, C.K. Vitamin A Toxicity Presenting as Bone Pain. Arch. Dis. Child. 2017, 102, 556–558. [Google Scholar] [CrossRef]
- Takeda, A.; Nyssen, O.P.; Syed, A.; Jansen, E.; Bueno-de-Mesquita, B.; Gallo, V. Vitamin A and Carotenoids and the Risk of Parkinson’s Disease: A Systematic Review and Meta-analysis. Neuroepidemiology 2013, 42, 25–38. [Google Scholar] [CrossRef]
- Sood, B.; Patel, P.; Keenaghan, M. Coenzyme Q10; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430685/ (accessed on 30 October 2025).
- Duarte-Jurado, A.P.; Gopar-Cuevas, Y.; Saucedo-Cardenas, O.; Loera-Arias, M.D.J.; Montes-de-Oca-Luna, R.; Garcia-Garcia, A.; Rodriguez-Rocha, H. Antioxidant Therapeutics in Parkinson’s Disease: Current Challenges and Opportunities. Antioxidants 2021, 10, 453. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Vinqvist, M.R.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A.; Uno, H. On the Antioxidant Mechanism of Curcumin: Classical Methods are Needed to Determine Antioxidant Mechanism and Activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef] [PubMed]
- Witika, B.A.; Makoni, P.A.; Matafwali, S.K.; Mweetwa, L.L.; Shandele, G.C.; Walker, R.B. Enhancement of Biological and Pharmacological Properties of an Encapsulated Polyphenol: Curcumin. Molecules 2021, 26, 4244. [Google Scholar] [CrossRef]
- Virk, T.L.; Liu, Q.; Yuan, Y.; Xu, X.; Chen, F. Curcumin as Therapeutic Modulator of Impaired Antioxidant Defense System: Implications for Oxidative Stress-Associated Reproductive Dysfunction. Biology 2025, 14, 750. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Alishiri, G.H.; Parvin, S.; Sahebkar, A. Mitigation of Systemic Oxidative Stress by Curcuminoids in Osteoarthritis: Results of a Randomized Controlled trial. J. Diet. Suppl. 2016, 13, 209–220. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Fan, J.; Feng, Z.; Song, X. Pharmacological Activity of Capsaicin: Mechanisms and Controversies. Mol. Med. Rep. 2024, 29, 38. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Cheng, K.; Zhang, L.; Wang, T. Capsaicin Alleviates the Intestinal Oxidative Stress via Activation of TRPV1/PKA/UCP2 and Keap1/Nrf2 Pathways in Heat-stressed Mice. J. Funct. Foods 2023, 108, 105749. [Google Scholar] [CrossRef]
- Nouchi, R.; Suiko, T.; Kimura, E.; Takenaka, H.; Murakoshi, M.; Uchiyama, A.; Aono, M.; Kawashima, R. Effects of Lutein and Astaxanthin Intake on the Improvement of Cognitive Functions among Healthy Adults: A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 617. [Google Scholar] [CrossRef]
- Yagi, A.; Nouchi, R.; Butler, L.; Kawashima, R. Lutein has a Positive Impact on Brain Health in Healthy Older Adults: A Systematic Review of Randomized Controlled Trials and Cohort Studies. Nutrients 2021, 13, 1746. [Google Scholar] [CrossRef]
- Huang, Y.M.; Dou, H.L.; Huang, F.F.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Effect of Supplemental Lutein and Zeaxanthin on Serum, Macular Pigmentation, and Visual Performance in Patients with Early Age-Related Macular Degeneration. BioMed Res. Int. 2015, 2015, 564738. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, M.; Wu, R.; Yang, Z.; Zhu, X.; Chen, J. The level and efficacy of lutein in patients with age-related macular degeneration: A comprehensive systematic review and meta-analysis. Ann. Transl. Med. 2022, 10, 299. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Zahabi, E.S.; Malekahmadi, M.; Zarrin, R.; Alizadeh, M. Carotenoids Supplementation and Inflammation: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 8161–8177. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular Evidence that Oral Supplementation with Lycopene or Lutein Protects Human Skin Against Ultraviolet Radiation: Results from a Double-Blinded, Placebo-Controlled, Crossover Study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.M.; Tharmarajah, S.; Jia, Y.; Semba, R.D.; Schaumberg, D.A.; Robinson, K.A. The Effect of Lutein/Zeaxanthin Intake on Human Macular Pigment Optical Density: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Smith, S.J. The Effects of Lutein/Zeaxanthin (Lute-gen®) on Eye Health, Eye Strain, Sleep Quality, and Attention in High Electronic Screen Users: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Nutr. 2025, 12, 1522302. [Google Scholar] [CrossRef]
- Hammond, B.R., Jr.; Miller, L.S.; Bello, M.O.; Lindbergh, C.A.; Mewborn, C.; Renzi-Hammond, L.M. Effects of Lutein/Zeaxanthin Supplementation on the Cognitive Function of Community Dwelling Older Adults: A Randomized, Double-Masked, Placebo-Controlled Trial. Front. Aging Neurosci. 2017, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Renzi-Hammond, L.M.; Bovier, E.R.; Fletcher, L.M.; Miller, L.S.; Mewborn, C.M.; Lindbergh, C.A.; Baxter, J.H.; Hammond, B.R. Effects of a Lutein and Zeaxanthin Intervention on Cognitive Function: A Randomized, Double-Masked, Placebo-Controlled Trial of Younger Healthy Adults. Nutrients 2017, 9, 1246. [Google Scholar] [CrossRef]
- Lindbergh, C.A.; Renzi-Hammond, L.M.; Hammond, B.R.; Terry, D.P.; Mewborn, C.M.; Puente, A.N.; Miller, L.S. Lutein and Zeaxanthin Influence Brain Function in Older Adults: A Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2018, 24, 77–90. [Google Scholar] [CrossRef]
- Petyaev, I.M.; Dovgalevsky, P.Y.; Klochkov, V.A.; Chalyk, N.E.; Pristensky, D.V.; Chernyshova, M.P.; Udumyan, R.; Kocharyan, T.; Kyle, N.H.; Lozbiakova, M.V.; et al. Effect of Lycopene Supplementation on Cardiovascular Parameters and Markers of Inflammation and Oxidation in Patients with Coronary Vascular Disease. Food Sci. Nutr. 2018, 6, 1770–1777. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, F. The Effects of Lycopene Supplementation on Serum Insulin-Like Growth Factor 1 (IGF-1) Levels and Cardiovascular Disease: A Dose-Response Meta-Analysis of Clinical Trials. Complement. Ther. Med. 2021, 56, 102632. [Google Scholar] [CrossRef]
- Wolak, T.; Sharoni, Y.; Levy, J.; Linnewiel-Hermoni, K.; Stepensky, D.; Paran, E. Effect of Tomato Nutrient Complex on Blood Pressure: A Double Blind, Randomized Dose–Response Study. Nutrients 2019, 11, 950. [Google Scholar] [CrossRef]
- Hayashi, M.; Ishibashi, T.; Maoka, T. Effect of Astaxanthin-Rich Extract Derived from Paracoccus carotinifaciens on Cognitive Function in Middle-Aged and Older Individuals. J. Clin. Biochem. Nutr. 2018, 62, 195–205. [Google Scholar] [CrossRef]
- Chan, K.C.; Chen, S.C.; Chen, P.C. Astaxanthin Attenuated Thrombotic Risk Factors in Type 2 Diabetic Patients. J. Funct. Foods 2019, 53, 22–27. [Google Scholar] [CrossRef]
- Baralic, I.; Andjelkovic, M.; Djordjevic, B.; Dikic, N.; Radivojevic, N.; Suzin-Zivkovic, V.; Radojevic-Skodric, S.; Pejic, S. Effect of Astaxanthin Supplementation on Salivary IgA, Oxidative Stress, and Inflammation in Young Soccer Players. Evid. Based Complement. Alternat. Med. 2015, 2015, 783761. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective effects of astaxanthin on skin deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef]
- Matsuura, B.; Miyake, T.; Yamamoto, S.; Furukawa, S.; Hiasa, Y. Usefulness of beta-cryptoxanthin for nonalcoholic fatty liver diseases. J. Food. Nutr. Disor. 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Jaffary, F.; Faghihi, G.; Mokhtarian, A.; Hosseini, S.M. Effects of oral vitamin E on treatment of atopic dermatitis: A randomized controlled trial. J. Res. Med. Sci. 2015, 20, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Tantavisut, S.; Tanavalee, A.; Honsawek, S.; Suantawee, T.; Ngarmukos, S.; Adisakwatana, S.; Callaghan, J.J. Effect of Vitamin E on Oxidative Stress Level in Blood, Synovial Fluid, and Synovial Tissue in Severe Knee Osteoarthritis: A Randomized Controlled Study. BMC Musculoskelet. Disord. 2017, 18, 281. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, Z.; Hejazi, N.; Dabbaghmanesh, M.H.; Dashtabi, A. Effects of Vitamin E on Fasting and Postprandial Oxidative Stress, Inflammatory Markers, Glucose Status, Insulin Resistance, Blood Pressure and Pulse Rate in Type-2 Diabetic Patients: A Randomized Clinical Trial. Galen. Med. J. 2015, 4, 67–74. [Google Scholar] [CrossRef]
- Muthu, S.K.; Narang, T.; Saikia, U.N.; Kanwar, A.J.; Parsad, D.; Dogra, S. Low-Dose Oral Isotretinoin Therapy in Lichen Planus Pigmentosus: An Open-Label Non-Randomized Prospective Pilot Study. Int. J. Dermatol. 2016, 55, 1048–1054. [Google Scholar] [CrossRef]
- Dhaked, D.R.; Meena, R.S.; Maheshwari, A.; Agarwal, U.S.; Purohit, S. A Randomized Comparative Trial of Two Low-Dose Oral Isotretinoin Regimens in Moderate to Severe Acne vulgaris. Indian. Dermatol. Online J. 2016, 7, 378–385. [Google Scholar] [CrossRef]
- Acmaz, G.; Cınar, L.; Acmaz, B.; Aksoy, H.; Kafadar, Y.T.; Madendag, Y.; Ozdemir, F.; Sahin, E.; Muderris, I. The Effects of Oral Isotretinoin in Women with Acne and Polycystic Ovary Syndrome. BioMed Res. Int. 2019, 2019, 2513067. [Google Scholar] [CrossRef]
- de Freitas, M.C.; Cholewa, J.M.; Gobbo, L.A.; de Oliveira, J.V.; Lira, F.S.; Rossi, F.E. Acute Capsaicin Supplementation Improves 1500-m Running Time-Trial Performance and Rate of Perceived Exertion in Physically Active Adults. J. Strength Cond. Res. 2018, 32, 572–577. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, M.C.; Billaut, F.; Panissa, V.L.G.; Rossi, F.E.; Figueiredo, C.; Caperuto, E.C.; Lira, F.S. Capsaicin Supplementation Increases Time to Exhaustion in High-Intensity Intermittent Exercise without Modifying Metabolic Responses in Physically Active Men. Eur. J. Appl. Physiol. 2019, 119, 971–979. [Google Scholar] [CrossRef]
- Grgic, J.; Memon, A.R.; Chen, S.; Ramirez-Campillo, R.; Barreto, G.; Haugen, M.E.; Schoenfeld, B.J. Effects of Capsaicin and Capsiate on Endurance Performance: A Meta-Analysis. Nutrients 2022, 14, 4531. [Google Scholar] [CrossRef]
- Oliver, J.M.; Stoner, L.; Rowlands, D.S.; Caldwell, A.R.; Sanders, E.; Kreutzer, A.; Mitchell, J.B.; Purpura, M.; Jäger, R. Novel Form of Curcumin Improves Endothelial Function in Young, Healthy Individuals: A Double-blind Placebo Controlled Study. J. Nutr. Metab. 2016, 2016, 1089653. [Google Scholar] [CrossRef]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin Supplementation Improves Vascular Endothelial Function in Healthy Middle-Aged and Older Adults by Increasing Nitric Oxide Bioavailability and Reducing Oxidative Stress. Aging 2017, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Osorio, A.S.; García-Niño, W.R.; González-Reyes, S.; Álvarez-Mejía, A.E.; Guerra-León, S.; Salazar-Segovia, J.; Falcón, I.; Montes de Oca-Solano, H.; Madero, M.; Pedraza-Chaverr, J. The Effect of Dietary Supplementation with Curcumin on Redox Status and Nrf2 Activation in Patients with Nondiabetic or Diabetic Proteinuric Chronic Kidney Disease: A Pilot Study. J. Ren. Nutr. 2016, 26, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Cur-cumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Mirzabeigi, P.; Mohammadpour, A.H.; Salarifar, M.; Gholami, K.; Mojtahedzadeh, M.; Javadi, M.R. The effect of Curcumin on Some of Traditional and Non-Traditional Cardiovascular Risk Factors: A Pilot Randomized, Double-blind, Placebo-Controlled Trial. Iran. J. Pharm. Res. 2015, 14, 479–486. [Google Scholar]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.T.; Gao, Z.Y.; Shi, D.Z. Effects of Coenzyme Q10 on Statin-Induced Myopathy: An Updated Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef]
- Borges, M.D. Translational Cardiology: Practical Insights into the Coenzyme Q10 Role as Potential Therapeutic Agent for Cardiovascular Disease Treatment via Systematic Review and Meta-Analysis of Prospective Cohort Studies. Trans. Med. Open Access 2024, 2, 1–9. [Google Scholar] [CrossRef]
- Sarmiento, A.; Diaz-Castro, J.; Pulido-Moran, M.; Moreno-Fernandez, J.; Kajarabille, N.; Chirosa, I.; Guisado, I.M.; Chirosa, L.J.; Guisado, R.; Ochoa, J.J. Short-Term Ubiquinol Supplementation Reduces Oxidative Stress Associated with Strenuous Exercise in Healthy Adults: A Randomized Trial. Biofactors 2016, 42, 612–622. [Google Scholar] [CrossRef]
- Sanoobar, M.; Dehghan, P.; Khalili, M.; Azimi, A.; Seifar, F. Coenzyme Q10 as a Treatment for Fatigue and Depression in Multiple Sclerosis Patients: A Double Blind Randomized Clinical Trial. Nutr. Neurosci. 2016, 19, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Rossi, A.; Consensi, A.; Giacomelli, C.; Bazzichi, L. Role for a Water-Soluble Form of CoQ10 in Female Subjects Affected by Fibromyalgia. A Preliminary Study. Clin. Exp. Rheumatol. 2017, 35, 20–27. [Google Scholar]
- Dahri, M.; Tarighat-Esfanjani, A.; Asghari-Jafarabadi, M.; Hashemilar, M. Oral Coenzyme Q10 Supplementation in Patients with Migraine: Effects on Clinical Features and Inflammatory Markers. Nutr. Neurosci. 2019, 22, 607–615. [Google Scholar] [CrossRef]
- Abrego-Guandique, D.M.; Bonet, M.L.; Caroleo, M.C.; Cannataro, R.; Tucci, P.; Ribot, J.; Cione, E. The Effect of Beta-Carotene on Cognitive Function: A Systematic Review. Brain Sci. 2023, 13, 1468. [Google Scholar] [CrossRef]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef]
- Hazarika, N. Acne Vulgaris: New Evidence in Pathogenesis and Future Modalities of Treatment. J. Dermatolog. Treat. 2021, 32, 277–285. [Google Scholar] [CrossRef]
- Scott-Emuakpor, R.; Vuthaluru, K.; Nagre, A.; Jawed, I.; Patel, P.A.; Sidhu, H.K. Role of Oral Retinoids in Treatment of Acne Vulgaris with a Bioinformatics-Based Perspective of Personalized Medicine. Cureus 2023, 15, e38019. [Google Scholar] [CrossRef]
- Choonara, B.F.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. A Review of Advanced Oral Drug Delivery Technologies Facilitating the Protection and Absorption of Protein and Peptide Molecules. Biotechnol. Adv. 2014, 32, 1269–1282. [Google Scholar] [CrossRef]
- Tyagi, P.; Pechenov, S.; Subramony, J.A. Oral Peptide Delivery: Translational Challenges Due to Physiological Rffects. J. Control. Release 2018, 287, 167–176. [Google Scholar] [CrossRef]
- Gonçalves, J.; Ramos, R.; Luís, A.; Rocha, S.; Rosado, T.; Gallardo, E.; Duarte, A.P. Assessment of the Bioaccessibility and Bioavailability of the Phenolic Compounds of Prunus Avium L. by in Vitro Digestion and Cell Model. ACS Omega 2019, 4, 7605–7613. [Google Scholar] [CrossRef]
- Yildiz, Z.I.; Topuz, F.; Kilic, M.E.; Durgun, E.; Uyar, T. Encapsulation of Antioxidant Beta-Carotene by Cyclodextrin Complex Electrospun Nanofibers: Solubilization and Stabilization of Beta-Carotene by Cyclodextrins. Food Chem. 2023, 423, 136284. [Google Scholar] [CrossRef]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef]
- Lilly, M.B.; Wu, C.; Ke, Y.; Chen, W.P.; Soloff, A.C.; Armeson, K.; Yokoyama, N.N.; Li, X.; Song, L.; Yuan, Y.; et al. A phase I study of docetaxel plus synthetic lycopene in metastatic prostate cancer patients. Clin. Transl. Med. 2024, 14, e1627. [Google Scholar] [CrossRef] [PubMed]
- Cakir, M.A.; Helvacioglu, I. Bioavailability and Health Effects of Some Carotenoids by Different Cooking Methods. Int. J. Gastron. Res. 2023, 2, 70–77. [Google Scholar] [CrossRef]
- Baghabrishami, R.G.; Goli, S.A.H. Tomato Seed Oil-Enriched Tomato Juice: Effect of Oil Addi-Tion Type and Heat Treatment on Lycopene Bioaccessibility and Oxidative Stability. Food Chem. 2023, 402, 134217. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Takada, T.; Kurauchi, R.; Tanaka, Y.; Komine, T.; Suzuki, H. Transporters for the Intestinal Absorption of Cholesterol, Vitamin E, and Vitamin K. J. Atheroscler. Thromb. 2017, 24, 347–359. [Google Scholar] [CrossRef]
- Abe-Matsumoto, L.T.; Sampaio, G.R.; Bastos, D.H.M. Stability of Antioxidant Vitamins in Commercial Vitamin Supplements. Braz. J. Pharm. Sci. 2019, 54, e17700. [Google Scholar] [CrossRef]
- Irías-Mata, A.; Sus, N.; Flory, S.; Stock, D.; Woerner, D.; Podszun, M.; Frank, J. α-Tocopherol Transfer Protein does not Regulate the Cellular Uptake and Intracellular Distribution of α-and γ-Tocopherols and-Tocotrienols in Cultured Liver Cells. Redox Biol. 2018, 19, 28–36. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols—Bioactive Dietary Compounds; What is Certain, What is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Leyden, J.; Del Rosso, J.; Baum, E. The Use of Isotretinoin in the Treatment of Acne Vulgaris-Clinical Considerations and Future Directions. J. Clin. Aesthet. Dermatol. 2014, 7, 3–21. [Google Scholar]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a Golden Spice with a Low Bioavailability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Salamt, N.; Yusuf, A.N.M.; Kashim, M.I.A.M.; Mokhtar, M.H. Potential Health Benefits of Curcumin on Female Reproductive Disorders: A review. Nutrients 2021, 13, 3126. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; He, Y. A comprehensive review on the benefits and problems of curcumin with respect to human health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef]
- Mantle, D.; Dybring, A. Bioavailability of Coenzyme Q10: An Overview of the Absorption Process and Subsequent Metabolism. Antioxidants 2020, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lilienfeldt, N.; Hekimi, S. Understanding Coenzyme Q. Physiol. Rev. 2024, 104, 1533–1610. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ribera, L.; Corredor, Z.; Silva, I.; Díaz, J.M.; Ballarín, J.; Marcos, R.; Pastor, S.; Coll, E. Vitamin E-coated Dialysis Membranes Reduce the Levels of Oxidative Genetic Damage in Hemodialysis Patients. Res. Genet. Toxicol. Environ. Mutagen. 2017, 815, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Turcuş, V.; Predoi, G.; Iordache, F. Nanoencapsulation Techniques for Compounds and Products with Antioxidant and Antimicrobial Activity—A Critical View. Eur. J. Med. Chem. 2018, 157, 1326–1345. [Google Scholar] [CrossRef]
- Simon, L.; Lapinte, V.; Lionnard, L.; Marcotte, N.; Morille, M.; Aouacheria, A.; Kissa, K.; Devoisselle, J.M.; Bégu, S. Polyoxazolines Based Lipid Nanocapsules for topical Delivery of Antioxidants. Int. J. Pharm. 2020, 579, 119126. [Google Scholar] [CrossRef]
- Tarone, A.G.; Cazarin, C.B.B.; Junior, M.R.M. Anthocyanins: New Techniques and Challenges in Microencapsulation. Food Res. Int. 2020, 133, 109092. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G. Attempts to Improve Lipophilic Drugs’ Solubility and Bioavailability: A Focus on Fenretinide. Pharmaceutics 2024, 16, 579. [Google Scholar] [CrossRef]
- Kurmaz, S.V.; Fadeeva, N.V.; Skripets, J.A.; Komendant, R.I.; Ignatiev, V.M.; Emel’yanova, N.S.; Soldatova, Y.V.; Faingold, I.I.; Poletaeva, D.A.; Kotelnikova, R.A. New Water-Soluble Forms of α-Tocopherol: Preparation and Study of Antioxidant Activity in Vitro. Mendeleev Commun. 2022, 32, 117–119. [Google Scholar] [CrossRef]
- Toydemir, G.; Gultekin Subasi, B.; Hall, R.D.; Beekwilder, J.; Boyacioglu, D.; Capanoglu, E. Effect of Food Processing on Antioxidants, their Bioavailability and Potential Relevance to Human Health. Food Chem. 2022, 14, 100334. [Google Scholar] [CrossRef]
- Abourashed, E.A. Bioavailability of Plant-Derived Antioxidants. Antioxidants 2013, 2, 309–325. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, H.D.; Jang, Y.J. Delivery Systems Designed to Enhance Stability and Suitability of Lipophilic Bioactive Compounds in Food Processing: A review. Food Chem. 2024, 437, 137910. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Li, L.; Xue, S.; Yang, D.; Wang, S. Stability of Flavonoid, Carotenoid, Soluble Sugar and Vitamin C in ‘Cara Cara’ Juice during Storage. Foods 2019, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Dehnad, D.; Emadzadeh, B.; Ghorani, B.; Rajabzadeh, G.; Kharazmi, M.S.; Jafari, S.M. Nanovesicular Carriers for Bioactive Compounds and their Applications in Food Formulations. Crit. Rev. Food Sci. Nutr. 2024, 64, 5583–5602. [Google Scholar] [CrossRef]
- Prabhakar, P.; Tripathy, S.; Verma, D.K.; Singh, S.; Thakur, M.; Singh, A.K.; Srivastav, P.P.; Banerjee, M.; Patel, A.R.; Chávez González, M.L.; et al. Trends and Advances in Liposome Formulation Technology with an Emphasis on Ensuring Safety and Quality in Food and Drug Applications. Food Biosci. 2025, 69, 106913. [Google Scholar] [CrossRef]
- Shafiepour, M.; Razavi, S.H.; Khanniri, E.; Jahan, F.M.; Nouri, M.; Afraei, M. Nanocarriers for foods: A review of niosomes and proniosomes in bioactive compounds. Food Humanit. 2025, 4, 100623. [Google Scholar] [CrossRef]
- da Silva Santos, V.; Badan Ribeiro, A.P.; Andrade Santana, M.H. Solid Lipid Nanoparticles as Carriers for Lipophilic Compounds for Applications in Foods. Food Res. Int. 2019, 122, 610–626. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of Hydrophobic and Low-Soluble Food Bioactive Compounds within Different Nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Gunawan, M.; Boonkanokwong, V. Current Applications of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Vehicles in Oral Delivery Systems for Antioxidant Nutraceuticals: A review. Colloids Surf. B Biointerfaces 2024, 233, 113608. [Google Scholar] [CrossRef] [PubMed]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef]
- Sevim, S.; Sanlier, N. Cyclodextrin as a Singular Oligosaccharide: Recent Advances of Health Benefit and in Food Applications. J. Food Sci. 2024, 89, 8215–8230. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, É.; Sohajda, T. Cyclodextrin-Enabled Green Environmental Biotechnologies. Environ. Sci. Pollut. Res. 2022, 29, 20085–20097. [Google Scholar] [CrossRef]
- Fuenmayor, C.A.; Baron-Cangrejo, O.G.; Salgado-Rivera, P.A. Encapsulation of Carotenoids as Food Colorants via Formation of Cyclodextrin Inclusion Complexes: A review. Polysaccharides 2021, 2, 454–476. [Google Scholar] [CrossRef]
- Garg, A.; Lai, W.C.; Chopra, H.; Agrawal, R.; Singh, T.; Chaudhary, R.; Dubey, B.N. Nanosponge: A Promising and Intriguing Strategy in Medical and Pharmaceutical Science. Heliyon 2024, 10, 23303. [Google Scholar] [CrossRef]
- Gujar, S.; Telange, D.; Pethe, A. Supramolecular Complexes of Phospholipids and β-Cyclodextrin with Bioactive β-Carotene: A Comparative Physico-Chemical and Functional Evaluation. Indian. J. Pharm. Edu. Res. 2020, 54, s220–s229. [Google Scholar] [CrossRef]
- Dela, C.J.; Flores, F. Evaluation of Physicochemical Characteristics, in Vitro Release and Anti-Oxidant Properties of Β-Carotene And Β-Cyclodextrin Inclusion Complexes with Rice as Food Matrix. Philipp. Agric. Sci. 2020, 103, 337–348. [Google Scholar] [CrossRef]
- Durante, M.; Milano, F.; De Caroli, M.; Giotta, L.; Piro, G.; Mita, G.; Frigione, M.; Lenucci, M.S. Tomato Oil Encapsulation by α-, β-, and γ-Cyclodextrins: A Comparative Study on the Formation of Supramolecular Structures, Antioxidant Activity, and Carotenoid Stability. Foods 2020, 9, 1553. [Google Scholar] [CrossRef]
- Flores, F.P.; Kong, F. Water Dispersibility of the β-Carotene Source and its Effect on the Physical, Thermal, and in vitro Release Properties of an Inclusion Complex. Int. J. Food Sci. Technol. 2021, 56, 3618–3626. [Google Scholar] [CrossRef]
- Kaur, M.; Bawa, M.; Singh, M. β-Carotene-β-Cyclodextrin Inclusion Complex: Towards Enhanced Aqueous Solubility. J. Glob. Biosci. 2016, 5, 3665–3675. [Google Scholar]
- de Lima Petito, N.; da Silva Dias, D.; Costa, V.G.; Falcão, D.Q.; de Lima Araujo, K.G. Increasing Solubility of Red Bell Pepper Carotenoids by Complexation with 2-Hydroxypropyl-β-Cyclodextrin. Food Chem. 2016, 208, 124–131. [Google Scholar] [CrossRef]
- Lobo, F.A.T.; Silva, V.; Domingues, J.; Rodrigues, S.; Costa, V.; Falcão, D.; de Lima Araújo, K.G. Inclusion Complexes of Yellow Bell Pepper Pigments with β-Cyclodextrin: Preparation, Characterisation and Application as Food Natural Colorant. J. Sci. Food Agric. 2018, 98, 2665–2671. [Google Scholar] [CrossRef]
- Nalawade, P.; Gajjar, A. Assessment of In-Vitro Bio Accessibility and Characterization of Spray Dried Complex of Astaxanthin with Methylated Betacyclodextrin. J. Incl. Phenom. Macrocyc. Chem. 2015, 83, 63–75. [Google Scholar] [CrossRef]
- Pinzón-García, A.D.; Orellano, L.A.A.; de Lazari, M.G.T.; Campos, P.P.; Cortes, M.E.; Sinisterra, R.D. Evidence of Hypoglycemic, Lipid-Lowering and Hepatoprotective Effects of the Bixin and Bixin: β-CD Inclusion Compound in High-Fat-Fed Obese Mice. Biomed. Pharmacother. 2018, 106, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Puebla-Duarte, A.L.; Bernal-Mercado, A.T.; Santos-Sauceda, I.; Acosta-Elias, M.; Fernández-Quiroz, D.; Burruel-Ibarra, S.E.; de Jesús Ornelas-Paz, J.; Pérez-Cabral, I.D.; Rodríguez-Félix, F.; Iturralde-García, R.D.; et al. The Characterization and Antioxidant and Erythroprotective Effects of β-Carotene Complexed in β-Cyclodextrin. Int. J. Mol. Sci. 2025, 26, 3902. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, J.; Liu, C.; Wang, D.; Wang, C.Y. Fabrication of Fucoxanthin/2-Hydroxypropyl-β-Cyclodextrin Inclusion Complex Assisted by Ultrasound Procedure to Enhance Aqueous Solubility, Stability and Antitumor Effect of Fucoxanthin. Ultrason. Sonochem. 2022, 90, 106215. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Zhu, H.; Wang, S.; Xing, J. Inclusion Complexes of Lycopene and β-Cyclodextrin: Preparation, Characterization, Stability and Antioxidant Activity. Antioxidants 2019, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Tavakoli, O.; Khoobi, M.; Wu, Y.S.; Faramarzi, M.A.; Gholibegloo, E.; Farkhondeh, S. Beta-Carotene/Cyclodextrin-Based Inclusion Complex: Improved Loading, Solubility, Stability, and Cytotoxicity. J. Incl. Phenom. Macrocyc. Chem. 2022, 102, 55–64. [Google Scholar] [CrossRef]
- Angi, R.; Kalóczkai, A.J.; Kovács, A.; Marton, A.D.; Bárdos, V.; Dormán, P.; Katona, G.; Agócs, A.; Csorba, A.; Nagy, Z.Z.; et al. Harnessing Cyclodextrins for Enhanced Ocular Delivery of Carotenoid Derivatives: From Development to Ex Vivo Characterization. Carbohydr. Polym. Technol. Appl. 2025, 9, 100718. [Google Scholar] [CrossRef]
- Wong, K.H.; Xie, Y.; Huang, X.; Kadota, K.; Yao, X.S.; Yu, Y.; Chen, X.; Lu, A.; Yang, Z. Delivering Crocetin Across the Blood-Brain Barrier by Using γ-Cyclodextrin to Treat Alzheimer’s Disease. Sci. Rep. 2020, 10, 3654. [Google Scholar] [CrossRef]
- Xu, P.X.; Dai, Z.Q.; Li, D.J.; Liu, C.Q.; Wu, C.E.; Song, J.F. Preparation, Optimization, Charac-terization, and In Vitro Bioaccessibility of a Lutein Microparticle Using Spray Drying with β-Cyclodextrin and Stevioside. J. Food Process. Preserv. 2021, 45, e15032. [Google Scholar] [CrossRef]
- Wang, H.; Yan, W.; Sun, Y.; Yang, C.S. δ-Tocotrienol is the Most Potent Vitamin E Form in Inhibiting Prostate Cancer Cell Growth and Inhibits Prostate Carcinogenesis in Ptenp−/− Mice. Cancer Prev. Res. 2022, 15, 233–245. [Google Scholar] [CrossRef]
- Braithwaite, M.C.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Tomar, L.K.; Tyagi, C.; Pillay, V. A novel Multi-Tiered Experimental Approach Unfolding the Mechanisms behind Cyclodextrin-Vitamin Inclusion Complexes for Enhanced Vitamin Solubility and Stability. Int. J. Pharm. 2017, 532, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Ferruzzi, M.G.; Campbell, W.W. Egg Consumption Increases Vitamin E Absorption from Co-Consumed Raw Mixed Vegetables in Healthy Young Men. J. Nutr. 2016, 146, 2199–2205. [Google Scholar] [CrossRef]
- Neunert, G.; Szwengiel, A.; Walejko, P.; Witkowski, S.; Polewski, K. Photostability of Alpha-Tocopherol Ester Derivatives in Solutions and Liposomes. Spectroscopic and LC–MS Studies. J. Photochem. Photobiol. B Biol. 2016, 160, 121–127. [Google Scholar] [CrossRef]
- Ali, H.M.; Attia, M.H.; Ramadan, K.M.; Rashed, E.N.; Bendary, E.S. Improving Stabilization of α-Tocopherol and α-Tocopheryl Acetate against Oxidation, Light and UV Radiation by Complexation with β-Cyclodextrin and Starch. J. Food Sci. Technol. 2025, 62, 75–87. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Cyclodextrins and antioxidants. Crit. Rev. Food Sci. Nutr. 2014, 54, 251–276. [Google Scholar] [CrossRef]
- Uekaji, Y.; Terao, K. Bioavailability Enhancement of Hydrophobic Nutraceuticals using γ-Cyclodextrin. J. Incl. Phenom. Macro. Chem. 2019, 93, 3–15. [Google Scholar] [CrossRef]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef]
- Iaconinoto, A.; Chicca, M.; Pinamonti, S.; Casolari, A.; Bianchi, A.; Scalia, S. Influence of Cy-clodextrin Complexation on the Photodegradation and Antioxidant Activity of α-Tocopherol. Die Pharm. 2004, 59, 30–33. [Google Scholar]
- Lange, K.; Gierlach-Hładon, T. Solid State Characterization of α-Tocopherol in Inclusion Complexes with Cyclodextrins. Acta Pol. Pharm. 2015, 72, 21–30. [Google Scholar]
- Celebioglu, A.; Uyar, T. Antioxidant Vitamin E/Cyclodextrin Inclusion Complex Clectrospun Nanofibers: Enhanced Water Solubility, Prolonged Shelf Life, and Photostability of Vitamin E. J. Agric. Food Chem. 2017, 65, 5404–5412. [Google Scholar] [CrossRef]
- Kuttiyawong, K.; Saehu, S.; Ito, K.; Pongsawasdi, P. Synthesis of Large-Ring Cyclodextrin from Tapioca Starch by Amylomaltase and Complex Formation with Vitamin E Acetate for Solubility Enhance-ment. Process Biochem. 2015, 50, 2168–2176. [Google Scholar] [CrossRef]
- Cao, C.; Xu, L.; Xie, P.; Hu, J.; Qi, J.; Zhou, Y.; Cao, L. The Characterization and Evaluation of the Synthesis of Large-Ring Cyclodextrins (CD9-CD22) and α-Tocopherol with Enhanced Thermal Stability. RSC Adv. 2020, 10, 6584–6591. [Google Scholar] [CrossRef]
- Ke, D.; Chen, W.; Chen, W.; Yun, Y.H.; Zhong, Q.; Su, X.; Chen, H. Preparation and Characterization of Octenyl Succinate β-Cyclodextrin and Vitamin E Inclusion Complex and its Application in Emulsion. Molecules 2020, 25, 654. [Google Scholar] [CrossRef]
- Singh, P.; Wu, L.; Ren, X.; Zhang, W.; Tang, Y.; Chen, Y.; Carrier, A.; Zhang, X.; Zhang, J. Hyaluronic-Acid-Based β-Cyclodextrin Grafted Copolymers as Biocompatible Supramolecular Hosts to Enhance the Water Solubility of Tocopherol. Int. J. Pharm. 2020, 586, 119542. [Google Scholar] [CrossRef]
- Eid, M.; Sobhy, R.; Zhou, P.; Wei, X.; Wu, D.; Li, B. β-Cyclodextrin-Soy Soluble Polysaccharide Based Core-Shell Bionanocomposites Hydrogel for Vitamin E Swelling Controlled Delivery. Food Hydrocoll. 2020, 104, 105751. [Google Scholar] [CrossRef]
- Cheng, C.; Yuan, C.; Cui, B.; Li, J.; Liu, G. β-Cyclodextrin Based Pickering Emulsions for α-Tocopherol Delivery: Antioxidation Stability and Bioaccessibility. Food. Chem. 2024, 438, 138000. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.; Jafari, S.M. Influence of Food Processing Operations on Vitamins. Encycl. Food. Chem. 2018, 2, 129–139. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-Encapsulation as a Promising Approach for Targeted Delivery and Controlled Release of Vitamins. Trends. Food. Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Vilanova, N.; Solans, C. Vitamin A Palmitate-β-Cyclodextrin Inclusion Complexes: Characterization, Protection and Emulsification Properties. Food. Chem. 2015, 175, 529–535. [Google Scholar] [CrossRef]
- Fathalla, Z.; Shoman, M.E.; Barakat, H.S.; Al Fatease, A.; Alamri, A.H.; Abdelkader, H. Cyclodextrins and Amino Acids Enhance Solubility and Tolerability of Retinoic Acid/Tretinoin: Molecular Docking, Physicochemical, Cytotoxicity, Scratch Assay, and Topical Gel Formulations Investigation. Pharmaceutics 2024, 16, 853. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Design of Polymer-Free Vitamin-A Acetate/Cyclodextrin Nanofibrous Webs: Antioxidant and Fast-Dissolving Properties. Food. Funct. 2020, 11, 7626–7637. [Google Scholar] [CrossRef]
- Kim, H.; Hu, Y.; Jeong, D.; Jun, B.H.; Cho, E.; Jung, S. Synthesis, Characterization, and Retinol Stabilization of Fatty Amide-β-Cyclodextrin Conjugates. Molecules 2016, 21, 963. [Google Scholar] [CrossRef]
- Zhang, G.; Meng, F.; Guo, Z.; Guo, T.; Peng, H.; Xiao, J.; Liu, B.; Singh, V.; Gui, S.; York, P.; et al. Enhanced Stability of Vitamin A Palmitate Microencapsulated by γ-Cyclodextrin Metal-Organic Frameworks. J. Microencapsul. 2018, 35, 249–258. [Google Scholar] [CrossRef]
- Gupta, A.; Joshi, R.; Dewangan, L.; Shah, K.; Soni, D.; Patil, U.K.; Chauhan, N.S. Capsaicin: Pharmacological Applications and Prospects for Drug Designing. J. Pharm. Pharmacol. 2025, 77, 459–474. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, C.; Shi, F.; Firempong, C.K.; Yu, J.; Xu, X.; Zhang, W. Preparation, Characterization, and Pharmacokinetics Study of Capsaicin via Hydroxypropyl-Beta-Cyclodextrin Encapsulation. Pharm. Biol. 2016, 54, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, X.; Ren, K.; Zhang, X.; Zhang, Z.; Gong, T. Enhanced Aqueous Solubility and Bioavailability of Capsaicin by the Preparation of an Inclusion Complex. Arzneimittelforschung 2010, 60, 571–574. [Google Scholar] [CrossRef]
- Couto, V.M.; de Oliveira-Nascimento, L.; Cabeça, L.F.; Geraldes, D.C.; Costa, J.S.R.; Riske, K.A.; Franz-Montan, M.; Yokaychiya, F.; Dias Franco, M.K.K.; de Paula, E. Capsaicin-Cyclodextrin Complex Enhances Mepivacaine Targeting and Improves Local Anesthesia in Inflamed Tissues. Int. J. Mol. Sci. 2020, 21, 5741. [Google Scholar] [CrossRef]
- Kadian, V.; Rao, R. Exploring the in Vitro Anti-Arthritic Potential of Capsaicin-Coordinated β-Cyclodextrin Nanosponges. J. Drug. Deliv. Sci. Technol. 2023, 87, 104801. [Google Scholar] [CrossRef]
- Paroha, S.; Chandel, A.K.S.; Dubey, R.D. Nanosystems for Drug Delivery of Coenzyme Q10. Environ. Chem. Lett. 2018, 16, 71–77. [Google Scholar] [CrossRef]
- Gao, X.; Nishimura, K.; Hirayama, F.; Arima, H.; Uekama, K.; Schmid, G.; Terao, K.; Nakata, D.; Fukumi, H. Enhanced Dissolution and Oral Bioavailability of Coenzyme Q10 in dogs Obtained by Inclusion Complexation with γ-Cyclodextrin. Asian. J. Pharm. Sci. 2006, 1, 95–102. [Google Scholar]
- Takahashi, H.; Bungo, Y.; Mikuni, K.; Beppu, H.; Ozaki, S.; Shimpo, K.; Itani, Y.; Sonoda, S. Improved Thermal Property and Absorption of Coenzyme Q10 in Humans Using Cyclodextrin. J. Appl. Glycosci. 2010, 57, 193–197. [Google Scholar] [CrossRef]
- Fir, M.M.; Smidovnik, A.; Milivojevic, L.; Zmitek, J.; Prosek, M. Studies of CoQ10 and Cyclodextrin Complexes: Solubility, Thermo- and Photo-Stability. J. Incl. Phenom. Macrocyc. Chem. 2009, 64, 225–232. [Google Scholar] [CrossRef]
- Paramera, E.I.; Konteles, S.J.; Karathanos, V.T. Stability and Release Properties of Curcumin Encapsulated in Saccharomyces Cerevisiae, β-Cyclodextrin and Modified Starch. Food. Chem. 2011, 125, 913–922. [Google Scholar] [CrossRef]
- Hu, Y.; Qiu, C.; Julian McClements, D.; Qin, Y.; Long, J.; Jiao, A.; Li, X.; Wang, J.; Jin, Z. Encapsulation, Protection, and Delivery of Curcumin Using Succinylated-Cyclodextrin Systems with Strong Resistance to Environmental and Physiological Stimuli. Food. Chem. 2022, 376, 131869. [Google Scholar] [CrossRef]
- Xu, H.; Ma, Q.; Qiu, C.; Wang, J.; Jin, Z.; Hu, Y. Encapsulation and Controlled Delivery of Curcumin by Self-Assembled Cyclodextrin Succinate/Chitosan Nanoparticles. Food Hydrocoll. 2024, 157, 110465. [Google Scholar] [CrossRef]
- Song, W.; Chen, X.; Dai, C.; Lin, D.; Pang, X.; Zhang, D.; Lin, J. Comparative study of preparation, evaluation, and pharmacokinetics in beagle dogs of curcumin β-cyclodextrin inclusion complex, curcumin solid dispersion, and curcumin phospholipid complex. Molecules 2022, 27, 2998. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Wong, L.R.; Xie, H.; Ho, P.C.L. In Vitro and In Vivo Comparison of Curcumin-Encapsulated Chitosan-Coated Poly (Lactic-Co-Glycolic Acid) Nanoparticles and Curcumin/Hydroxypropyl-β-Cyclodextrin Inclusion Complexes Administered Intranasally as Therapeutic Strategies for Alzheimer’s Disease. Mol. Pharm. 2020, 17, 4256–4269. [Google Scholar] [CrossRef]
- Zhang, L.; Man, S.; Qiu, H.; Liu, Z.; Zhang, M.; Long, M.; Gao, W. Curcumin-Cyclodextrin Complexes Enhanced the Anti-Cancer Effects of Curcumin. Environ. Toxicol. Pharmacol. 2016, 48, 31–38. [Google Scholar] [CrossRef]
- Patro, N.M.; Sultana, A.; Terao, K.; Nakata, D.; Jo, A.; Urano, A.; Ishida, Y.; Gorantla, R.N.; Pandit, V.; Devi, K.; et al. Comparison and Correlation of In Vitro, In Vivo and In Silico Evaluations of Alpha, Beta and Gamma Cyclodextrin Complexes of Curcumin. J. Incl. Phenom. Macrocyc. Chem. 2014, 78, 471–483. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and Characterization of Curcumin-β-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers 2021, 13, 4073. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Fast-dissolving Antioxidant Curcumin/Cyclodextrin Inclusion Complex Electrospun Nanofibrous Webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Sheng, J.; Yang, R. Encapsulation of Curcumin in CD-MOFs: Promoting its Incorporation into Water-Based Products and Consumption. Food Funct. 2021, 12, 10795–10805. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Chen, L.; Yang, X.; Wang, Y.; Xu, J.; Zhang, R.; Ling, S.; Liu, Y. Active Curcumin-Loaded γ-Cyclodextrin-Metal Organic Frameworks as Nano Respiratory Channels for Reinforcing Chitosan/Gelatin Films in Strawberry Preservation. Food Hydrocoll. 2025, 159, 110656. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, Y.; Lei, M.; Qin, Y.; Wang, Z.; Chen, Z.; Zhang, L.; Zhu, Y. Development of Oral Curcumin Based on pH-Responsive Transmembrane Peptide-Cyclodextrin Derivative Nanoparticles for Hepatoma. Carbohydr. Polym. 2022, 277, 118892. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Vyas, A.; Ahmad, A.; Banerjee, S.; Deshpande, J.; Swamy, K.V.; Jamadar, A.; Dumhe-Klaire, A.C.; Padhye, S.; Sarkar, F.H. Inclusion complex of novel curcumin analogue CDF and β-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm. Res. 2012, 29, 1775–1786. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).