Impact of C-Terminal PKC Phosphorylation on TRPC6 Current Kinetics
Abstract
1. Introduction
2. Results
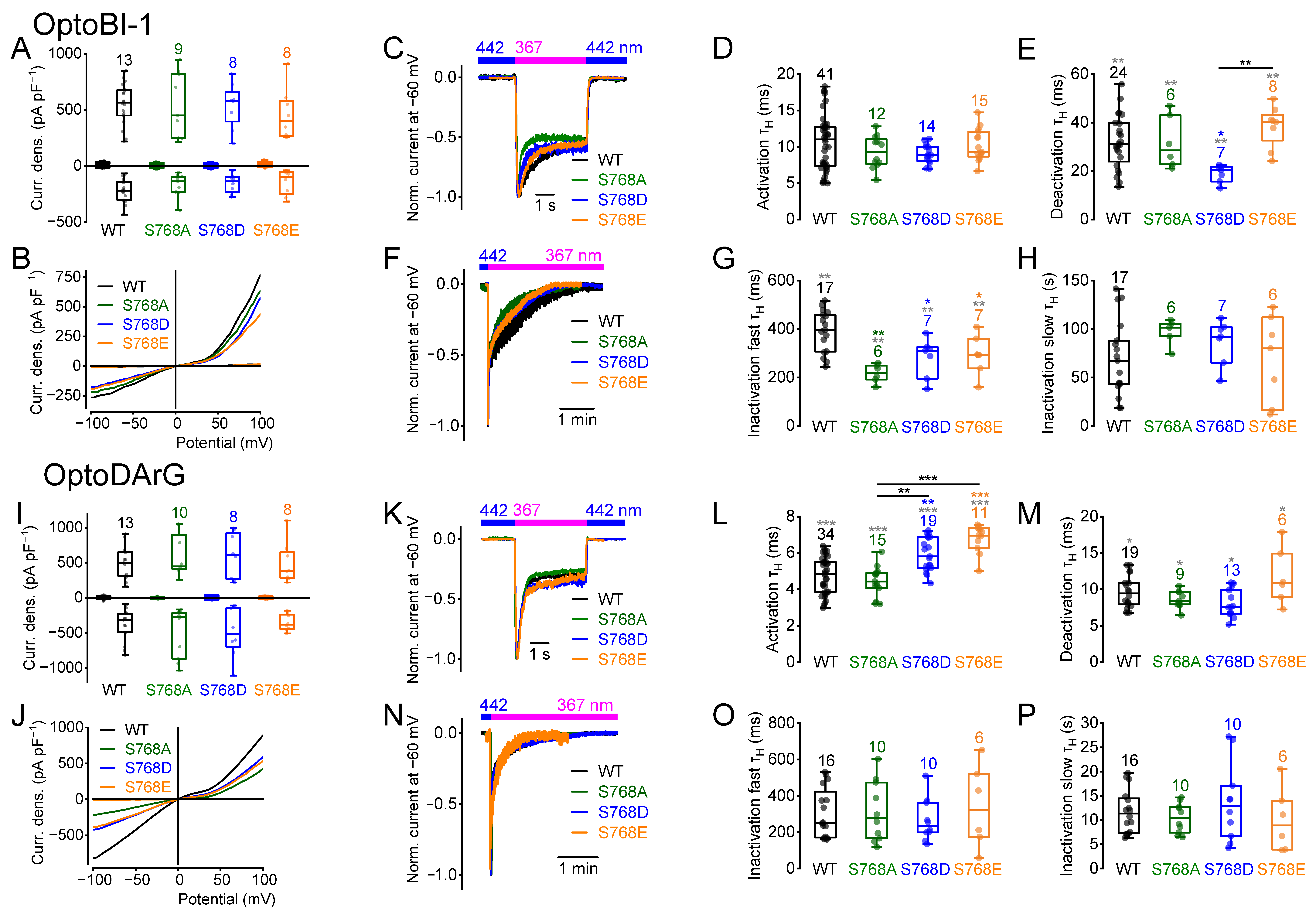
2.1. Amino Acid Exchanges of Serine 768 Result in Distinct Current Kinetics
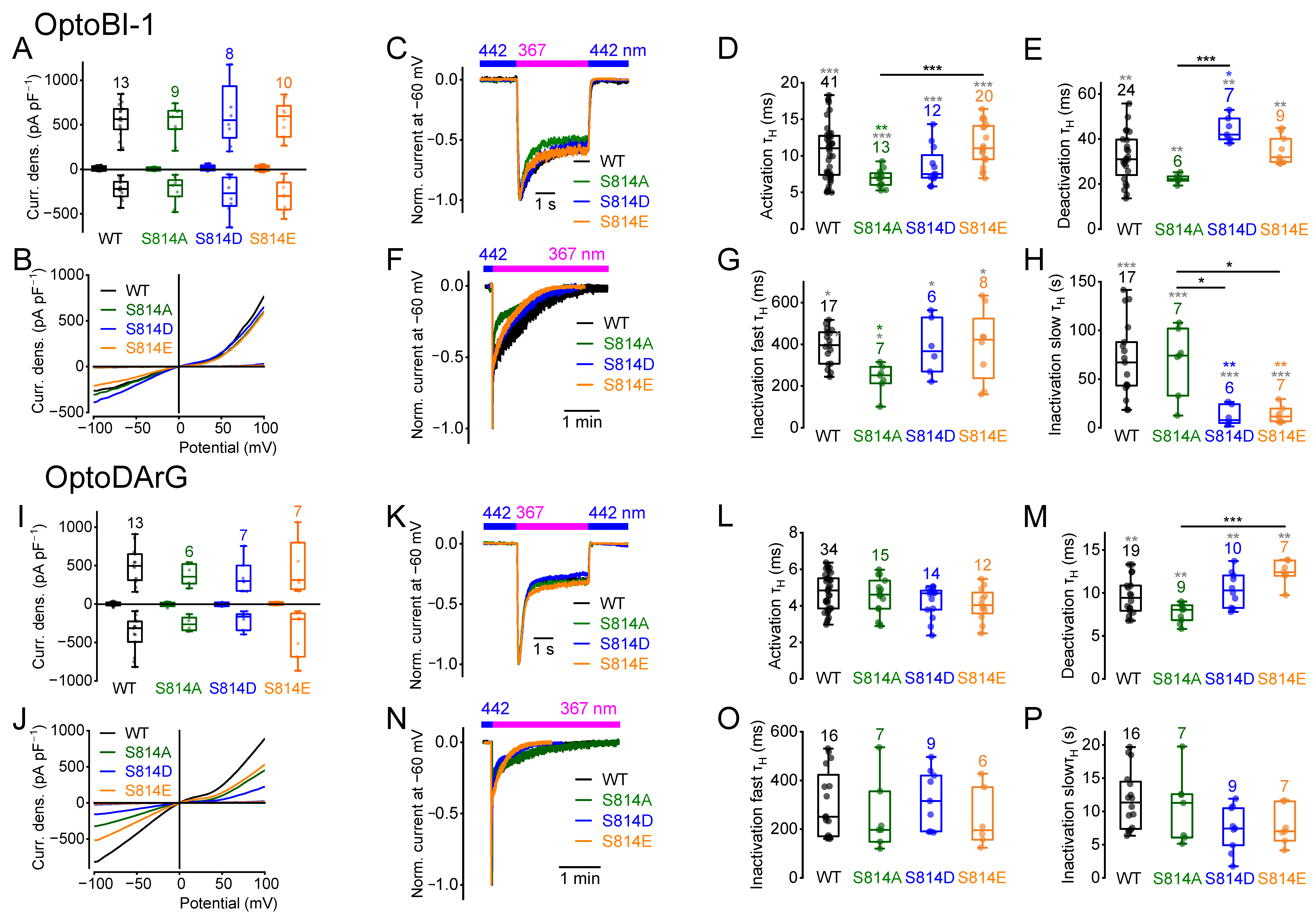
2.2. Amino Acid Exchanges of Serine 814 Influence the Current Kinetics
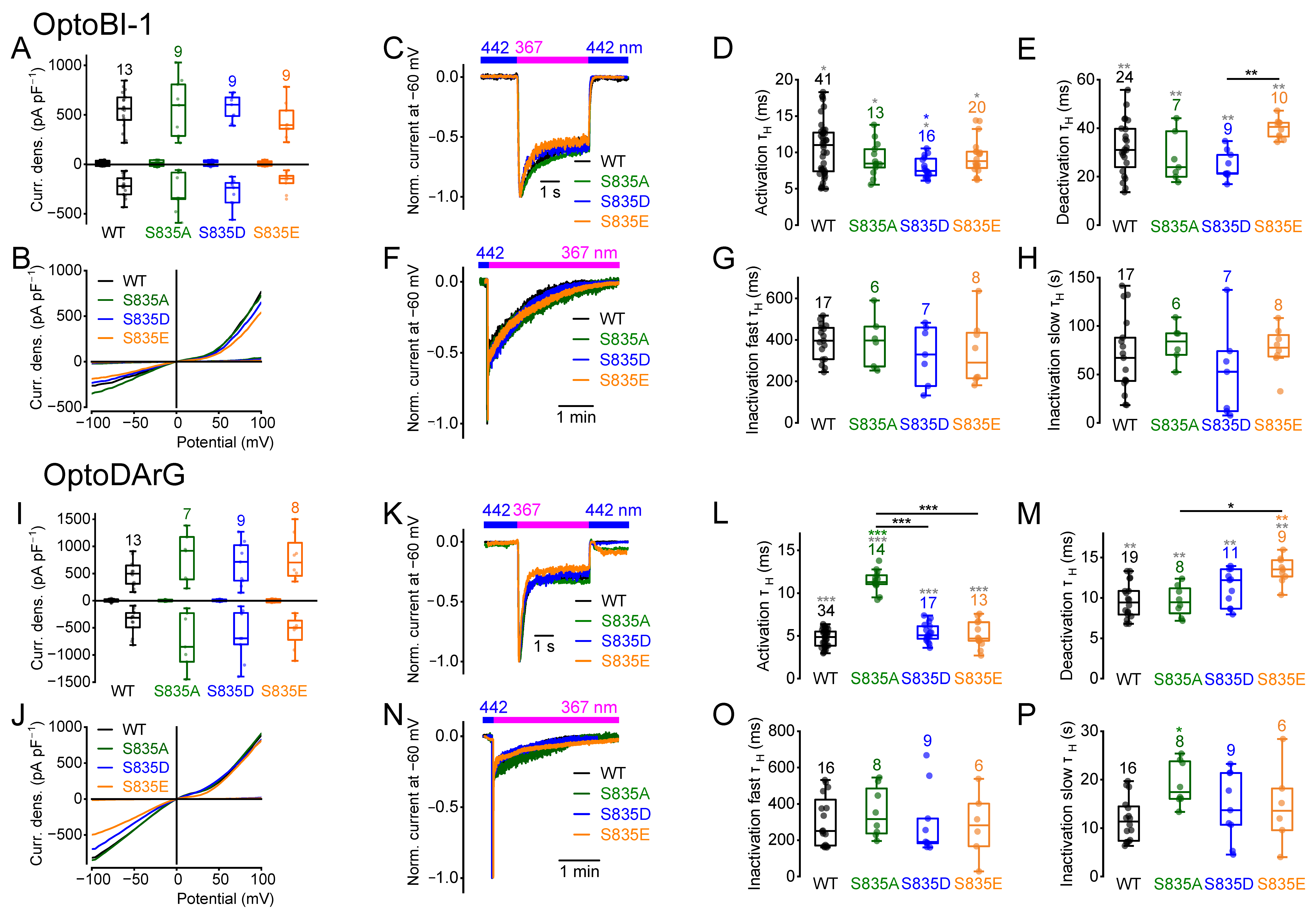
2.3. Amino Acid Exchanges of Serine 835 Alter the Current Kinetics
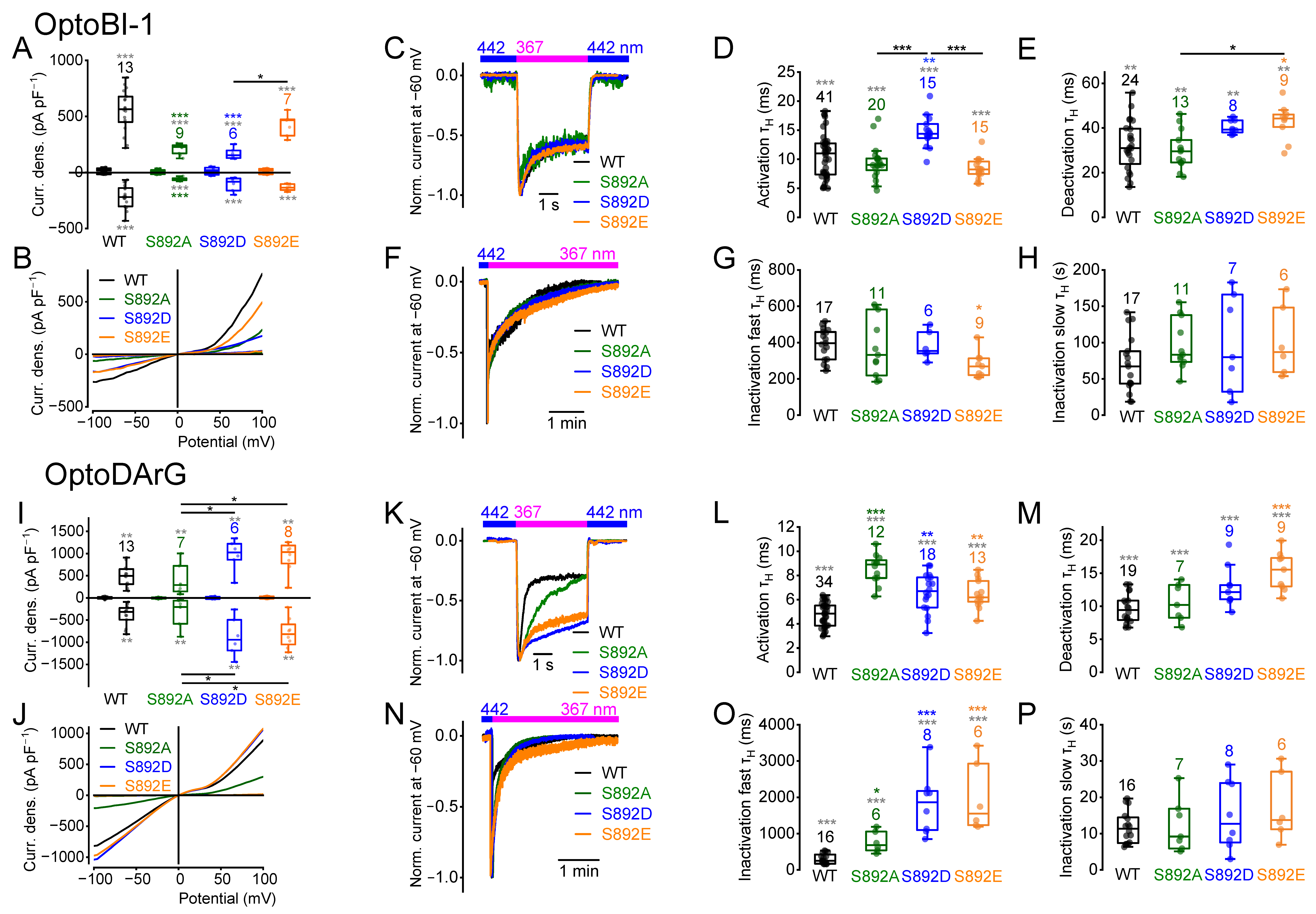
2.4. Amino Acid Exchanges of Serine 892 Influence Current Densities and Current Kinetics
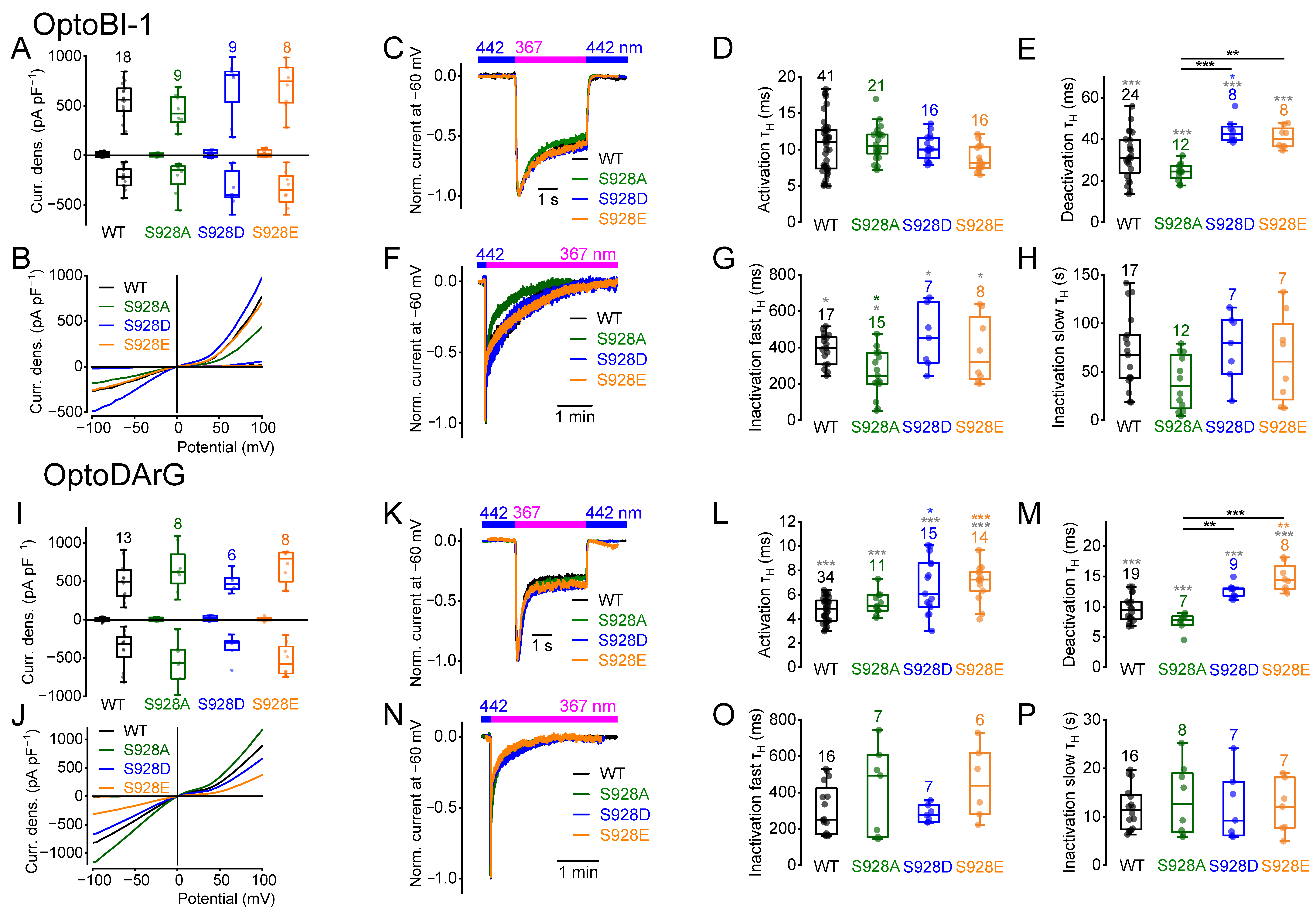
2.5. Amino Acid Exchanges of Serine 928 Cause Changes in the Current Kinetics
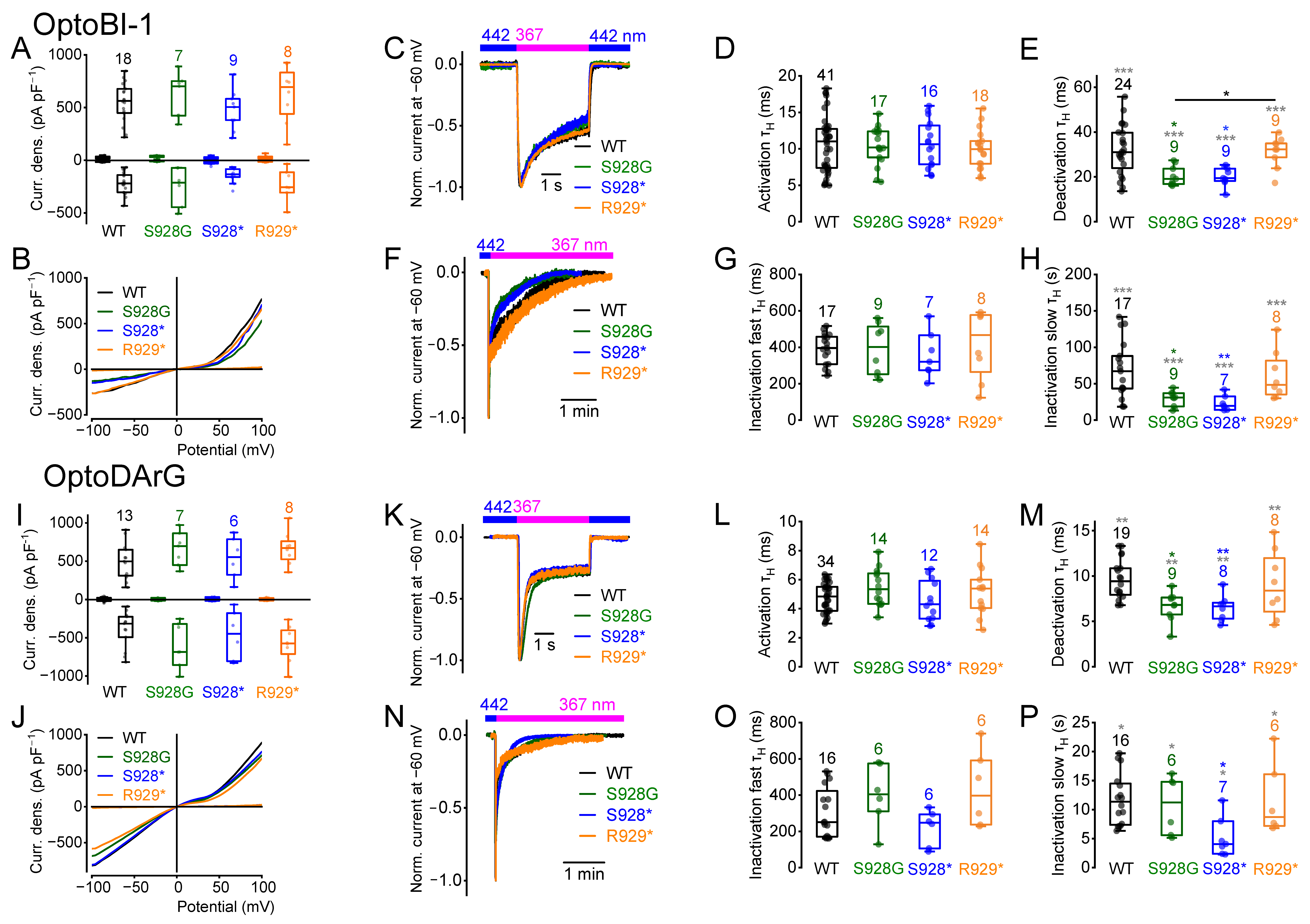
2.6. Amino Acid Exchange from Serine 928 to Glycine and C-Terminal Truncations Influence the Current Kinetics
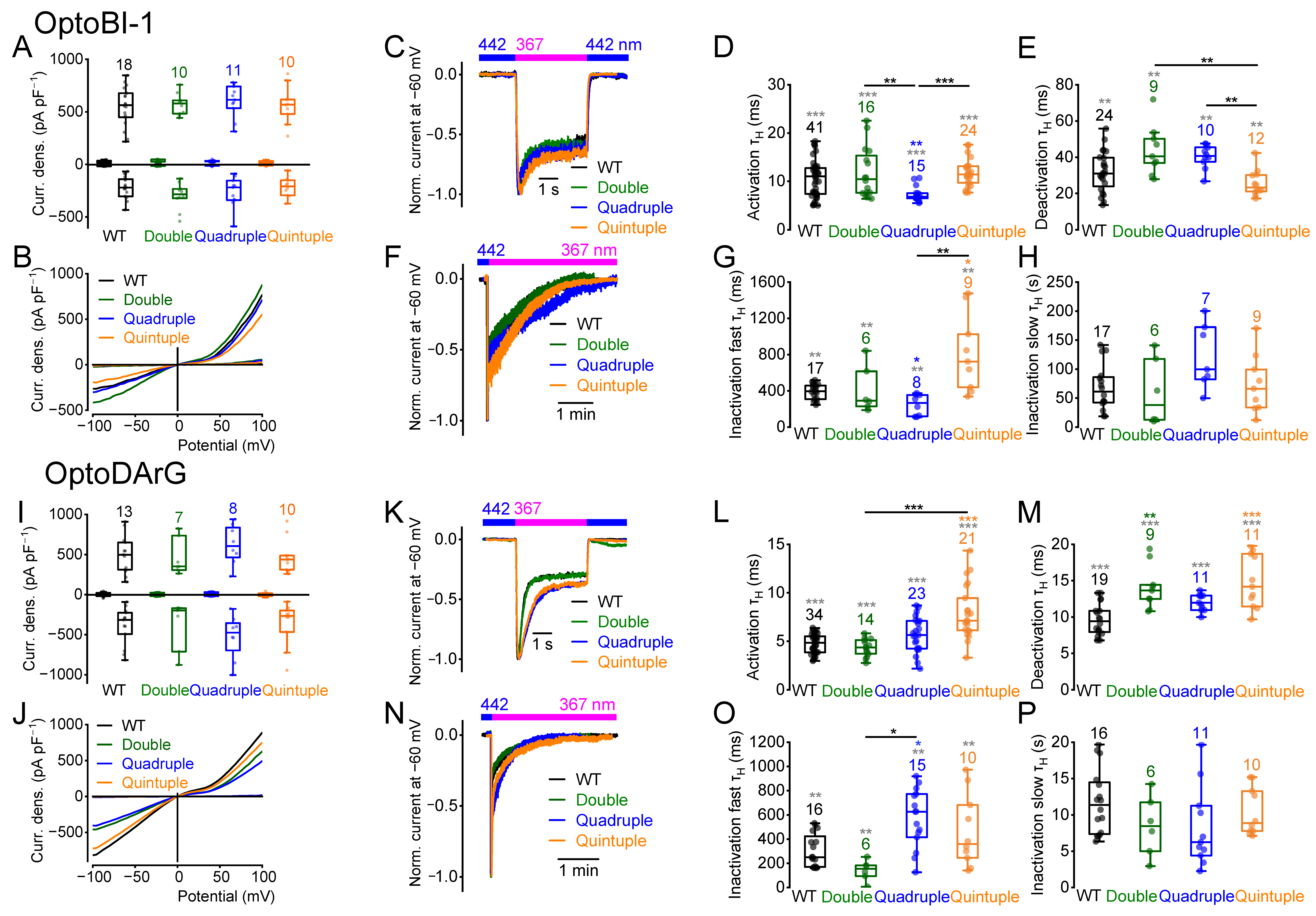
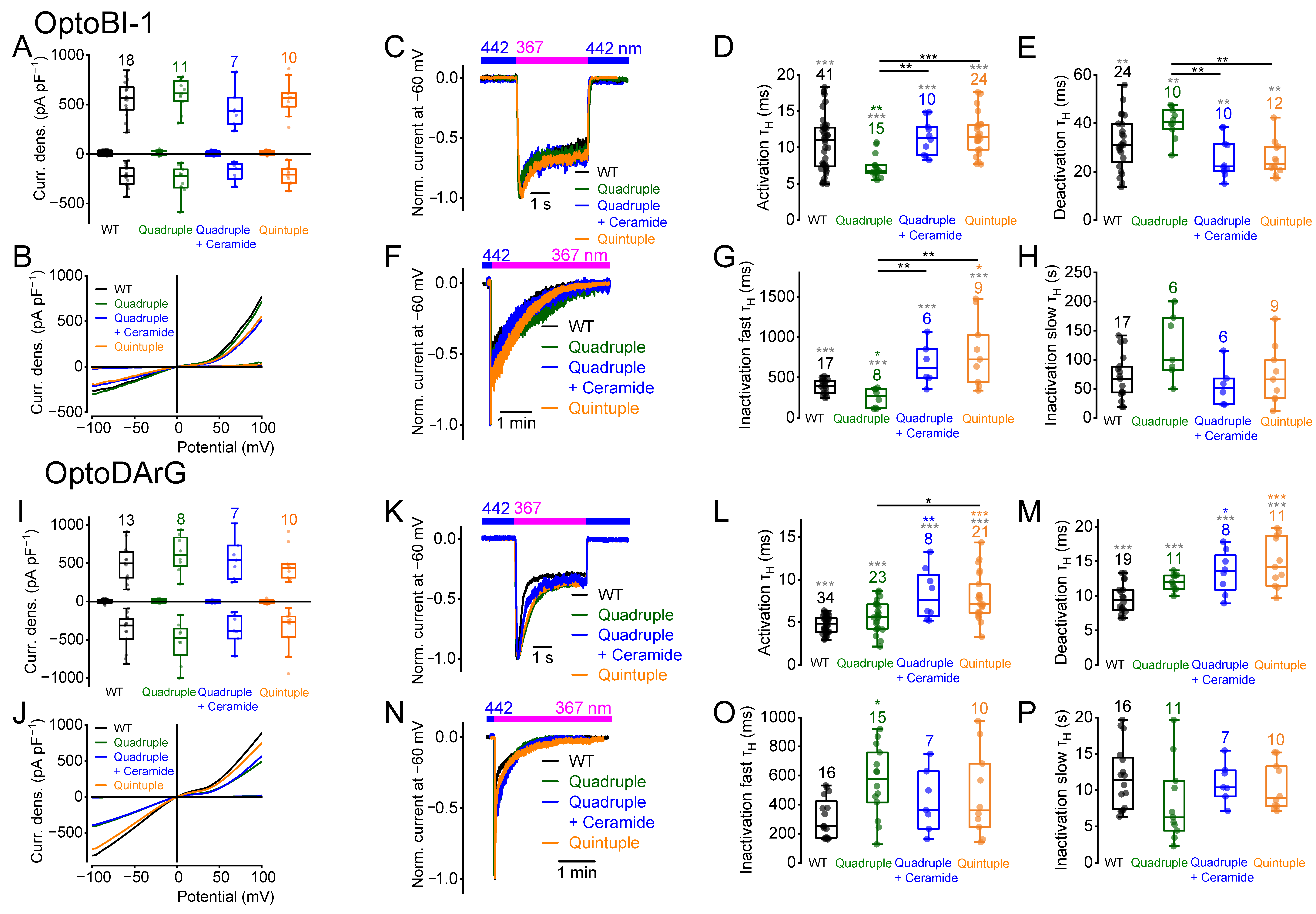
2.7. Multiple Amino Acid Exchanges Influence the Current Kinetics
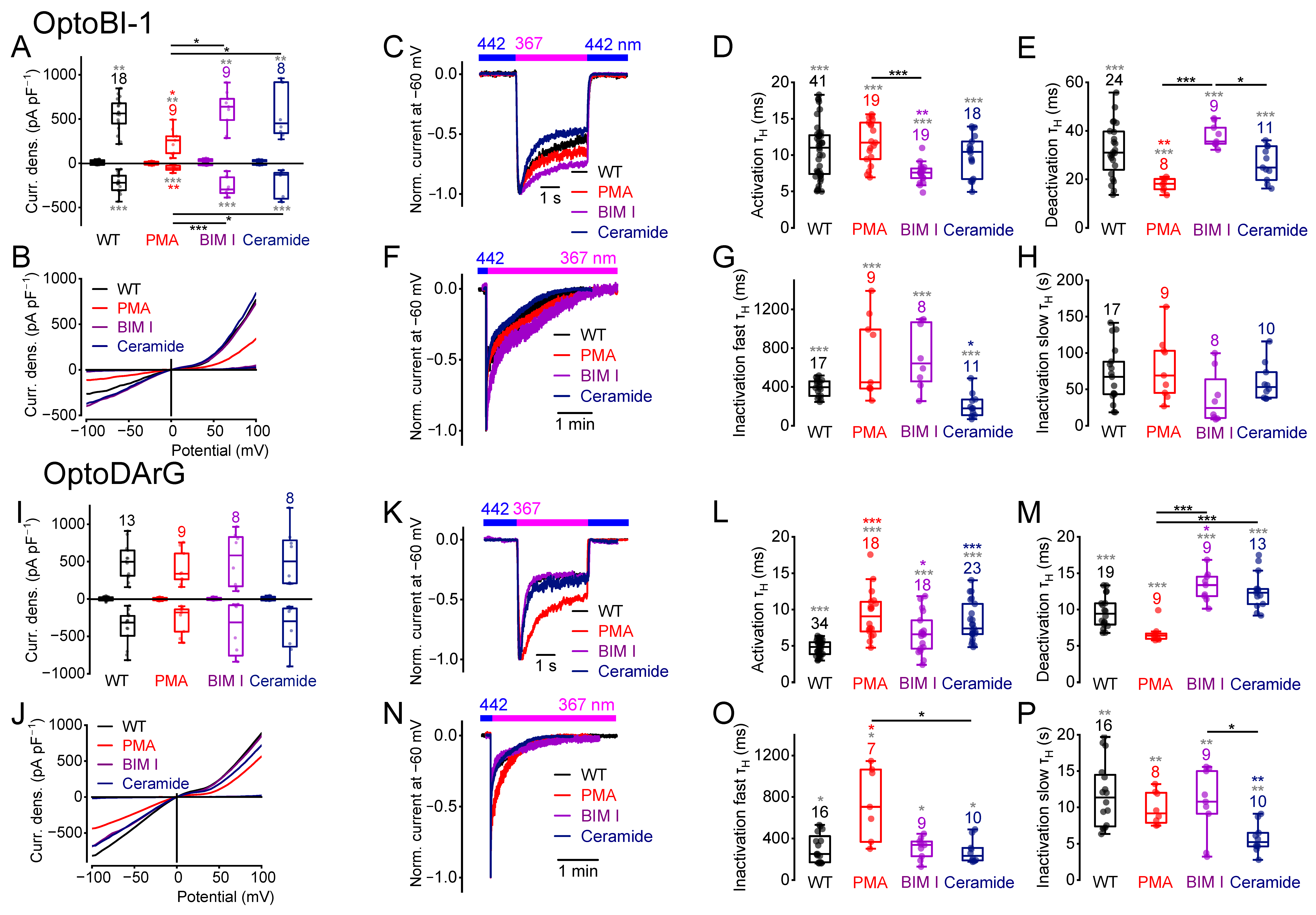
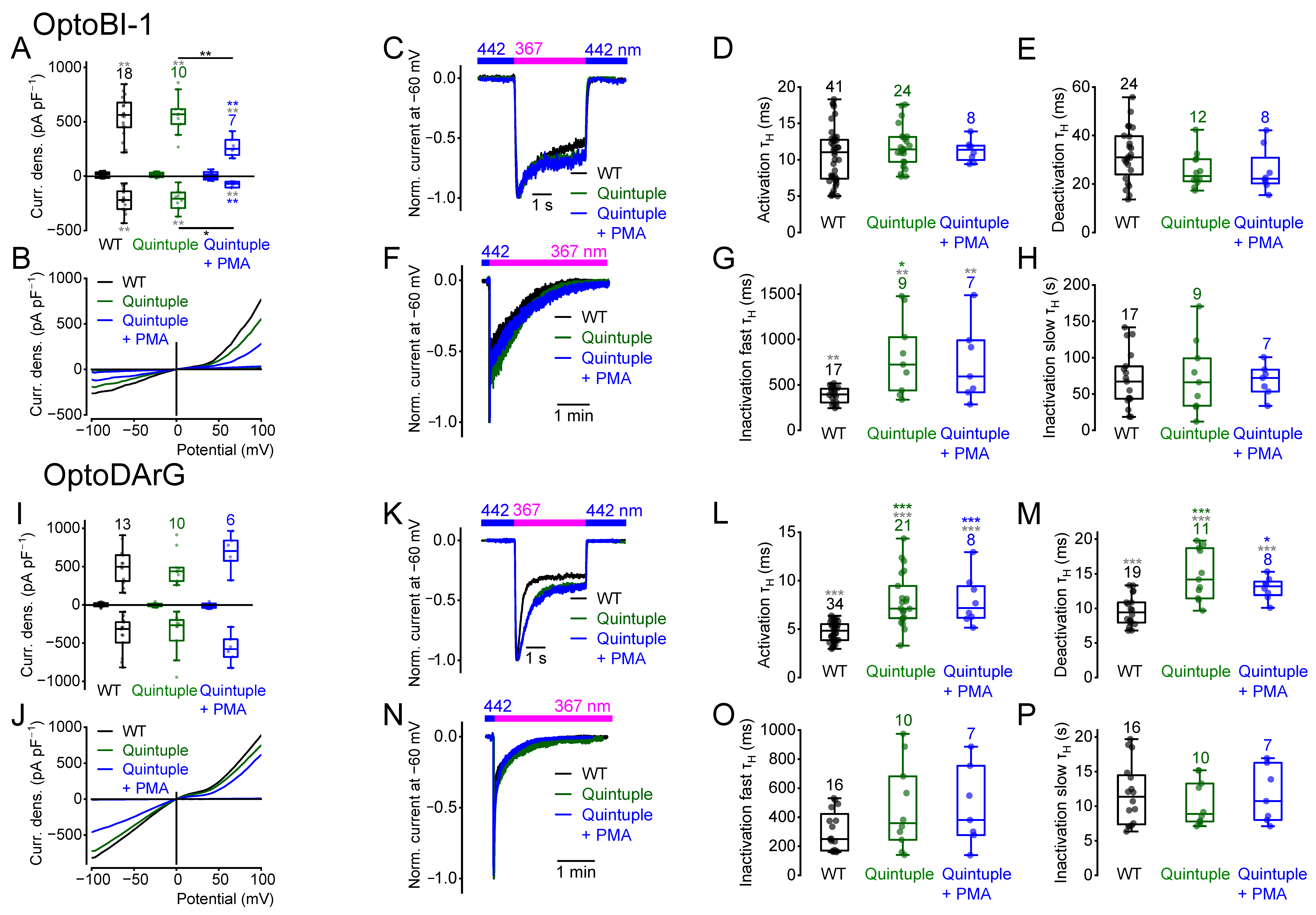
2.8. PKC Phosphorylation or Dephosphorylation Alters the Current Kinetics
2.9. The Quadruple Mutant Incubated with Ceramide Behaves Like the Quintuple Mutant
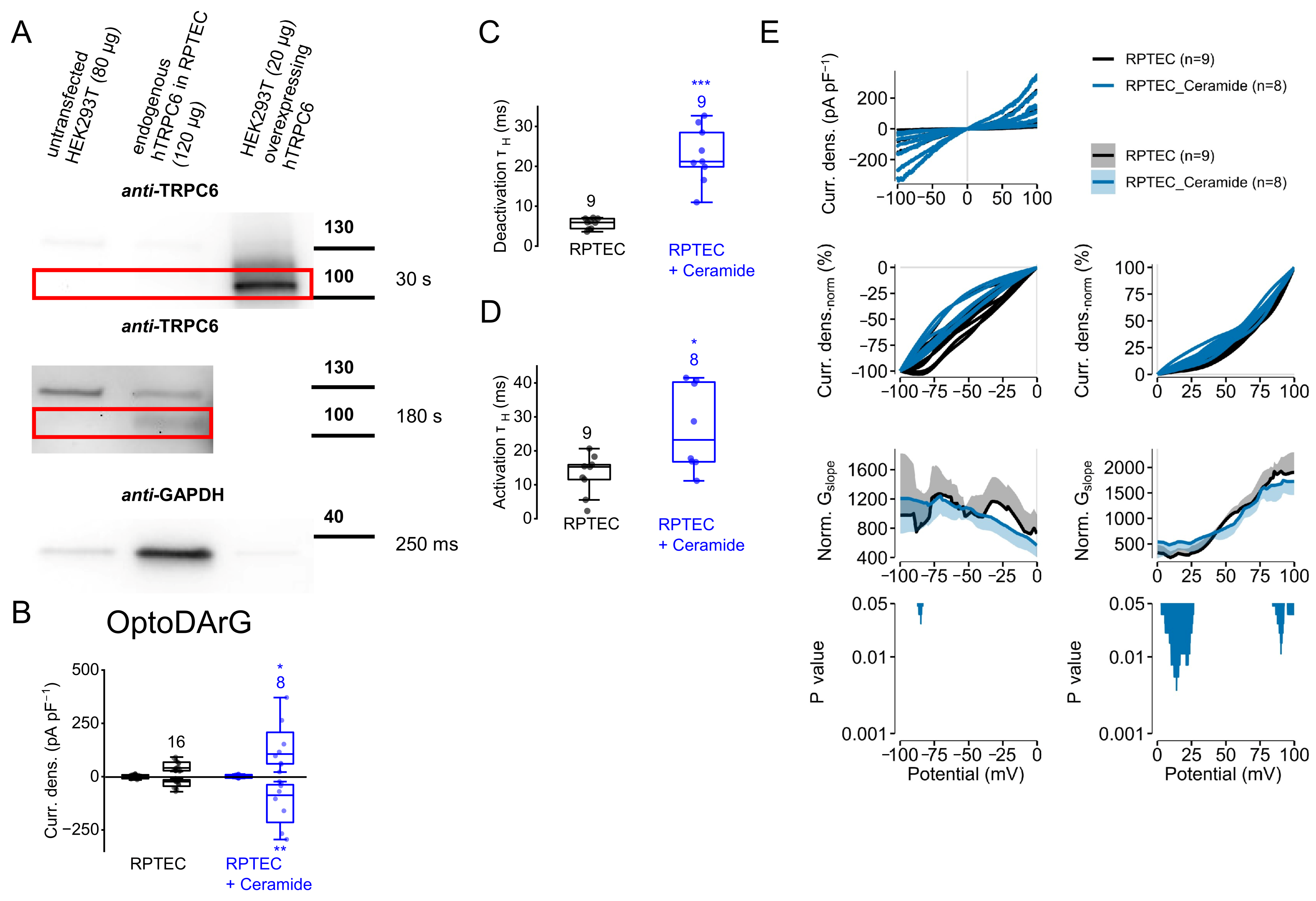
2.10. Dephosphorylation of Endogenously Expressed TRPC6 Channels Results in Increased Current Density Amplitudes and in Slower Current Kinetics
3. Discussion
4. Material and Methods
4.1. Data Availability
4.2. Cell Lines Used in the Study
4.3. Materials
4.4. Molecular Biology and Mutagenesis
4.5. Cell Culture and Transfection
4.6. Light Stimulation
4.7. Patch-Clamp Recordings
4.8. Normalized Slope Conductance
4.9. Fit Routine
4.10. Western Blot
4.11. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Sooch, G.; Demaree, I.S.; White, F.A.; Obukhov, A.G. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells 2020, 9, 1983. [Google Scholar] [CrossRef]
- Mukerji, N.; Damodaran, T.V.; Winn, M.P. TRPC6 and FSGS: The latest TRP channelopathy. Biochim. Biophys. Acta 2007, 1772, 859–868. [Google Scholar] [CrossRef]
- Winn, M.P.; Conlon, P.J.; Lynn, K.L.; Farrington, M.K.; Creazzo, T.; Hawkins, A.F.; Daskalakis, N.; Kwan, S.Y.; Ebersviller, S.; Burchette, J.L.; et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 2005, 308, 1801–1804. [Google Scholar] [CrossRef]
- Reiser, J.; Polu, K.R.; Moller, C.C.; Kenlan, P.; Altintas, M.M.; Wei, C.; Faul, C.; Herbert, S.; Villegas, I.; Avila-Casado, C.; et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 2005, 37, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, X.; Tsang, S.Y. Post-Translational Modification and Natural Mutation of TRPC Channels. Cells 2020, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Wang, Z.; Zubcevic, L.; Hsu, A.L.; He, Q.; Borgnia, M.J.; Ji, R.-R.; Lee, S.-Y. Structural insights into electrophile irritant sensing by the human TRPA1 channel. Neuron 2019, 105, 882–894.e885. [Google Scholar] [CrossRef]
- Lin, B.L.; Matera, D.; Doerner, J.F.; Zheng, N.; Del Camino, D.; Mishra, S.; Bian, H.; Zeveleva, S.; Zhen, X.; Blair, N.T.; et al. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc. Natl. Acad. Sci. USA 2019, 116, 10156–10161. [Google Scholar] [CrossRef]
- Welsh, D.G.; Morielli, A.D.; Nelson, M.T.; Brayden, J.E. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ. Res. 2002, 90, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; Mederos, Y.S.M.; Gollasch, M.; Gross, V.; Storch, U.; Dubrovska, G.; Obst, M.; Yildirim, E.; Salanova, B.; Kalwa, H.; et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol. Cell Biol. 2005, 25, 6980–6989. [Google Scholar] [CrossRef]
- Yu, Y.; Fantozzi, I.; Remillard, C.V.; Landsberg, J.W.; Kunichika, N.; Platoshyn, O.; Tigno, D.D.; Thistlethwaite, P.A.; Rubin, L.J.; Yuan, J.X. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc. Natl. Acad. Sci. USA 2004, 101, 13861–13866. [Google Scholar] [CrossRef]
- Yu, Y.; Keller, S.H.; Remillard, C.V.; Safrina, O.; Nicholson, A.; Zhang, S.L.; Jiang, W.; Vangala, N.; Landsberg, J.W.; Wang, J.-Y.; et al. A functional single-nucleotide polymorphism in the TRPC6 gene promoter associated with idiopathic pulmonary arterial hypertension. Circulation 2009, 119, 2313–2322. [Google Scholar] [CrossRef]
- Joshi, N.; Vaidya, B.; Sharma, S.S. Transient receptor potential channels as an emerging target for the treatment of Alzheimer’s disease: Unravelling the potential of pharmacological interventions. Basic. Clin. Pharmacol. Toxicol. 2024, 135, 375–400. [Google Scholar] [CrossRef]
- Sharma, A.; Patel, S.; Rajput, M.S. Emerging Trends in Modulation of Transient Receptor Potential Canonical 6 Channels as Therapeutic Targets. J. Biochem. Mol. Toxicol. 2025, 39, e70203. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Obukhov, A.G.; Schaefer, M.; Harteneck, C.; Gudermann, T.; Schultz, G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999, 397, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Mederos y Schnitzler, M.; Gudermann, T.; Storch, U. Emerging Roles of Diacylglycerol-Sensitive TRPC4/5 Channels. Cells 2018, 7, 218. [Google Scholar] [CrossRef]
- Storch, U.; Forst, A.L.; Pardatscher, F.; Erdogmus, S.; Philipp, M.; Gregoritza, M.; Mederos y Schnitzler, M.; Gudermann, T. Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol. Proc. Natl. Acad. Sci. USA 2017, 114, E37–E46. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.; Ukhanov, K.; Leinders-Zufall, T.; Zufall, F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: Mechanism of pheromone transduction. Neuron 2003, 40, 551–561. [Google Scholar] [CrossRef]
- Song, K.; Wei, M.; Guo, W.; Quan, L.; Kang, Y.; Wu, J.X.; Chen, L. Structural basis for human TRPC5 channel inhibition by two distinct inhibitors. eLife 2021, 10, e63429. [Google Scholar] [CrossRef]
- Erkan-Candag, H.; Clarke, A.; Tiapko, O.; Gsell, M.A.; Stockner, T.; Groschner, K. Diacylglycerols interact with the L2 lipidation site in TRPC3 to induce a sensitized channel state. EMBO Rep. 2022, 23, e54276. [Google Scholar] [CrossRef]
- Erkan-Candag, H.; Krivic, D.; Gsell, M.A.F.; Aleksanyan, M.; Stockner, T.; Dimova, R.; Tiapko, O.; Groschner, K. Characterization of DAG Binding to TRPC Channels by Target-Dependent cis-trans Isomerization of OptoDArG. Biomolecules 2022, 12, 799. [Google Scholar] [CrossRef]
- Lichtenegger, M.; Tiapko, O.; Svobodova, B.; Stockner, T.; Glasnov, T.N.; Schreibmayer, W.; Platzer, D.; de la Cruz, G.G.; Krenn, S.; Schober, R.; et al. An optically controlled probe identifies lipid-gating fenestrations within the TRPC3 channel. Nat. Chem. Biol. 2018, 14, 396–404. [Google Scholar] [CrossRef]
- Lee-Kwon, W.; Wade, J.B.; Zhang, Z.; Pallone, T.L.; Weinman, E.J. Expression of TRPC4 channel protein that interacts with NHERF-2 in rat descending vasa recta. Am. J. Physiol. Cell Physiol. 2005, 288, C942–C949. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, J.; Chen, Z.; Trost, C.; Flockerzi, V.; Li, M.; Ramesh, V.; Zhu, M.X. Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J. Biol. Chem. 2000, 275, 37559–37564. [Google Scholar] [CrossRef]
- Obukhov, A.G.; Nowycky, M.C. TRPC5 activation kinetics are modulated by the scaffolding protein ezrin/radixin/moesin-binding phosphoprotein-50 (EBP50). J. Cell. Physiol. 2004, 201, 227–235. [Google Scholar] [CrossRef]
- Ningoo, M.; Plant, L.D.; Greka, A.; Logothetis, D.E. PIP2 regulation of TRPC5 channel activation and desensitization. J. Biol. Chem. 2021, 296, 100726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.H.; Chae, M.; Kim, H.J.; Lee, Y.M.; Kim, M.J.; Jin, N.G.; Yang, D.K.; So, I.; Kim, K.W. Desensitization of canonical transient receptor potential channel 5 by protein kinase C. Am. J. Physiol. Cell Physiol. 2005, 289, C591–C600. [Google Scholar] [CrossRef]
- Kazanietz, M.G.; Cooke, M. Protein kinase C signaling “in” and “to” the nucleus: Master kinases in transcriptional regulation. J. Biol. Chem. 2024, 300, 105692. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Kumar, S.; Gautam, P.K.; Tomar, M.S.; Verma, P.K.; Singh, S.P.; Kumar, S.; Acharya, A. Protein kinase C-alpha and the regulation of diverse cell responses. Biomol. Concepts 2017, 8, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Capuani, B.; Pacifici, F.; Pastore, D.; Palmirotta, R.; Donadel, G.; Arriga, R.; Bellia, A.; Di Daniele, N.; Rogliani, P.; Abete, P.; et al. The role of epsilon PKC in acute and chronic diseases: Possible pharmacological implications of its modulators. Pharmacol. Res. 2016, 111, 659–667. [Google Scholar] [CrossRef]
- Chen, L.; Shi, D.; Guo, M. The roles of PKC-delta and PKC-epsilon in myocardial ischemia/reperfusion injury. Pharmacol. Res. 2021, 170, 105716. [Google Scholar] [CrossRef]
- Bertram, A.; Ley, K. Protein kinase C isoforms in neutrophil adhesion and activation. Arch. Immunol. Ther. Exp. 2011, 59, 79–87. [Google Scholar] [CrossRef]
- Brezar, V.; Tu, W.J.; Seddiki, N. PKC-Theta in Regulatory and Effector T-cell Functions. Front. Immunol. 2015, 6, 530. [Google Scholar] [CrossRef]
- Pilo, C.A.; Newton, A.C. Two Sides of the Same Coin: Protein Kinase C gamma in Cancer and Neurodegeneration. Front. Cell Dev. Biol. 2022, 10, 929510. [Google Scholar] [CrossRef] [PubMed]
- Estacion, M.; Li, S.; Sinkins, W.G.; Gosling, M.; Bahra, P.; Poll, C.; Westwick, J.; Schilling, W.P. Activation of human TRPC6 channels by receptor stimulation. J. Biol. Chem. 2004, 279, 22047–22056. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Saffen, D. Activation of M1 muscarinic acetylcholine receptors stimulates the formation of a multiprotein complex centered on TRPC6 channels. J. Biol. Chem. 2005, 280, 32035–32047. [Google Scholar] [CrossRef]
- Bousquet, S.M.; Monet, M.; Boulay, G. Protein kinase C-dependent phosphorylation of transient receptor potential canonical 6 (TRPC6) on serine 448 causes channel inhibition. J. Biol. Chem. 2010, 285, 40534–40543. [Google Scholar] [CrossRef]
- Bousquet, S.M.; Monet, M.; Boulay, G. The serine 814 of TRPC6 is phosphorylated under unstimulated conditions. PLoS ONE 2011, 6, e18121. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Jedrychowski, M.P.; Elias, J.E.; Goswami, T.; Rad, R.; Beausoleil, S.A.; Villen, J.; Haas, W.; Sowa, M.E.; Gygi, S.P. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell 2010, 143, 1174–1189. [Google Scholar] [CrossRef] [PubMed]
- Manes, N.P.; Dong, L.; Zhou, W.; Du, X.; Reghu, N.; Kool, A.C.; Choi, D.; Bailey, C.L.; Petricoin, E.F., 3rd; Liotta, L.A.; et al. Discovery of mouse spleen signaling responses to anthrax using label-free quantitative phosphoproteomics via mass spectrometry. Mol. Cell Proteom. 2011, 10, M110.000927. [Google Scholar] [CrossRef]
- Lundby, A.; Secher, A.; Lage, K.; Nordsborg, N.B.; Dmytriyev, A.; Lundby, C.; Olsen, J.V. Quantitative maps of protein phosphorylation sites across 14 different rat organs and tissues. Nat. Commun. 2012, 3, 876. [Google Scholar] [CrossRef]
- Shiromizu, T.; Adachi, J.; Watanabe, S.; Murakami, T.; Kuga, T.; Muraoka, S.; Tomonaga, T. Identification of missing proteins in the neXtProt database and unregistered phosphopeptides in the PhosphoSitePlus database as part of the Chromosome-centric Human Proteome Project. J. Proteome Res. 2013, 12, 2414–2421. [Google Scholar] [CrossRef]
- Schweppe, D.K.; Rigas, J.R.; Gerber, S.A. Quantitative phosphoproteomic profiling of human non-small cell lung cancer tumors. J. Proteom. 2013, 91, 286–296. [Google Scholar] [CrossRef]
- Trebak, M.; Hempel, N.; Wedel, B.J.; Smyth, J.T.; Bird, G.S.; Putney, J.W., Jr. Negative regulation of TRPC3 channels by protein kinase C-mediated phosphorylation of serine 712. Mol. Pharmacol. 2005, 67, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Poteser, M.; Schleifer, H.; Lichtenegger, M.; Schernthaner, M.; Stockner, T.; Kappe, C.O.; Glasnov, T.N.; Romanin, C.; Groschner, K. PKC-dependent coupling of calcium permeation through transient receptor potential canonical 3 (TRPC3) to calcineurin signaling in HL-1 myocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 10556–10561, Erratum in Proc. Natl. Acad. Sci. USA 2011, 108, 13876–13878. [Google Scholar] [PubMed]
- Gopal, S.; Sogaard, P.; Multhaupt, H.A.; Pataki, C.; Okina, E.; Xian, X.; Pedersen, M.E.; Stevens, T.; Griesbeck, O.; Park, P.W.; et al. Transmembrane proteoglycans control stretch-activated channels to set cytosolic calcium levels. J. Cell Biol. 2015, 210, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, G.U.; Mehta, D.; Vogel, S.; Holinstat, M.; Paria, B.C.; Tiruppathi, C.; Malik, A.B. Protein kinase Calpha phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J. Biol. Chem. 2004, 279, 20941–20949. [Google Scholar]
- Saleh, S.N.; Albert, A.P.; Large, W.A. Obligatory role for phosphatidylinositol 4,5-bisphosphate in activation of native TRPC1 store-operated channels in vascular myocytes. J. Physiol. 2009, 587, 531–540. [Google Scholar]
- Bodiga, V.L.; Kudle, M.R.; Bodiga, S. Silencing of PKC-alpha, TRPC1 or NF-kappaB expression attenuates cisplatin-induced ICAM-1 expression and endothelial dysfunction. Biochem. Pharmacol. 2015, 98, 78–91. [Google Scholar] [CrossRef]
- Shi, J.; Miralles, F.; Birnbaumer, L.; Large, W.A.; Albert, A.P. Store depletion induces Galphaq-mediated PLCbeta1 activity to stimulate TRPC1 channels in vascular smooth muscle cells. FASEB J. 2016, 30, 702–715. [Google Scholar]
- Xu, J.; Zhang, W.; Cui, W.; Shi, B.; Wang, H. PKCalpha promotes insulin secretion via TRPC1 phosphorylation in INS-1E cells. Biosci. Biotechnol. Biochem. 2019, 83, 1676–1682. [Google Scholar]
- Keck, M.; Hermann, C.; Lutzel, K.; Gudermann, T.; Konrad, D.B.; Mederos, Y.S.M.; Storch, U. Photoswitchable TRPC6 channel activators evoke distinct channel kinetics reflecting different gating behaviors. iScience 2024, 27, 111008. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Ellerkmann, C.S.; Schmelzer, H.; Hermann, C.; Lutzel, K.; Gudermann, T.; Konrad, D.B.; Trauner, D.; Storch, U.; Mederos, Y.S.M. Optical Control of TRPM8 Channels with Photoswitchable Menthol. Angew. Chem. Int. Ed. Engl. 2025, 64, e202416549. [Google Scholar] [CrossRef] [PubMed]
- Tiapko, O.; Shrestha, N.; Lindinger, S.; Guedes de la Cruz, G.; Graziani, A.; Klec, C.; Butorac, C.; Graier, W.F.; Kubista, H.; Freichel, M.; et al. Lipid-independent control of endothelial and neuronal TRPC3 channels by light. Chem. Sci. 2019, 10, 2837–2842. [Google Scholar] [CrossRef]
- Frank, J.A.; Yushchenko, D.A.; Hodson, D.J.; Lipstein, N.; Nagpal, J.; Rutter, G.A.; Rhee, J.S.; Gottschalk, A.; Brose, N.; Schultz, C.; et al. Photoswitchable diacylglycerols enable optical control of protein kinase C. Nat. Chem. Biol. 2016, 12, 755–762. [Google Scholar] [CrossRef]
- Müller, M.; Niemeyer, K.; Urban, N.; Ojha, N.K.; Zufall, F.; Leinders-Zufall, T.; Schaefer, M.; Thorn-Seshold, O. BTDAzo: A Photoswitchable TRPC5 Channel Activator. Angew. Chem. 2022, 61, e202201565. [Google Scholar] [CrossRef] [PubMed]
- Leinders-Zufall, T.; Storch, U.; Bleymehl, K.; Mederos y Schnitzler, M.; Frank, J.A.; Konrad, D.B.; Trauner, D.; Gudermann, T.; Zufall, F. PhoDAGs Enable Optical Control of Diacylglycerol-Sensitive Transient Receptor Potential Channels. Cell Chem. Biol. 2018, 25, 215–223.e3. [Google Scholar] [CrossRef]
- Leinders-Zufall, T.; Storch, U.; Mederos y Schnitzler, M.; Ojha, N.K.; Koike, K.; Gudermann, T.; Zufall, F. A diacylglycerol photoswitching protocol for studying TRPC channel functions in mammalian cells and tissue slices. STAR Protoc. 2021, 2, 100527. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, Y.; Luo, X.; Wang, J.; Zhou, Q.; Han, X.; Ren, J.; Wang, L.; Liang, G. Angiotensin II-induced phosphorylation of CHK1 at serine-280 drives cardiac remodelling by direct phosphorylation of JAK1, thus activating JAK1-STAT signalling in murine cardiomyocytes. Br. J. Pharmacol. 2025, 182, 6120–6135. [Google Scholar] [CrossRef]
- Pearlman, S.M.; Serber, Z.; Ferrell, J.E., Jr. A mechanism for the evolution of phosphorylation sites. Cell 2011, 147, 934–946. [Google Scholar] [CrossRef]
- Lutzel, K.; Laqua, H.; Sathian, M.B.; Nissl, B.; Szanto, J.K.; Senser, C.A.; Savasci, G.; Allmendinger, L.; Kicin, B.; Ruf, V.; et al. A Platform for the Development of Highly Red-Shifted Azobenzene-Based Optical Tools. Angew. Chem. 2025, 137, e202501779. [Google Scholar]
- Hermann, C.; Treder, A.; Naher, M.; Geiseler, R.; Gudermann, T.; Mederos y Schnitzler, M.; Storch, U. The normalized slope conductance as a tool for quantitative analysis of current-voltage relations. Biophys. J. 2022, 121, 1435–1448. [Google Scholar] [CrossRef]
- Bourbon, N.A.; Yun, J.; Berkey, D.; Wang, Y.; Kester, M. Inhibitory actions of ceramide upon PKC-epsilon/ERK interactions. Am. J. Physiol. Cell Physiol. 2001, 280, C1403–C1411. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hannun, Y.A.; Obeid, L.M. Ceramide inactivates cellular protein kinase Calpha. J. Biol. Chem. 1996, 271, 13169–13174. [Google Scholar] [CrossRef]
- Wang, Y.M.; Seibenhener, M.L.; Vandenplas, M.L.; Wooten, M.W. Atypical PKC zeta is activated by ceramide, resulting in coactivation of NF-kappaB/JNK kinase and cell survival. J. Neurosci. Res. 1999, 55, 293–302. [Google Scholar] [CrossRef]
- Bourbon, N.A.; Yun, J.; Kester, M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J. Biol. Chem. 2000, 275, 35617–35623. [Google Scholar] [CrossRef]
- Englisch, C.N.; Paulsen, F.; Tschernig, T. TRPC Channels in the Physiology and Pathophysiology of the Renal Tubular System: What Do We Know? Int. J. Mol. Sci. 2022, 24, 181. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Kallam, A. Clear Cell Renal Carcinoma (Archived). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hofmann, T.; Schaefer, M.; Schultz, G.; Gudermann, T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7461–7466. [Google Scholar] [CrossRef]
- Frank, J.A.; Antonini, M.J.; Chiang, P.H.; Canales, A.; Konrad, D.B.; Garwood, I.C.; Rajic, G.; Koehler, F.; Fink, Y.; Anikeeva, P. In Vivo Photopharmacology Enabled by Multifunctional Fibers. ACS Chem. Neurosci. 2020, 11, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, L.; Olah, A.; Olah, T.; Balla, G.; Saleem, M.A.; Orosz, P.; Zsuga, J.; Biro, K.; Csernoch, L.; Biro, T.; et al. Inhibition of TRPC6 by protein kinase C isoforms in cultured human podocytes. J. Cell. Mol. Med. 2015, 19, 2771–2779. [Google Scholar] [CrossRef]
- Bharath, L.P.; Ruan, T.; Li, Y.; Ravindran, A.; Wan, X.; Nhan, J.K.; Walker, M.L.; Deeter, L.; Goodrich, R.; Johnson, E.; et al. Ceramide-Initiated Protein Phosphatase 2A Activation Contributes to Arterial Dysfunction In Vivo. Diabetes 2015, 64, 3914–3926. [Google Scholar] [CrossRef] [PubMed]
- Bourbon, N.A.; Sandirasegarane, L.; Kester, M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: Implications for growth arrest. J. Biol. Chem. 2002, 277, 3286–3292. [Google Scholar] [CrossRef]
- Vetterkind, S.; Lin, Q.Q.; Morgan, K.G. A novel mechanism of ERK1/2 regulation in smooth muscle involving acetylation of the ERK1/2 scaffold IQGAP1. Sci. Rep. 2017, 7, 9302. [Google Scholar] [CrossRef] [PubMed]
- Saouaf, S.J.; Mahajan, S.; Rowley, R.B.; Kut, S.A.; Fargnoli, J.; Burkhardt, A.L.; Tsukada, S.; Witte, O.N.; Bolen, J.B. Temporal differences in the activation of three classes of non-transmembrane protein tyrosine kinases following B-cell antigen receptor surface engagement. Proc. Natl. Acad. Sci. USA 1994, 91, 9524–9528. [Google Scholar] [CrossRef]
- Nishizuka, Y. Perspectives on the role of protein kinase C in stimulus-response coupling. J. Natl. Cancer Inst. 1986, 76, 363–370. [Google Scholar] [PubMed]
- Violin, J.D.; Zhang, J.; Tsien, R.Y.; Newton, A.C. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J. Cell Biol. 2003, 161, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, T.; Higa, T.; Aoyagi, H.; Nishiya, T.; Terada, K.; Miwa, S. Adenylate cyclase/cAMP/protein kinase A signaling pathway inhibits endothelin type A receptor-operated Ca2+ entry mediated via transient receptor potential canonical 6 channels. J. Pharmacol. Exp. Ther. 2012, 340, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Lin, H.; Geshi, N.; Mori, Y.; Kawarabayashi, Y.; Takami, N.; Mori, M.X.; Honda, A.; Inoue, R. Nitric oxide-cGMP-protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. J. Physiol. 2008, 586, 4209–4223. [Google Scholar] [CrossRef]
- Nishioka, K.; Nishida, M.; Ariyoshi, M.; Jian, Z.; Saiki, S.; Hirano, M.; Nakaya, M.; Sato, Y.; Kita, S.; Iwamoto, T.; et al. Cilostazol suppresses angiotensin II-induced vasoconstriction via protein kinase A-mediated phosphorylation of the transient receptor potential canonical 6 channel. Arter. Thromb. Vasc. Biol. 2011, 31, 2278–2286. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, X.Y.; Aoudjit, L.; Mouawad, F.; Baldwin, C.; Nattel, S.; Takano, T. Nuclear factor of activated T cells mediates RhoA-induced fibronectin upregulation in glomerular podocytes. Am. J. Physiol. Ren. Physiol. 2013, 304, F849–F862. [Google Scholar] [CrossRef]
- Koitabashi, N.; Aiba, T.; Hesketh, G.G.; Rowell, J.; Zhang, M.; Takimoto, E.; Tomaselli, G.F.; Kass, D.A. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. J. Mol. Cell. Cardiol. 2010, 48, 713–724. [Google Scholar] [CrossRef]
| Mutation | Primer Name | Primer Sequence (5′-3′) | Annealing Temperature [°C] |
|---|---|---|---|
| S768A | mTRPC6_S768A_f | TCTTGTACCAgccCCAAAATCCTTGCTTTATC | 61 |
| mTRPC6_S768A_r | TTGAAGGGGACAGGAAGTG | ||
| S768D | mTRPC6_S768D_f | TCTTGTACCAgacCCAAAATCCTTGCTTTATC | 61 |
| mTRPC6_S768D_r | TTGAAGGGGACAGGAAGTG | ||
| S768E | mTRPC6_S768E_f | TCTTGTACCAgaaCCAAAATCCTTGCTTTATC | 61 |
| mTRPC6_S768E_r | TTGAAGGGGACAGGAAGTG | ||
| S814A | mTRPC6_S814A_f | AATTTCAGGAgccCACGAAGACCTTTC | 57 |
| mTRPC6_S814A_r | CCAAATTTCTTTTCTTCATTTCTC | ||
| S814D | mTRPC6_S814D_f | AATTTCAGGAgacCACGAAGACCTTTC | 57 |
| mTRPC6_S814D_r | CCAAATTTCTTTTCTTCATTTCTC | ||
| S814E | mTRPC6_S814E_f | AATTTCAGGAgaaCACGAAGACCTTTC | 57 |
| mTRPC6_S814E_r | CCAAATTTCTTTTCTTCATTTCTC | ||
| S835A | mTRPC6_S835A_F | CAAACAATCAgccACAAGGAGCTCAGAAG | 60 |
| mTRPC6_S835A_R | TTGTGTGCCAACTGATTTTTG | ||
| S835D | mTRPC6_S835D_f | CAAACAATCAgacACAAGGAGCTCAGAAG | 59 |
| mTRPC6_S835D_r | TTGTGTGCCAACTGATTTTTG | ||
| S835E | mTRPC6_S835E_f | CAAACAATCAgaaACAAGGAGCTCAGAAG | 59 |
| mTRPC6_S835E_r | TTGTGTGCCAACTGATTTTTG | ||
| S892A | mTRPC6_S892A_f | AGACATCTCAgccCTCCGTTATGAAC | 56 |
| mTRPC6_S892A_r | TGCTTAATTTCCTTCAATTC | ||
| S892D | mTRPC6_S892D_f | AGACATCTCAgacCTCCGTTATGAAC | 56 |
| mTRPC6_S892D_r | TGCTTAATTTCCTTCAATTC | ||
| S892E | mTRPC6_S892E_f | AGACATCTCAgaaCTCCGTTATGAAC | 56 |
| mTRPC6_S892E_r | TGCTTAATTTCCTTCAATTC | ||
| S928A | mTRPC6_S928A_f | GCTGGAGGAAgccCGCAGATAGC | 62 |
| mTRPC6_S928A_r | TTTGGCTCTAACGACAGTC | ||
| S928D | mTRPC6_S928D_f | GCTGGAGGAAgacCGCAGATAGC | 63 |
| mTRPC6_S928D_r | TTTGGCTCTAACGACAGTC | ||
| S928E | mTRPC6_S928E_f | GCTGGAGGAAgaaCGCAGATAGC | 58 |
| mTRPC6_S928E_r | TTTGGCTCTAACGACAGTC | ||
| S928G | mTRPC6_S928G_f | GCTGGAGGAAggcCGCAGATAGC | 67 |
| mTRPC6_S928G_r | TTTGGCTCTAACGACAGTCTCTC | ||
| S928* | mTRPC6_S928Stopp_f | GCTGGAGGAAtaaCGCAGATAGCC | 60 |
| mTRPC6_S928Stopp_r | TTTGGCTCTAACGACAGTC | ||
| R929* | mTRPC6_R929Stopp_f | GGAGGAAAGCtaaAGATAGCCCTAC | 59 |
| mTRPC6_R929Stopp_r | AGCTTTGGCTCTAACGAC |
| Parameter | Value |
|---|---|
| MaxFunEvals | 5000 |
| MaxIter | 10,000 |
| TolX | 1 × 10−10 |
| TolFun | 1 × 10−6 |
| Initial values activation | a = 0.01; τH = 1; c = 0.01 |
| Initial values deactivation | a = 0.7; τH = 7; c = 0.1 |
| Initial values inactivation | a1 = 1; τH1 = 0.02; c = 0.1, a2 = 1, τH2 = 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keck, M.; Pöll, S.; Schmelzer, H.; Kressmann, T.; Hermann, C.; Mederos y Schnitzler, M.; Storch, U. Impact of C-Terminal PKC Phosphorylation on TRPC6 Current Kinetics. Int. J. Mol. Sci. 2025, 26, 11482. https://doi.org/10.3390/ijms262311482
Keck M, Pöll S, Schmelzer H, Kressmann T, Hermann C, Mederos y Schnitzler M, Storch U. Impact of C-Terminal PKC Phosphorylation on TRPC6 Current Kinetics. International Journal of Molecular Sciences. 2025; 26(23):11482. https://doi.org/10.3390/ijms262311482
Chicago/Turabian StyleKeck, Maximilian, Sebastian Pöll, Hannah Schmelzer, Tabea Kressmann, Christian Hermann, Michael Mederos y Schnitzler, and Ursula Storch. 2025. "Impact of C-Terminal PKC Phosphorylation on TRPC6 Current Kinetics" International Journal of Molecular Sciences 26, no. 23: 11482. https://doi.org/10.3390/ijms262311482
APA StyleKeck, M., Pöll, S., Schmelzer, H., Kressmann, T., Hermann, C., Mederos y Schnitzler, M., & Storch, U. (2025). Impact of C-Terminal PKC Phosphorylation on TRPC6 Current Kinetics. International Journal of Molecular Sciences, 26(23), 11482. https://doi.org/10.3390/ijms262311482






