Abstract
The current WHO grading of central nervous system tumors relies exclusively on histopathological criteria for diagnosing lower-grade, IDH-mutant astrocytomas (LGIMAs), overlooking genetic features. The TP53 R273C mutation, frequently observed in brain tumors, may influence LGIMA biology and aggressiveness. We analyzed 14 TP53-mutant LGIMAs using NGS. Five tumors (33.3%) carried the R273C mutation; these were mostly of grade 2 and all from female patients. Ki-67 levels in R273C-mutant tumors were higher compared with those in other TP53-mutant grade 2 tumors but lower than those in grade 3 tumors, which may suggest that R273C defines a more aggressive grade 2 profile. This mutation was linked to loss of the wild-type allele, supporting a loss-of-function mechanism. Its frequency was found to be potentially higher in women, and this sex-based difference reached statistical significance when incorporating TCGA LGIMA data. Overall, the R273C mutation, although mechanistically unclear, is more prevalent than other TP53 variants and defines a distinct biological subset of LGIMAs, marked by increased Ki-67 and female predominance. Incorporating TP53 and broader genetic profiling via NGS could improve our understanding of LGIMAs and support a refined classification system, enhancing diagnostic and prognostic accuracy.
1. Introduction
Infiltrative astrocytic tumors are the most frequent primary malignant neoplasms of the central nervous system (CNS), and the lack of effective therapeutic approaches against them explains their high mortality and morbidity rates [1]. Astrocytomas show a wide range of biologic behavior and different outcomes among the four grades in which they are classified by the World Health Organization (WHO) system, from pilocytic astrocytoma (grade 1) to glioblastoma (grade 4) [2]. In the latest updates, this classification tends to highlight genetic tumor patterns as critical features in order to determine the grades of these entities. Thus, mutations in the isocitrate dehydrogenase genes IDH1 and IDH2 define specific entities, associated with lower histologic grades and better prognosis when compared to IDH-wild-type astrocytomas [3].
IDH-mutant astrocytomas (IMAs) are diffusely infiltrating gliomas that frequently present mutations in other genes, TP53 (in over 90% of tumors) and ATRX (in over 70% of tumors), and do not show the 1p/19q codeletion [4,5].
The ubiquity of TP53 mutations suggests that they play an essential role in the initiation and progress of growth in these relatively less aggressive tumors, which is an interesting and unexplained issue, considering that other brain and non-CNS tumors show highly malignant behavior when TP53 mutations are present. For instance, fewer than 30% of IDH-wild-type glioblastomas show TP53 mutations; however, their acquisition occurs later in tumor progression, their behavior is more aggressive, and their grade is higher [2,6,7], thus suggesting a different and critical role of TP53 mutations in IMA. Of all the mutations described for this locus, there is a single dominant hotspot at codon 273, which accounts for 38% of all TP53 mutations in IMA, whereas its frequency in IDH-wild-type tumors is much lower, around 5% [3]. Moreover, of all the codon 273 TP53 mutations, there is one that stands out for its high prevalence: the one that codes for the R273C amino acid substitution or TP53 R273C. This mutation accounts for 20 to 30% of all TP53 mutations in IMA [3].
According to the WHO grading system for CNS tumors, IMA can be classified into three grades: 2, 3 and 4. Grades 2 and 3 comprise low-grade, IDH-mutant astrocytomas (LGIMAs), which have less aggressive behavior than grade 4 gliomas [2]. Considering prognosis, some LGIMAs are stable for a long time, while others progress to higher degrees fast, within months [8].
Grade 4 IDH-mutant astrocytomas are discriminated from their LGIMA counterparts based on the presence of the homozygous deletion of CDKN2A/2B, the existence of microvascular proliferation or necrosis, or any combination of these three features. Instead, LGIMAs are classified according to the absence of any of these grade 4 features and histopathologic criteria.
Grade 2 IDH-mutant astrocytoma is an LGIMA with a lower grade and thus a less aggressive one. The histopathologic features that define it are (i) good differentiation (low cell density, no anaplastic features) and (ii) absence or a very low rate of mitotic and proliferative activity (Figure 1) [2]. However, the histopathological classification of grades 2 and 3 cannot accurately predict aggressiveness nor survival. Additional genetic characterization and classification could be useful in order to better estimate aggressiveness and guide treatment [9]. TP53 is one of the most frequently mutated genes in LGIMAs, with the TP53 R273C mutation being predominant [10,11]. The biological role of TP53 R273C is still not well understood. In several cancers and in vitro models with tumor cells, this mutation has shown contradictory effects [12,13,14,15]. In LGIMAs, it seems clear that TP53 R273C may play a fundamental role in aggressiveness, and it has been postulated that this mutation could be an unfavorable prognostic biomarker for LGIMA patients and associated with higher tumor mutation burden values [9].
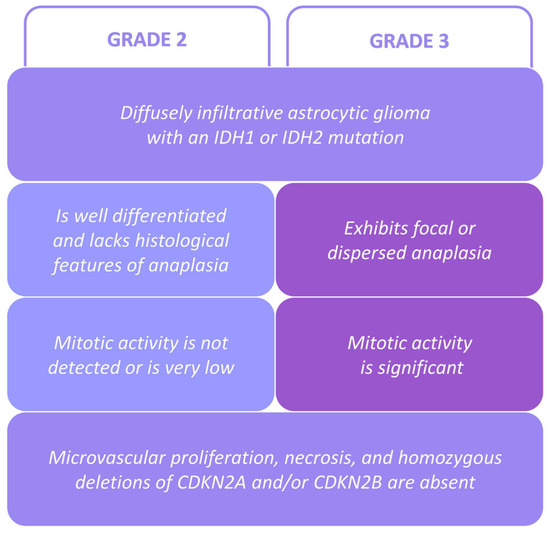
Figure 1.
LGIMA classification criteria according to the 2021 WHO grading system [2].
The ATRX mutation, the second main mutation present in IMA, is associated with an abnormal telomere maintenance mechanism known as alternative lengthening of telomeres. It is known that this mutation can induce p53-dependent cell death in some contexts. Therefore, TP53 mutations in IMA may enable tumor cell survival in the setting of ATRX loss [16].
In the present study, we describe a cohort of 14 patients with LGIMAs harboring TP53 mutations, with a long clinical follow-up, and we assess whether these mutations had a beneficial or malignant effect on the prognosis or our patients, considering all other gene mutations which could be simultaneously present.
The predominant mutation in our cohort, R273C, appears to define a more aggressive tumor subtype, with higher prevalence in female patients, compared to other LGIMAs lacking this mutation. Similarly to R273C, reported mutations in TP53 or in other genes may also play a role in determining LGIMA phenotype, yet they are not considered under the current WHO classification criteria. Therefore, we propose next-generation sequencing (NGS) as a valuable tool for improving characterization and classification of LGIMAs, with the potential to better predict tumor behavior and patient survival.
2. Results
From our neuropathology archives, we retrieved data on fourteen patients diagnosed with LGIMAs, which were classified as grade 2 or 3, according to the WHO 5th Edition grading system criteria (Figure 1) [2]. Our cohort included five men and nine women (two cases were from the same woman, with a surgical resected relapsed tumor), with ages from 29 to 70 years old (45.6 ± 12.8), without significant differences between sexes (45.0 ± 14.9 in men against 46.0 ± 12.5 in women). The median follow-up was 88 months (with a range of 24–240 months). All patients were alive at the end of this study.
Ten LGIMAs from our cohort were diagnosed as grade 2 IDH-mutant astrocytomas, while the other four were diagnosed as grade 3. Histologically, they were diffuse glial cell tumors with low/moderate density, mild or medium nuclear atypia, and infiltrative growth. No mitotic figures were detected in grade 2 tumors, whereas there were some mitosis or anaplastic features in grade 3 tumors (Figure 2a,b). Neither necrosis nor vascular proliferation was detected.

Figure 2.
IDH-mutant astrocytomas of grades 2 and 3, demonstrating histological differences: (a) Absence of anaplasia and mitotic figures in grade 2 (HE). (b) Focal anaplasia and low mitotic activity (arrow) in grade 3 (HE). (c) Low Ki67 in grade 2 (2%). Oval indicates representative area where Ki-67 expression is detected. (d) Higher Ki67 levels in grade 3 (12%). Oval indicates representative area where Ki-67 expression is detected. (e) Absence of ATRX expression in both types of LGIMA. Micrographs are representative of samples of our cohort. Magnification: 40×. HE: hematoxylin–eosin.
Immunohistochemistry showed low Ki-67 values in grade 2 tumors (range of 1–7%, average of 4%), whereas grade 3 tumors had higher levels (range of 5–12%, average of 9.0%). Nuclear p53 expression was present in all cases (range of 1–80%, average of 45%). We observed ATRX in nine cases: seven cases lost nuclear expression of ATRX, but two cases were focally positive. These two cases and the five ones with no available immunohistochemical information were studied for 1p/19q codeletion, confirming a negative result (no co-deletion present) (Figure 2c–e).
Table 1 summarizes the most relevant features of the 14 cases of our cohort, including IDH1/2 and TP53 mutations. Molecular diagnosis via NGS confirmed IDH1 R132H (in 11 tumors), IDH1 R132G (1 tumor), IDH1 R132L (1 tumor), and IDH2 R172G (1 tumor) mutations. All the 14 LGIMAs presented TP53 mutations, most of them at the hotspot codon 273 (46.6% of the mutations), with a median variant allele frequency (VAF) of 59,5% (13–96%). Five tumors presented the TP53 R273C mutation (33.3%), three the TP53 R248Q mutation (20%), and two the TP53 R175H mutation (13.3%), and the other four showed different TP53 mutations (Table 1, Figure 3a). Association with Li–Fraumeni syndrome was ruled out for all the samples.

Table 1.
Tumor grades, sex, age, relevant mutations, follow-up after surgery, and comorbidities of the 14 patients and LGIMAs.

Figure 3.
(a) Frequency of TP53 mutations in the LGIMA tumors of our cohort. (b) Predominance of the TP53 R273C mutation in brain tumors compared to cancers from other tissues, determined through TCGA studies.
We performed a TCGA analysis of only LGIMA IDH1/2 and TP53-mutant tumors. Of 115 LGIMAs with both mutations (TCGA cohort), 25 tumors presented the R273C mutation (21.7%). The percentage of tumors with this mutation in our cohort (33.3%) is in line with that in the TCGA cohort (21.7%), despite being higher; it is also congruent with the frequency in all brain tumors, in which the hotspot mutation stands out when compared with that in other cancer types, according to the TCGA database (Figure 3b).
Then, as shown in Table 2, we compared the features of cases with the TP53 R273C mutation from our cohort with those of other cases, in order to find differences that could be related to this specific mutation in LGIMA. Interestingly, all the five tumors with the TP53 R273C mutation came from female patients. In contrast, tumors with other TP53 mutations had a similar distribution between both sexes: five were from men and four from women. Additionally, most of the tumors with the TP53 R273C mutation were of grade 2 (four of the five), while tumors with other mutations were more evenly distributed between grades 2 (six cases) and 3 (three cases). Tumors with the TP53 R273C mutation were diagnosed in patients with older ages (51.6 ± 13.9 vs. 42.3 ± 11.7) and showed similar sizes (34.4 cm3 ± 27.7 vs. 32.7 cm3 ± 47.0) when compared to tumors with other TP53 mutations.

Table 2.
Comparison of clinical data between LGIMAs with TP53 R273C mutation and LGIMAs with other TP53 mutations, from our cohort.
All grade 2 LGIMA tumors had low proliferation indexes, ranging from 1% to 7%, whereas grade 3 LGIMAs had values ranging from 5% to 12%, according to the Ki-67 labeling index. Remarkably, if we consider LGIMAs with the TP53 R273C mutation as a specific group, independent of the canonical grading, we find that these tumors had Ki67 levels between those of tumors of grades 2 and 3. There was an interesting tendency in cell proliferation between TP53 R273C LGIMAs and the grade 2 tumors with other TP53 mutations, since the Ki-67 index was higher in TP53 R273C tumors than it was in other grade 2 tumors (5.8 ± 3.0 vs. 3.5 ± 2.3), and it was lower in TP53 R273C tumors than it was in grade 3 LGIMAs (5.8 ± 3.0 vs. 8.7 ± 3.5) (Figure 4). Although these differences are not significant due to the small size of our cohort, the Ki67 values show three levels of cell proliferation if LGIMAs with the R273C mutation are considered a specific category. This finding may suggest the existence of grade 2 LGIMAs with different proliferative behaviors, depending on TP53 and perhaps other gene mutations, thereby promoting improving the current LGIMA classification through advanced genetic studies via NGS.

Figure 4.
Ki67 levels in grade 2 LGIMA with other mutations in TP53, LGIMA with the TP53 R273C mutation, and grade 3 LGIMA with other mutations in TP53. The results are the mean ± S.D. Grade 2: n = 6 cases; R273C: n = 5 cases; grade 3: n = 3 cases. * p < 0.05, Student’s t-test. n.s.: not significant.
Then, we investigated whether TP53 R273C mutations were mostly heterozygous or homozygous in our tumors. It is known that IDH1/2 mutations are ubiquitous in IDH-mutant astrocytomas, which means that all tumor cells present a heterozygous mutation of IDH1 or IDH2 and retain a wild-type allele, necessary for tumor cell survival. For this reason, the IDH mutation frequency in each tumor sample is considered a reference measure of tumor cellularity [17,18]. According to this, we established the ratios between the variant allele frequencies (VAFs) of TP53 mutations and IDH1/2 mutations for all tumors, in order to elucidate whether the tumors had homozygous (ratio of 2/1) or heterozygous (ratio of 1/1) TP53 mutations (Figure 5a). In our cohort, the majority of tumors showed homozygous mutations (9 of 14), which is concordant with data obtained across all kinds of cancers, where it has been shown that the loss of the wild-type TP53 allele, or second hit, occurs in approximately 90% of cases [19]. TP53 R273C mutations were homozygous (in three cases of five) and heterozygous (in two of five). Having one or two mutant alleles did not correlate with the Ki-67 index, the size of the tumor, or the main histologic features of the two grades. In order to evaluate the proportions of homozygous and heterozygous R273C mutations in LGIMAs, we studied the VAF ratios between TP53 R273C and IDH1/2 mutations in the TCGA cohort. Of the 25 cases with TP53 R273C, 19 were homozygous (76%), and 6 were heterozygous (24%) (Figure 5b). Remarkably, half of the heterozygous cases (three of six) were compound heterozygotes, with a second deleterious mutation in the other TP53 allele and, therefore, closer to homozygous features.
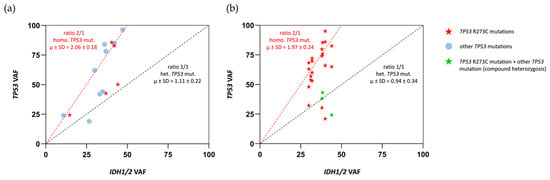
Figure 5.
(a) VAF ratios between TP53 and IDH1/2 mutations for each tumor in our CHGUV cohort. According to these, tumors were identified as homozygous (ratio of 2/1) or heterozygous (ratio of 1/1) for TP53 mutations. (b) VAF ratios between TP53 R273C and IDH1/2 mutations for each tumor in the TCGA cohort. According to these, tumors were identified as homozygous (ratio of 2/1) or heterozygous (ratio of 1/1) for TP53 R273C mutations.
Previous works revealed that TP53 R273C mutations are more common in women than in men in IDH-mutant astrocytomas of all grades. These mutations account for 26% of all TP53 mutations in women and only 11% in men [3]. In our cohort, only with LGIMAs, 55.6% of tumors from women had the TP53 R273C mutation (five cases of nine), and this mutation was not present in any of the tumors from men. In contrast, LGIMAs with other TP53 mutations were equally distributed between sexes (four in women and five in men). This difference was statistically significant (* p < 0.05, chi-squared test) (Figure 6a). In the TCGA LGIMA cohort, 25 tumors of 115 presented the R273C mutation (21.7%), while the other 90 (78.3%) had other TP53 mutations. The TP53 R273C mutation was slightly more frequent in women, and the other TP53 mutations were more frequent in men, but the differences between the two groups were not significant. Interestingly, the addition of our 14 LGIMA cases to the TCGA cohort made the differences significant, as shown in Figure 6b (** p < 0.01; chi-squared test). These findings unveil an apparent sex-dependent bias in the presence of TP53 R273C in IDH-mutant astrocytomas and, more prominently, in LGIMAs, which needs to be explored.
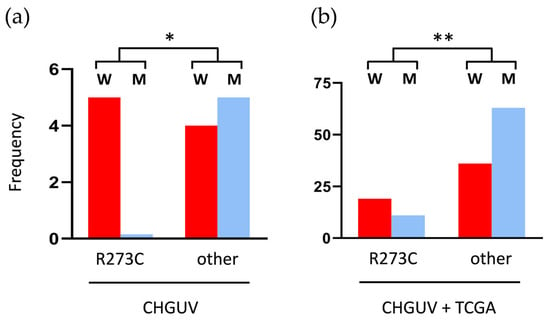
Figure 6.
Sex differences related to TP53 mutations in LGIMAs: (a) in all the LGIMAs of our CHGUV cohort; (b) in all the LGIMAs of our cohort plus 221 IDH-mutant and TP53-mutant LGIMAs from the TCGA database. * p < 0.05 and ** p < 0.01 (chi-squared test). W: women; M: men.
3. Discussion
The WHO glioma grading system has progressively improved in recent years, increasingly incorporating genetic criteria, which, together with histopathological parameters, allow for a more accurate classification of tumors [2,20]. The stratification of gliomas based on the presence or absence of mutations in IDH1 or IDH2 has represented a major advancement in the understanding of glioma biology and has improved diagnostic and prognostic accuracy, to the extent that this criterion alone is sufficient to discriminate between astrocytomas and glioblastomas. Additional genetic alterations widely reported in the literature are now considered in the refined classification of high-grade gliomas, such as mutations in TP53 and ATRX and EGFR amplification, further underscoring the predominant role of tumor genetics [21,22,23].
However, regarding LGIMAs, classification still relies exclusively on histopathological criteria and disregards genetic factors. Thus, the principal features distinguishing grade 2 from grade 3 IDH-mutant astrocytomas according to the WHO are histological anaplasia and increased mitotic activity. However, no studies on LGIMA cohorts have established a clear mitotic count threshold for stratifying risk between both grades [2]. Similarly, studies assessing proliferation based on the Ki-67 index have not defined criteria that unequivocally stratify risk among patients with IDH-mutant astrocytomas [2]. Although LGIMAs are less aggressive than grade 4 gliomas, their clinical outcomes vary widely and are difficult to predict; some tumors remain stable for extended periods, while others may progress to higher grades within months [8]. These observations suggest the need for more consistent classification criteria for LGIMAs, integrating both genetic and histopathological features for more accurate tumor characterization.
One gene that could be of particular relevance for LGIMA stratification is TP53. Among TP53 mutations, R273C stands out in brain tumors, especially in IDH-mutant astrocytomas. This mutation is present in approximately 25% of IDH-mutant astrocytomas; it is significantly less prevalent in other cancer types, where, although also considered a hotspot mutation, it occurs in only 2.7% of cases [24,25,26].
In our initial cohort of 72 infiltrative astrocytic tumors, 14 were diagnosed as LGIMAs (ten were grade 2 and four were grade 3), all of which harbored TP53 mutations. Of these, 33.3% carried the TP53 R273C mutation. This proportion is slightly higher than that observed in the TCGA cohort (21.7%), although both values are consistent with previous reports of a 20–30% prevalence in IDH-mutant astrocytomas [3] and with the high frequency of this mutation in brain tissue (Figure 3b).
The R273C mutation is associated with worse prognosis, faster progression, and shorter survival, both in IDH-mutant astrocytomas and other cancers [3]. It is known that LGIMAs are characterized by low proliferative activity, typically reflected by low Ki-67 values. R273C is also linked to poor outcomes in these tumors, despite their relatively less aggressive nature [9]. In our LGIMA cohort, grade 3 tumors showed higher Ki-67 indices than grade 2 tumors. Interestingly, the tumors harboring the R273C mutation exhibited higher Ki-67 values than those of grade 2 and with other TP53 mutations. In fact, tumors with the R273C mutation appear to constitute a category different from grade 2 and grade 3, according to the Ki67 profiles. This suggests that the current grade 2 classification may include tumors with higher biological aggressiveness that are not being accurately diagnosed. Our findings support the distinct role for R273C in grade 2 LGIMAs, making Ki-67 levels higher, possibly explaining the notably more aggressive behavior observed in these cases.
The higher frequency of TP53 R273C in IDH-mutant astrocytomas compared to other TP53 mutations could be related to the production of D-2-hydroxyglutarate (D-2HG) by these tumors. The neomorphic activity of the mutant IDH1/2 enzymes produces this metabolite, which promotes a hypermethylated state of DNA and histones [7,17], potentially favoring the occurrence of R273C. Enrichment of TP53 R273C may occur via two possible mechanisms—selective mutagenesis or selective advantage—with the latter being, apparently, more likely [3].
Recent studies have shown that over 90% of all cancers with TP53 mutations eventually lose the wild-type allele [19], consistent with TP53’s canonical role as a tumor suppressor. Marker et al. reported that all gliomas with the R273C mutation in their cohort, regardless of subtype or grade, lost their wild-type allele [3]. Our data, both from the CHGUV and TCGA cohorts, indicate that most R273C-mutant tumors were either homozygous for this mutation or harbored R273C in one allele and a different TP53 mutation in the other (compound heterozygosis). A minority of cases exhibited R273C in only one allele, without other mutational findings. Two scenarios are possible here: (i) hemizygosity for TP53 R273C with loss of the wild-type allele, consistent with tumor suppressor gene dynamics; (ii) true heterozygosity with retention of the wild-type allele. Based on our observations, the most probable scenario is loss of the wild-type allele in the vast majority of cases, resulting in only one allele carrying the R273C mutation.
Structural studies and in vitro models have demonstrated that the R273C mutation leads to a dramatic reduction in the DNA binding affinity of p53, with no p53-mediated transcriptional activity, although the protein retains wild-type stability [27,28]. This R273C p53 protein, having lost its original function, may gain novel functions with distinct biological effects in tumors. It is well established that loss of p53 activity is associated with increased tumor mutation burden [29]. In the case of R273C, this burden is higher than that for other TP53 mutations in LGIMAs and has been linked to differential expression of up to 13 genes from the HOX family, which are involved in embryogenesis. The R273C p53 protein may contribute to these transcriptional changes [9]. Additionally, R273C has been implicated in mechanisms activating NF-κB, which may influence tumor behavior [30]. Nevertheless, the role of R273C remains incompletely understood, and its effects on various cancers are still under debate [12,14,15,31]. Mounting evidence suggests that p53 R273C may exert unique effects on tumor cell biology, conferring selective advantages to these cells within the tumor context [3].
Our data suggest a possible association between the TP53 R273C mutation and female sex in patients with LGIMAs, as all five tumors with this mutation in our cohort occurred in female patients. Analysis of existing data from IDH-mutant astrocytomas of various grades in the TCGA database revealed a similar trend: R273C mutations were present in 26% of tumors in women compared to 11% in men [3]. In our LGIMA-specific cohort, the mutation was found in 55.5% of tumors from female patients (five of nine) and was absent in male tumors (zero of five), although the limited number of male cases may have been a confounding factor. To address this limitation, we examined the TCGA dataset. Among 115 IDH- and TP53-mutant LGIMAs, most tumors with the R273C mutation occurred in females, whereas other TP53 mutations were more common in males. While these differences were not statistically significant in the TCGA dataset alone, combining our 14 LGIMAs with the TCGA cohort rendered the trends statistically significant. The increased frequency of TP53 R273C in female LGIMA patients remains unexplained, although sex-based differences in gene expression or tumor microenvironments may play a role in selective pressures affecting LGIMAs.
As with glioblastomas and grade 4 astrocytomas, the classification of LGIMAs should be revised to incorporate genetic findings, alongside new refined and unambiguous histopathological criteria. Beyond TP53 mutations, other recurrently mutated genes in LGIMAs include ATRX, CIC, FUBP1, MUC16, and NOTCH, as reported in the TCGA database. Additional relevant mutations may remain undiscovered, requiring more powerful genomic diagnostic tools. NGS is a modern approach that can aid in several ways: First, it can identify novel target genes missed by current testing strategies, but this requires broad gene panels that go beyond the most predictable glioma-associated genes. Second, it can distinguish between different mutations within the same gene, since, as demonstrated for R273C, specific mutations may have distinct biological implications. This may represent a major advancement over traditional histological techniques that detect only selected mutations. Third, NGS could improve the WHO classification, particularly for LGIMAs, and help explain why tumors of the same histological grade can behave so differently across patients.
In conclusion, we show that the TP53 R273C mutation confers a distinct biological profile in LGIMAs, characterized by a level of cellular proliferation that might be intermediate between grades 2 and 3 (as measured by Ki-67) and more prevalent among female patients. These findings support a proposed update to the current WHO classification to incorporate the genetic profiles of LGIMAs, especially those harboring R273C, which may be underestimated and may require closer follow-up in order to provide more accurate diagnostic and prognostic guidance for patients.
4. Materials and Methods
4.1. Cohort Description
Fourteen low-grade, IDH-mutant astrocytomas were selected from the archives of the Department of Pathology, Consorcio Hospital General Universitario de Valencia (CHGUV), from a list of 72 adults with IDH-mutant astrocytomas. The cohort data were collected according to the protocol approved by the CHGUV Biobank (B.0001392), and this study was approved by the Institutional Research Ethics Committee of the CHGUV on 24 November 2023 (Registration No. 97/2023), based on the following selection criteria: adults over 18 years, grade 2 or 3 diffuse IDH-mutant astrocytomas, complete surgical resection, follow-up of more than 24 months, and available histological and molecular studies.
4.2. Clinical, Histological, and Immunohistochemical Data
Clinical and histological data were collected from pathology reports and information on clinical history: (i) clinical data: sex (male/female), patient age (years at diagnosis), clinical status (death due to disease, alive with disease, alive free of disease, relapses), and survival in months; (ii) histological data: resected tumor size, histological grade, mitotic rate in 2 mm2, % Ki-67 (proliferative index), % p53 neoplastic nuclei, ATRX neoplastic nuclear expression, and IDH1 neoplastic nuclear expression; (iii) FISH: 1p/19q codeletion. Slides were reviewed by two pathologists. Briefly, tumors were formalin-fixed and paraffin-embedded, and sections were stained with hematoxylin and eosin. An immunohistochemical study was carried using a Ventana Autostainer (Roche Diagnostics, Barcelona, Spain) with Ventana antibodies (IDH1 antibody (Ref: MAD-00047SGLD; clone H09; 1.5 μg/mL); p53 antibody (Ref: (92)800-2912; clone DO-7; 0.5 μg/mL); anti-Ki-67 antibody (Ref: (92)790-4286, clone 30-9, 2 μg/mL)) and a Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA) antibody (ATRX (Ref: HPA001906-100; rabbit polyclonal; 1/50 dilution)). The FISH study on 1p/19q was conducted using paraffin sections from 7 cases, using LSI 1p36/19q13 probe sets from Vysis (Abbott Laboratories, Downers Grove, IL, USA).
4.3. Molecular Studies
Molecular analyses were performed using the OncomineTM Precision Assay next-generation sequencing panel (Thermo Fisher Scientific, Valencia, Spain). This panel detects hotspot mutations (substitutions, insertions, and deletions), copy number variations (CNVs), and gene fusions across 50 cancer driver genes.
In order to compare our CHGUV cohort with other larger cohorts, we studied additional LGIMA cases from the Cancer Genome Atlas (TCGA, https://www.cancer.gov/ccg/research/genome-sequencing/tcga, accessed on 14 July 2025). We selected a cohort with 115 LGIMA cases with IDH1/2 and TP53 mutated (TCGA cohort). Molecular data were obtained from cBioPortal (https://www.cbioportal.org, last accessed 29 April 2025).
4.4. Statistical Analysis
Statistical analyses were performed using GraphPad Prism software, version 8.0.1. (San Diego, CA, USA), with the results presented as the mean ± S.D. These analyses were conducted using one-way analysis of variance (ANOVA) followed by Student’s t-test for dual comparisons. The chi-squared test was used to examine the correlation between TP53 mutations and the sex of the patients. A p-value < 0.05 was considered statistically significant.
Author Contributions
Conceptualization, T.S.-M., E.R.-S., and J.M.; methodology, L.N., I.S.-S., M.S.-P., and J.M.; software, L.N., E.R.-S., T.S.-M., and J.M.; validation, T.S.-M., E.R.-S., and J.M.; formal analysis, L.N., E.R.-S., T.S.-M., and J.M.; investigation, J.M., T.S.-M., E.R.-S., L.N., I.S.-S., and M.S.-P.; resources, T.S.-M. and E.R.-S.; data curation, L.N., E.R.-S., T.S.-M., and J.M.; writing—original draft preparation, J.M., T.S.-M., and E.R.-S.; writing—review and editing, J.M., T.S.-M., and E.R.-S.; visualization, J.M., T.S.-M., and E.R.-S.; supervision, J.M., T.S.-M., and E.R.-S.; project administration, T.S.-M. and E.R.-S.; funding acquisition, T.S.-M. and E.R.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This original research was funded by Generalitat Valenciana, Conselleria de Educación, Cultura, Universidades y Empleo, grant number CIGE/2023/64 (PBL00194); Generalitat Valenciana, grant number CISEJI/2026/049; and Consorcio Hospital General Universitario de Valencia & Fundación HGU (FIHGUV), grant number Premio López-Trigo n.8 (PLV000723).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the CHGUV Biobank (B.0001392) and by the Institutional Ethics Committee of CHGUV (protocol code 97/2023) (24 November 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors acknowledge Consorcio Hospital General Universitario de Valencia and Fundación para la Investigación Hospital General Universitario de Valencia for their continuous support for this research.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| CHGUV | Consorcio Hospital General Universitario de Valencia |
| CNS | Central nervous system |
| IDH | Isocitrate dehydrogenase |
| IMA | IDH-mutant astrocytoma |
| LGIMA | Low-grade, IDH-mutant astrocytoma |
| NGS | Next-generation sequencing |
| TCGA | The Cancer Genome Atlas |
| WHO | World Health Organization |
References
- Rajesh, Y.; Pal, I.; Banik, P.; Chakraborty, S.; Borkar, S.A.; Dey, G.; Mukherjee, A.; Mandal, M. Insights into Molecular Therapy of Glioma: Current Challenges and next Generation Blueprint. Acta Pharmacol. Sin. 2017, 38, 591–613. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Marker, D.F.; Agnihotri, S.; Amankulor, N.; Murdoch, G.H.; Pearce, T.M. The Dominant TP53 Hotspot Mutation in IDH -Mutant Astrocytoma, R273C, Has Distinctive Pathologic Features and Sex-Specific Prognostic Implications. Neuro Oncol. Adv. 2022, 4, vdab182. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Aoki, K.; Chiba, K.; Sato, Y.; Shiozawa, Y.; Shiraishi, Y.; Shimamura, T.; Niida, A.; Motomura, K.; Ohka, F.; et al. Mutational Landscape and Clonal Architecture in Grade II and III Gliomas. Nat. Genet. 2015, 47, 458–468. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Berzero, G.; Di Stefano, A.L.; Ronchi, S.; Bielle, F.; Villa, C.; Guillerm, E.; Capelle, L.; Mathon, B.; Laurenge, A.; Giry, M.; et al. IDH-Wildtype Lower-Grade Diffuse Gliomas: The Importance of Histological Grade and Molecular Assessment for Prognostic Stratification. Neuro Oncol. 2021, 23, 955–966, Erratum in Neuro Oncol. 2023, 25, 1011–1012. [Google Scholar] [CrossRef]
- Cairns, R.A.; Mak, T.W. Oncogenic Isocitrate Dehydrogenase Mutations: Mechanisms, Models, and Clinical Opportunities. Cancer Discov. 2013, 3, 730–741. [Google Scholar] [CrossRef]
- van den Bent, M.J.; Brandes, A.A.; Taphoorn, M.J.B.; Kros, J.M.; Kouwenhoven, M.C.M.; Delattre, J.-Y.; Bernsen, H.J.J.A.; Frenay, M.; Tijssen, C.C.; Grisold, W.; et al. Adjuvant Procarbazine, Lomustine, and Vincristine Chemotherapy in Newly Diagnosed Anaplastic Oligodendroglioma: Long-Term Follow-up of EORTC Brain Tumor Group Study 26951. J. Clin. Oncol. 2013, 31, 344–350. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Fang, Y.; Li, J.; Chen, Y.; Jiao, S. TP53 R273C Mutation Is Associated With Poor Prognosis in LGG Patients. Front. Genet. 2022, 13, 720651. [Google Scholar] [CrossRef]
- Petitjean, A.; Mathe, E.; Kato, S.; Ishioka, C.; Tavtigian, S.V.; Hainaut, P.; Olivier, M. Impact of Mutant P53 Functional Properties on TP53 Mutation Patterns and Tumor Phenotype: Lessons from Recent Developments in the IARC TP53 Database. Hum. Mutat. 2007, 28, 622–629. [Google Scholar] [CrossRef]
- Salnikova, L.E. Clinicopathologic Characteristics of Brain Tumors Are Associated with the Presence and Patterns of TP53 Mutations: Evidence from the IARC TP53 Database. NeuroMolecular Med. 2014, 16, 431–447. [Google Scholar] [CrossRef]
- Chan, K.-T.; Lung, M.L. Mutant P53 Expression Enhances Drug Resistance in a Hepatocellular Carcinoma Cell Line. Cancer Chemother. Pharmacol. 2004, 53, 519–526. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Qi, F.; Hu, X.; Luo, J. Exploration of the Relationships between Tumor Mutation Burden with Immune Infiltrates in Clear Cell Renal Cell Carcinoma. Ann. Transl. Med. 2019, 7, 648. [Google Scholar] [CrossRef]
- Rasti, M.; Azimi, T. TP53 Binding to BRCA1 and RAD51 in MCF7 and MDA-MB-468 Breast Cancer Cell Lines In Vivo and In Vitro. Avicenna J. Med. Biotechnol. 2015, 7, 76–79. [Google Scholar]
- Román-Rosales, A.A.; García-Villa, E.; Herrera, L.A.; Gariglio, P.; Díaz-Chávez, J. Mutant P53 Gain of Function Induces HER2 Over-Expression in Cancer Cells. BMC Cancer 2018, 18, 709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Hakin-Smith, V.; Teo, M.; Xinarianos, G.E.; Jellinek, D.A.; Carroll, T.; McDowell, D.; MacFarlane, M.R.; Boet, R.; Baguley, B.C.; et al. Association of Mutant TP53 with Alternative Lengthening of Telomeres and Favorable Prognosis in Glioma. Cancer Res. 2006, 66, 6473–6476. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH Mutation in Glioma: Molecular Mechanisms and Potential Therapeutic Targets. Br. J. Cancer 2020, 122, 1580. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gurav, M.; Dhanavade, S.; Shetty, O.; Epari, S. Diffuse Glioma—Rare Homozygous IDH Point Mutation, Is It an Oncogenetic Mechanism? Neuropathology 2017, 37, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Donehower, L.A.; Soussi, T.; Korkut, A.; Liu, Y.; Schultz, A.; Cardenas, M.; Li, X.; Babur, O.; Hsu, T.-K.; Lichtarge, O.; et al. Integrated Analysis of TP53 Gene and Pathway Alterations in The Cancer Genome Atlas. Cell Rep. 2019, 28, 1370–1384.e5. [Google Scholar] [CrossRef]
- Wesseling, P.; Capper, D. WHO 2016 Classification of Gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef]
- Galbraith, K.; Snuderl, M. Molecular Pathology of Gliomas. Surg. Pathol. Clin. 2021, 14, 379–386. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, C.; Gibert, M.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The P53 Pathway in Glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef]
- López-Ginés, C.; Muñoz-Hidalgo, L.; San-Miguel, T.; Megías, J.; Triviño, J.C.; Calabuig, S.; Roldán, P.; Cerdá-Nicolás, M.; Monleón, D. Whole-Exome Sequencing, EGFR Amplification and Infiltration Patterns in Human Glioblastoma. Am. J. Cancer Res. 2021, 11, 5543–5558. [Google Scholar]
- Li, J.; Yang, L.; Gaur, S.; Zhang, K.; Wu, X.; Yuan, Y.-C.; Li, H.; Hu, S.; Weng, Y.; Yen, Y. Mutants TP53 p.R273H and p.R273C but Not p.R273G Enhance Cancer Cell Malignancy. Hum. Mutat. 2014, 35, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Baugh, E.H.; Ke, H.; Levine, A.J.; Bonneau, R.A.; Chan, C.S. Why Are There Hotspot Mutations in the TP53 Gene in Human Cancers? Cell Death Differ. 2018, 25, 154–160. [Google Scholar] [CrossRef]
- Freed-Pastor, W.A.; Prives, C. Mutant P53: One Name, Many Proteins. Genes Dev. 2012, 26, 1268–1286. [Google Scholar] [CrossRef]
- Eldar, A.; Rozenberg, H.; Diskin-Posner, Y.; Rohs, R.; Shakked, Z. Structural Studies of P53 Inactivation by DNA-Contact Mutations and Its Rescue by Suppressor Mutations via Alternative Protein-DNA Interactions. Nucleic Acids Res. 2013, 41, 8748–8759. [Google Scholar] [CrossRef] [PubMed]
- Baroni, T.E.; Wang, T.; Qian, H.; Dearth, L.R.; Truong, L.N.; Zeng, J.; Denes, A.E.; Chen, S.W.; Brachmann, R.K. A Global Suppressor Motif for P53 Cancer Mutants. Proc. Natl. Acad. Sci. USA 2004, 101, 4930–4935. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 Human Cancer Genomes Reveals the Landscape of Tumor Mutational Burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef]
- Soubannier, V.; Stifani, S. NF-κB Signalling in Glioblastoma. Biomedicines 2017, 5, 29. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Liu, W.; Mao, Y.; Fu, Z.; Liu, J.; Huang, W.; Zhang, Z.; An, D.; Li, B. Human Papillomavirus Infection Increases the Chemoradiation Response of Esophageal Squamous Cell Carcinoma Based on P53 Mutation. Radiother. Oncol. 2017, 124, 155–160. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).